Abstract
Objective
The present study aims to explore the correlation of the transforming growth factor β (TGF-β), drosophila mothers against decapentaplegic protein gene (SMAD) 2/3/4, and leukemia inhibitory factors (LIF) with the cyst formation of hepatic Echinococcus granulosus in young children.
Methods
A total of 40 patients who met the diagnostic criteria for children's hydatid disease in people's Hospital of Xinjiang Uygur Autonomous Region between January 2020 and June 2021 were enrolled a s the study subjects. The cystic fluid of these children was collected as the case group and the corresponding infected viscera or pericystic tissue as the control group, with 40 cases in each group. In vitro cultured protoscolice of hydatid cyst, four groups including control group, LIF siRNA group, LIF factor group and SMAD4 siRNA group were divided by inhibiting TGF-β/SMADs signal pathway. Each assay was performed in triplicate. The expression of TGF-β, SMAD2/3/4 and LIF were detected.
Results
The results of the clinical trial showed that the contents of SMAD2 and SMAD3 were increased in the case group compared with the control group; the differences were statistically significant (P < 0.05). The expression levels of TGF-β, Smad4, and LIF increased in the case group compared with the control group; however, the differences were not statistically significant. The results of further in vitro experiments, the expression levels of TGF-β, SMAD 2/3/4, and LIF after adding siRNA to interfere with Smad4 decreased in the case group compared with the control group; the differences were statistically significant (P < 0.05). Compared with the control group, the expression levels of TGF-β, SMAD2/3/4, and LIF increased after treatment with added LIF in the case group, and the expression levels of TGF-β, SMAD2/3/4, and LIF decreased after adding siRNA to interfere with LIF in the case group; the differences were all statistically significant (P < 0.05).
Conclusion
SMAD2 and SMAD3 have a certain clinical relevance with hydatidosis in young children. The LIF expression level may be related to the cystic transformation of protoscoleces. It has been suggested that the TGF-β/Smads/LIF signaling pathway may be present in the process of protoscoleces cyst formation; this provides a research basis for the prevention and treatment of post-infection parasitism of E. multilocularis eggs in young children.
Similar content being viewed by others
Introduction
Hydatidosis or echinococcosis is a disease caused by the larva of Echinococcus granulosus (E. granulosus) infecting the human body [1]. In Northwest China, especially in large pastoral areas (such as Xinjiang and Tibet), children are infected with cystic echinococcosis at a high prevalence, seriously endangering their health. Large hydatid cysts are common in the detection of hydatidosis in children, and the complication rate of cyst rupture is higher than in adults [2, 3]. At present, the regulation mechanism of Echinococcus multilocularis (E. multilocularis) eggs forming cysts in a human host after infection is unclear. Relevant studies revealed that there was a complex molecular regulation mechanism in the process from the infection of E. multilocularis eggs to the successful parasitic development, and there was a close mutual response between the host’s body and the larvae. Furthermore, the transforming growth factor β (TGF-β) played a crucial role in these interactive responses [4,5,6]. However, at present, studies on hydatidosis are mostly focused on adults, and there are few reports on its occurrence in children. It is unclear whether children’s hydatidosis contains the same molecular regulation mechanism. Therefore, the TGF-β/LIF signaling pathway was chosen as the research object in this study in order to conduct a clinical trial and in vitro experiments to further explore the role of this signaling pathway in the in vivo cyst formation process after the infection of E. multilocularis eggs in children; this is done with the aim to (1) reveal the relevant molecular mechanism of the cyst formation process of hydatidosis parasites in children and (2) hopefully provide a research basis for the prevention and treatment of cystic echinococcosis in children.
Data and methods
Clinical study
General information
After obtaining the approval of the ethics committee of people's Hospital of Xinjiang Uygur Autonomous Region and the signed informed consent of the children's parents, the cystic fluid (20 ml) and the corresponding paracystal tissues of infected viscera or tissues were collected from children with hydatidosis who met the diagnostic criteria [7] of pediatric hydatidosis in our hospital from January 2020 to June 2021. The paracystal tissues of infected viscera or tissues were used as the control group, and the cystic fluid was used as the case group (n = 40 each). All operations were performed with the consent of the children and their families, and relevant informed documents were signed.
Experimental methods
Sample processing
The tissues were washed with pre-cooled phosphate-buffered saline (PBS), cut into pieces, and weighed. The small pieces of tissue were mixed with the corresponding volume of PBS (generally at the weight/volume ratio of 1:9) and fully ground.
Experimental steps
Blank wells (blank control wells without samples and enzyme-labeled reagents) and wells for the sample to be tested were set, respectively. A volume of 40 μl of sample diluent was added into the wells for the sample to be tested on the enzyme-labeled coating plate; next, 10 μl of the sample to be tested was added (the final dilution of the sample was 5×). After sealing the plate with plate sealing membrane, it was incubated at 37°C for 30 minutes. The 30× concentrated washing solution was diluted with distilled water 30× and set aside. The sealing membrane was carefully removed, the liquid was discarded, and the plate was swayed for drying. Each well was filled with detergent, left standing for 30 seconds, and discarded; this procedure was repeated 5×, then the plate was patted dry.
A volume of 50 μL of enzyme-labeled reagent was added to each well, with the exception of the blank well. The plate was incubated at a warm temperature again and washed. Each well was first added with 50 μL of developer A, then with 50 μL of developer B, and was then slightly shaken to mix; the contents then underwent color development at 37°C in the dark for 10 minutes. Each well was added with 50 μL of termination solution, and the reaction was stopped after the blue turned yellow. Zero adjustments were carried out based on the blank well, and the absorbance (optic density) of each well was measured at 450 nm of wavelength according to the number. The measurements were all carried out within 15 minutes after the termination solution was added.
Test indexes
The levels of TGF-β, SMAD2, SMAD3, SMAD4, and LIF in the cystic fluid of children with hydatidosis and the corresponding paracystal tissues of infected viscera or tissues were measured by the enzyme-linked immunosorbent assay (ELISA).
In vitro study
In vitro culture and grouping of protoscoleces
The method of Liying Yuan [8] was adopted to digest, separate, and culture protoscoleces obtained from the inner cyst of the hydatid cyst in children with parasitic Echinococcus. The activity of the protoscoleces was detected using 0.1% eosin staining. Protoscoleces with >90% activity were used for testing. Four groups were established for this experiment: the control group (control), the LIF siRNA group (LIF siRNA), the LIF factor group (LIF factor), and the Smad4 siRNA group (SMAD4 siRNA). Each group was triplicate.
Experimental methods
The isolated protoscoleces cultured in a six-well plate were observed. Under the visual field, the number, proportion, and activity of protoscoleces with cystic characteristics were examined and recorded. The protoscoleces were treated with LIF, LIF siRNA interference, and SMAD4 siRNA interference, respectively. After the treatment, the number, proportion, and activity of protoscoleces with cystic characteristics were examined under the visual field and recorded. After microscopic examination, the cultured protoscoleces of each group were killed, and the total protein was extracted. The expression of LIF was detected by ELISA. The correlation between LIF expression after interference and the regulation of protoscoleces cyst formation was statistically analyzed.
Test indexes
Expression levels of TGF-β, SMAD2, SMAD3, SMAD4, and LIF in the samples of Echinococcus Taenia protoscoleces of each group were detected using ELISA. The ELISA method was the same as in the clinical experiment.
Statistical methods
Data were statistically analyzed using the SPSS 17.0 software, and statistical analysis and charting were conducted using the GraphPad Prism 7.00 software. In the comparison of the clinical trial data, measurement data were expressed as mean ± standard deviation (\(\overline{\mathrm x}\) ± SD), and count data were expressed as percentages (%); comparisons were made using the t-test and rank-sum test, respectively. The data obtained from the in vitro experiment were compared among groups using a one-way analysis of variance (F test). The inspection level was set at α = 0.05.
Results
Clinical trials
Expression levels of TGF-β, SMAD2, SMAD3, SMAD4, and LIF
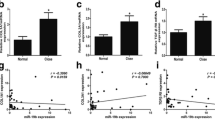
The expression levels of TGF-β, SMAD2, SMAD3, SMAD4, and leukemia inhibitory factors (LIF) in the cystic fluid and the corresponding paracystal tissues of infected viscera or tissues in children with hydatidosis were measured using the ELISA kit. The contents of SMAD2 and SMAD3 increased in the case group compared with the control group; the differences were statistically significant (P < 0.05, P < 0.01). The TGF-β, SMAD4, and LIF expression levels increased in the case group; however, the differences were not statistically significant (Table 1 and Fig. 1).
In vitro experiment
Expression levels of TGF-β, SMAD2, SMAD3, SMAD4, and LIF
After adding siRNA to interfere with SMAD4, the expression levels of TGF-β, SMAD2/3/4, and LIF decreased in the case group compared with the control group; the differences were statistically significant (P < 0.05, P < 0.01, P < 0.001). The expression levels of TGF-β, SMAD2/3/4, and LIF increased after treatment with added LIF in the case group compared with the control group; the differences were statistically significant (P < 0.01, P < 0.001, P < 0.05). After adding siRNA to interfere with LIF, the expression levels of TGF-β, SMAD2/3/4, and LIF decreased in the case group compared with the control group; the differences were statistically significant (P < 0.01, P < 0.05) (Table 2 and Fig. 2).
Discussion
Characteristics and hazards of hydatidosis in young children
(1) As the liver tissue of young children has a loose structure and is active in development, it provides a relatively good living environment for the activation of E. granulosus eggs [7]; eggs can be activated and can grow rapidly after entering the hepatic portal vein through the intestinal mucosa. (2) A hydatid cyst in young children is in the early cyst stage, with a high activity of cyst fluid and great tension of the cyst wall, which is prone to rupture and infection. (3) The morphological characteristics of hepatic cystic Echinococcosis in young children are that the cyst grows faster than in adults, the cyst wall is thin, and the infection often simultaneously occurs in both the liver and the lung. (4) Due to the poor self-protection awareness of young children, the probability of intraperitoneal implantation due to the rupture of the hydatid cyst is higher than in adults, and the cure is difficult [9]. In summary, after the formation of a hepatic hydatid cyst in children, they are either surgically removed or eventually rupture, resulting in abdominal implantation; this causes serious trauma to the child’s physical and mental health. Blocking the cyst formation in the early stages in the early stage after the hydatid eggs enter the children’s body is very important for the treatment of children’s hydatidosis.
TGF-β/ Smads/LIF signaling pathway
A large number of previous studies revealed that serum expression levels of TGF-α and TGF-β were significantly higher in patients with hydatidosis than in non-patients, and the expression levels of TGF-α and TGF-β in cystic tissue effusion were significantly higher than in paracystal tissue or the outer capsule in patients. Researchers also found through studies on the inflammatory factors of alveolar hepatic hydatidosis that TGF-β and other cytokines played important roles in the progression of hepatic hydatidosis [4,5,6]. Leukemia inhibitory factors (LIF) are a cytokine with multiple functions [10]. The most important discovery is that LIF can maintain the pluripotency of mouse embryonic stem cells [11, 12]. The loss of cell pluripotency is a necessary process during the development and maturation of the body, and cell pluripotency is gradually lost with the degree of cell differentiation and the maturity of body development. Only a few cells in a mature organism can maintain pluripotency. In fact, TGF-β is not only an important anti-inflammatory factor but also a very important transforming growth factor in the body; it regulates important signaling pathways in cells. The regulatory network that TGF-β participates in within the body is very complex. The members of Smads protein family in the pathway mainly include SMAD2, SMAD3 and SMAD4. The SMAD protein is a very important transcription regulatory factor of the TGF-β superfamily; it can regulate the expression of many factors in cells, one of which is LIF. Previous studies revealed that when Smad4 or SMAD2/3 is interfered in by siRNA, the intracellular expression of LIF induced by TGF-β will be significantly inhibited [13,14,15].
Pathological and physiological differences between cystic fluid and paracystic tissue
Echinococcus contains many protoscolice (scolex). Chen et al. [16] stimulated spleen cells of mice with protoscolice and hydatid cyst fluid respectively, and the results showed that the increase of TGF-β is consistent at the protein level and the molecular level, thus affecting the immune escape from the host. Zhou et al. [17] stimulated mouse spleen cells with hydatid cyst fluid, The increase of SMAD4, the downstream signal transduction protein of TGF-β, suggests that the differentiation of Treg cells and the SMAD4 signal pathway may be involved in the immune escape process of the cyst fluid to the host, which is consistent with the results of this study in vitro.
Therefore, we speculate that the SMAD pathway in pericystic tissues (hepatocytes) is inactive under normal conditions, and can specifically activate TGF/Smads signal pathway under the stimulation of hydatid cyst stimulates Stimulate the phosphorylation of TGF-β receptors distributed on the liver cell membrane, and then induce the expression of receptor regulated SMAD2/3/4 in the cytoplasm to increase, activate LIF factor. The SMAD protein transmits the pathogenic signal into the nucleus, and the corresponding target genes in the nucleus are activated and transcribed, leading to pathological liver injury. The host's immune ability may be down regulated, thus avoiding the host's immune attack.
Analysis of study results
In the clinical part of this study, the levels of SMAD2 and SMAD3 in the cystic fluid were significantly higher in the case group than in the control group; the differences were statistically significant (P < 0.05, P < 0.01). Further in vitro experiment verified that after adding siRNA to interfere with SMAD4, the expression levels of TGF-β, SMAD 2/3/4, and LIF decreased in the case group compared with the control group; the differences were statistically significant (P < 0.05). Compared with the control group, the expression levels of TGF-β, SMAD2/3/4, and LIF increased after treatment with added LIF in the case group. After adding siRNA to interfere with LIF, the expression levels of TGF-β, SMAD2/3/4, and LIF decreased in the case group compared with the control group; the differences were all statistically significant (P < 0.05). Although the expression of TGF-β was not statistically significant in clinical trials, its content in the case group showed an upward trend. The reasons for this are as follows: (1) the sample size was small; (2) in this study, the paracystal tissues of the corresponding infected viscera or tissues in the body of children were greatly interfered in by the factors of different infection states in the host. In future clinical trials, the in vivo infection factors in the host need to be further eliminated and the sample size needs to be increased to optimize the experiment. Therefore, it is speculated that in the process of human infection with hydatid eggs in this study, the eggs hatched into larvae and then stopped developing into cysts; during this process, the pluripotency of hydatid cells was partially lost. The key factor that could maintain this pluripotency may be the LIF factor activated by a surging expression of TGF-β. Due to the small sample size of this study, whether it is generalizability and applicability for the general pediatrics population with cystic echinoisis is unclear and still needs to be verified by a large amount of research data.
Conclusions
The present study suggests that SMAD2 and SMAD3 have certain clinical relevance to hydatidosis in young children. The LIF may be related to the cystic transformation of protoscoleces. It is suggested that the TGF-β/Smads/LIF signaling pathway may be present in the process of protoscoleces cyst formation. This provides a research basis for the prevention and treatment of post-infection parasitism of E. multilocularis eggs in young children.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
Petropoulos AS, Chatzoulis GA. EchinococcusGranulosus in Childhood: A Retrospective Study of 187 Cases and Newer Data. Clin Pediatr. 2019;58(8):864–88. https://doi.org/10.1177/0009922819847032.
Tartar T, Bakal U, Sarac M, Kazez A. Laboratory results and clinical findings of children with hydatid cyst disease. Niger J Clin Pract. 2020;23(7):1008–12. https://doi.org/10.4103/njcp.njcp_531_19.
Demir S, Ilikan GB, Erturk A, Oztorun CI, Guney D, Azili MN, et al. A serious complicaton of liver hydatid cysts in children: cystobiliaryfistulas. Pediatr Surg Int. 2020;36(5):611–20. https://doi.org/10.1007/s00383-020-04637-9.
Mejri N, Hemphill A, Gottstein B. Triggering and modulation of the host-parasite interplay by Echinococcusmultilocularis: a review. Parasitology. 2010;137(3):557–68. https://doi.org/10.1017/S0031182009991533.
Mejri N, Müller J, Gottstein B. Intraperitoneal murineEchinococcusmultilocularis infection induces differentiation of TGF-beta expressing DCs that remain immature. Parasite Immunol. 2011;33(9):471–82. https://doi.org/10.1111/j.1365-3024.2011.01303.x.
Mejri N, Müller N, Hemphill A, Gottstein B. Intraperitoneal Echinococcus multilocularis infection in mice modulates peritoneal CD4+ and CD8+ regulatory T cell development. Parasitol Int. 2011;60(1):45–53. https://doi.org/10.1016/j.parint.2010.10.002.
Chinese Doctor Association, Chinese College of Surgeons (CCS), Chinese Committee for Hadytidology (CCH). Expert consensus on diagnosis and treatment of hepatic cystic and alveolar echinococcosis (2019 edition). Chin J Dig Surg. 2019;18(8):711–21. https://doi.org/10.3760/cma.j.issn.1673-9752.2019.08.002.
Yuan LY, Zhang ZZ, Shi BX, Gunul T, Mi XY, Wang JC, et al. Establishment of in vitro cultivation model of protoscolex of Echinococcus cyst. Animal Husbandry Vet Med. 2009;41(07):29–31.
Tersigni C, Venturini E, Montagnani C, Bianchi L, Chiappini E, de Martino M, et al. Should Pediatricians Be Aware of Cystic Echinococcosis?A Literature Review. J Pediatr Gastroenterol Nutr. 2019;68(2):161–8. https://doi.org/10.1097/MPG.0000000000002182.
Bamber BA, Masters BA, Hoyle GW, Brinster RL, Palmiter RD. Leukemia Inhibitory Factor Induces Neurotransmitter Switching in Transgenic Mice. Pro Natl Acad Sci USA. 1994;91(17):7839–43. https://doi.org/10.1073/pnas.91.17.7839.
Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25(19):2697–707. https://doi.org/10.1038/sj.onc.1209307.
Torres J, Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFκB and cooperating with Stat3. Nat Cell Biol. 2008;10(2):194–201. https://doi.org/10.1038/ncb1680.
Dong CY, Mi RF, Jin GS, Zhou YQ, Zhang J, Liu FS. Identification of the proliferative effect of Smad2 and 3 in the TGF β2/Smad signaling pathway using RNA interference in a glioma cell line. Mol Med Rep. 2015;12(2):1824–8. https://doi.org/10.3892/mmr.2015.3614.
Jonckheere N, Van Der Sluis M, Velghe A, Buisine MP,Sutmuller M,Ducourouble MP,etal.Transcriptional activation of the murine Muc5ac mucin gene in epithelial cancer cells by TGF-beta/Smad4 signalling pathway is potentiated by Sp1. Biochem J, 2004;377(Pt 3):797-808. https://doi.org/10.1042/BJ20030948.
Wang SQ, Wang S, Li HB, Zhu LJ, Wang YH. Inhibition of the TGF-β/Smads signaling pathway attenuates pulmonary fibrosis and induces anti-proliferative effect on synovial fibroblasts in rheumatoid arthritis. Int J Clin Exp Pathol. 2019;12(5):1835–45.
Chen XL, Yin SH, Wu XW, Chen XL. Protoscoleces and hydatid fluid promote expression of TGF-βfrom mouse spleen cells in vitro. J Pathogen Biol. 2013;8(6):523–6. https://doi.org/10.13350/j.cjpb.2013.06.013.
Zhou Y, Jin YB, Wang J, Turgunjan T, Wei XL, Zhang Z, et al. The influence of Echinococcus granulosus fluid on the expression of Foxp 3 and Smad 4 genes of mouse spleen cells in vitro. J Pathogen Biol. 2011;6(3):193–6, 182. https://doi.org/10.13350/j.cjpb.2011.03.012.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This work was supported by Xinjiang Uygur Autonomous Region Science and Technology Support Project (No.2020E0287) and Hospital project of People's Hospital of Xinjiang Uygur Autonomous Region (No.20190301).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: SLQ, SXL. Acquisition of data: YG, HXY. Analysis and interpretation of the data: SLQ, LZ. Statistical analysis: AM. Obtaining financing: SLQ, SXL. Writing of the manuscript: SLQ. Critical revision of the manuscript for intellectual content: YA, JH. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of the people's Hospital of Xinjiang Uygur Autonomous Region (No. ky2019120601). This study was conducted in accordance with the declaration of Helsinki. All operations were performed with the consent of the children and their families, and relevant informed documents were signed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qin, Sl., Guo, Y., Li, SX. et al. The role of the TGF-β/LIF signaling pathway mediated by SMADs during the cyst formation of Echinococcus in young children. BMC Mol and Cell Biol 23, 50 (2022). https://doi.org/10.1186/s12860-022-00452-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12860-022-00452-3