Abstract
Background
Rhodopsin is a seven-transmembrane protein covalently linked with retinal chromophore that absorbs photons for energy conversion and intracellular signaling in eukaryotes, bacteria, and archaea. Haloarchaeal rhodopsins are Type-I microbial rhodopsin that elicits various light-driven functions like proton pumping, chloride pumping and Phototaxis behaviour. The industrial application of Ion-pumping Haloarchaeal rhodopsins is limited by the lack of full-length rhodopsin sequence-based classifications, which play an important role in Ion-pumping activity. The well-studied Haloarchaeal rhodopsin is a proton-pumping bacteriorhodopsin that shows promising applications in optogenetics, biosensitized solar cells, security ink, data storage, artificial retinal implant and biohydrogen generation. As a result, a low-cost computational approach is required to identify Ion-pumping Haloarchaeal rhodopsin sequences and its subtype.
Results
This study uses a support vector machine (SVM) technique to identify these ion-pumping Haloarchaeal rhodopsin proteins. The haloarchaeal ion pumping rhodopsins viz., bacteriorhodopsin, halorhodopsin, xanthorhodopsin, sensoryrhodopsin and marine prokaryotic Ion-pumping rhodopsins like actinorhodopsin, proteorhodopsin have been utilized to develop the methods that accurately identified the ion pumping haloarchaeal and other type I microbial rhodopsins. We achieved overall maximum accuracy of 97.78%, 97.84% and 97.60%, respectively, for amino acid composition, dipeptide composition and hybrid approach on tenfold cross validation using SVM. Predictive models for each class of rhodopsin performed equally well on an independent data set. In addition to this, similar results were achieved using another machine learning technique namely random forest. Simultaneously predictive models performed equally well during five-fold cross validation. Apart from this study, we also tested the own, blank, BLAST dataset and annotated whole-genome rhodopsin sequences of PWS haloarchaeal isolates in the developed methods. The developed web server (https://bioinfo.imtech.res.in/servers/rhodopred) can identify the Ion Pumping Haloarchaeal rhodopsin proteins and their subtypes. We expect this web tool would be useful for rhodopsin researchers.
Conclusion
The overall performance of the developed method results show that it accurately identifies the Ionpumping Haloarchaeal rhodopsin and their subtypes using known and unknown microbial rhodopsin sequences. We expect that this study would be useful for optogenetics, molecular biologists and rhodopsin researchers.
Similar content being viewed by others
Background
Rhodopsin is present in a wide range of organisms, from vertebrates to bacteria. Rhodopsin consists of seven retinal chromophore-associated transmembrane helix proteins belonging to the superfamily of GPCRs that act as photoreceptors [1, 2]. Based on the seven transmembrane topology, the rhodopsins are classified into two groups: type-I Microbial Rhodopsin and type-II animal Rhodopsin. Type-I microbial rhodopsins consist of seven transmembrane domain that is covalently associated with retinal chromophore functions like proton pumping, chloride pumping, and phototaxis behaviour. The type-I microbial rhodopsins used in this study, such as actinorhodopsin, bacteriorhodopsin, proteorhodopsin, xanthorhodopsin, belong to the proton pumping type-I microbial rhodopsins family. Halorhodopsin and sensory rhodopsin functions like non-proton-pumping type-I Microbial rhodopsin, such as chloride pumps and photoreceptors. Bacteriorhodopsin is the first microbial rhodopsin to be isolated and well-characterized from the Halobacterium salinarium in the 1970s by Oesterhelt and stockineus group [3]. The Light driven proton pump bacteriorhodopsin extensively used in several biophotonics and Bioelectronics applications [4]. Proton pump proteorhodopsins were first discovered during environmental sequencing of pacific coastal waters and deep ocean samples. Proteorhodopsins are the largest subfamily of type-I rhodopsins. 13% of proteorhodopsins harboring bacterial cells live in the photic zone of oceanic marine samples. Proteorhodopsin is the largest type-I microbial rhodopsin subfamily among marine proteobacteria [5, 6]. Xanthorhodopsin, originally found in Salinibacter ruber binds to salinixanthin-like carotenoids that bind specifically to the rhodopsin protein. These carotenoids contain a retinal chromophore that absorbs light and transfers energy to the rhodopsin protein in hypersaline Haloarchaea. The light-driven proton pump was transformed into halorhodopsin due to Asp 85 single mutation which acts as proton acceptor [7, 8]. ActR gene lineage is also the one of the globally abundant Type-I microbial rhodopsin gene. Actinorhodopsin was first reported in the freshwater lakes in the actinobacteria. Subsequent findings suggested that actinorhodopsin is present abundantly in the terrestrial and ocean environments [9, 10]. Light-modulated swimming behavior is a well-known feature of sensory rhodopsins I. Takahashi and colleagues suggested the existence of a second sensory photoregulatory receptor, rhodopsin II, present in Halobacterium salinarium for their repellent response under highly aerobic conditions and showed slow photocyclic processes [11, 12]. Many computational methods have been developed to identify or predict the proteins and their functions, based on protein structure, DNA binding sites, glycosylation sites, subcellular localization and hybridization-based prediction methods [13,14,15]. Recently a research group Jeanthon from France has developed a MicRhoDE is a comprehensive database that categorize the different types of microbial rhodopsins and their taxonomy classification [16]. Research group Kandori and Takeuchi from Japan developed a machine learning approach to predict the light absorption properties of microbial rhodopsin [17]. Classification and prediction of GPCRs based on amino acid sequences have been reported using a three-layer approach [18, 19]. The isolation of rhodopsin proteins from wild type Haloarchaeal culture is laborious, expensive involves lengthy procedures. The well studied bacteriorhodopsin protein from Haloarchaeal strains has a wide range of applications in Biophotonics and bioelectronic applications. Therefore, it is necessary to identify the bacterial rhodopsin proteins that express in their wild type as well as additional microbial rhodopsin proteins with restricted expression at the mg/l expression level. The full length bacteriorhodopsin sequence also plays a crucial role in the ion pumping activity of recombinant bacteriorhodopsin, which helps to facilitate the development of recombinant bacteriorhodopsin. Full length microbial rhodopsin expressed at high levels is useful for finding new rhodopsin proteins with ion pumping capabilities through crystallography studies.
Currently, GPCR is the only rhodopsin superfamily that has been studied in detail using support vector machine learning by multiple research groups [20]. As per our knowledge, there were no reports on the classification of microbial rhodopsin proteins by support vector machine (SVM). Here, we have developed a method for identification of Ion pumping Haloarchaeal rhodopsin using amino acid composition (AAC), dipeptide composition (DPC), and hybrid models. Support vector machine is a supervised machine learning method that has been used in various bioinformatics studies to classify GPCR, proteins of oxygen-binding, plasminogen activators and evolutionary relationship of receptor-associated proteins (RAPs) [21,22,23]. SVM is a powerful predictor tool that has been extended to many clinical investigations beyond protein studies [15]. It is well-established that sequence-based SVM statistical predictors for biological systems are susceptible to the following rules: (a) Data set construction, (b) Program the biological sequence in mathematical terms (c) Develop a robust algorithm (d) Perform cross-validation to evaluate prediction accuracy (e) Run the algorithm using the server user-friendly online web [24]. SVM models have been created for bacteriorhodopsin, actinorhodopsin, xanthorhodopsin, proteorhodopsin, sensory rhodopsin, and halorhodopsin. To run the SVM to generate models, a sequence of subclasses is labelled as positive and negative every other classes are labelled as negative [25]. When creating classification models, it is repeated for all classes. Each of the five SVM models was developed by employing a fivefold cross validation procedure that is identical in both techniques. To recognise the classes depicted in the prediction score graphs, Each and every sequence in the dataset was analyzed using recently constructed models. Haloarchaeal rhodopsin proteins and subtypes were also identified using the blind dataset. The accuracy (ACC), sensitivity (SN), and specificity (SP) of the prediction results were compared with in the classes [26]. SVM classifiers integrated with rhodopred webserver correctly identified the subtype of Ion pumping Haloarchaeal rhodopsin and experimentally validated whole-genome Haloarchaeal rhodopsin sequences extracted from NCBI and Haloweb Genome web databases (https://www.haloweb.org/) [27]. This SVM method focuses on the prediction and analysis of various ion-pumping Haloarchaea rhodopsins of recently isolated Haloarchaeal strains whole genome data available in the NCBI (https://www.ncbi.nlm.nih.gov/genome/) database using the Rhodopred web server. Among the Type-I Ion pumping Microbial rhodpsins the sensory rhodopsins were out grouped from the chloride pumping rhodopsins were different from Ion Pumping rhodopsin amino acid sequences.
The developed SVM models suggest that full-length rhodopsin sequences are responsible for Ion pumping properties of type-I microbial rhodopsin, which would be helpful in heterologous protein expression and optogenetics studies [28].
Methods
The present method classifies the ion pumping type-I microbial rhodopsin by combining the amino acid composition (AAC) and the dipeptide composition (DPC) in order to get a higher level of precision. These predictive models were developed to compare the type-I microbial rhodopsin amino acid sequences using -5-fold and 10-fold cross-validation methods. Amino acid composition (AAC), dipepide composition (DPC), and hybrid (HYB) approach were used to build the predictive models. The known and experimentally verified rhodopsin sequences extracted from NCBI, and Haloweb genome database were given as input in the rhodopred web server. Based on the AAC, DPC, HYB scores, the outcome of the predictor clearly shows that amino acid sequences belong to type-I microbial rhodopsins proteins. This indirectly indicates the information that those rhodopsin proteins belongs to Haloarcheal rhodopsins or Prokaryotic rhodopsins. Among Haloarchaeal rhodopsins, we can also predict the above amino acid sequence belongs to proton pumping or non-proton pumping rhodopsin proteins
Data set preparation
The most-reported proton-pumping rhodopsins are in NCBI databases as bacteriorhodopsin, actinorhodopsin, proteorhodopsin and xanthorhodopsin. We retrieved the various microbial rhodopsin sequences from the uniport database using the protein's keyword (https://www.uniprot.org/). The sequences labelled “fragments,” “isoforms,” “potentials,” “similarity,” or “probables” were removed. Furthermore, the CD-hit programme was used to reduce redundancy with a cutoff of 90% ensuring that no two sequences in the dataset share more than 90 percent of redundancy [29].
The final dataset includes 366, 139, 23, 191, 16, and 167 sequences from bacteriorhodopsin, actinorhodopsin, halorhodopsin, proteorhodopsin, sensoryrhodopsin and xanthorhodopsin respectively, the complete datasets are available publicly at the following link (https://bioinfo.imtech.res.in/servers/rhodopred/download.php)
Amino acid composition
AAC was initially computed by dividing the fraction of each amino acid in a protein by the total number of amino acids. The AAC profile generated a final output of 20. The DPC was calculated by dividing the fraction of each dipeptide in a protein by the total number of dipeptides with a pattern length of 400(20X20) [30].
The percentage of each amino acid present in a protein is referred to as its amino acid composition (AAC).
Data must be encoded into vectors in order for the SVM light to run. The following equation was used to determine the percentage of each of the 20 naturally occurring amino acids:
Dipeptide composition
DPC was calculated in the same way, using a vector with a fixed length of 400 (20 × 20) dimensions. The following equation was used to determine the fraction of each dipeptide composition [31]:
Hybrid approach
To increase the prediction accuracy the HYB approach was developed. A prediction model that combines two or more profiles is known as a hybrid model. This study used 420 vector lengths to create hybrid models that included AAC and DPC. The GPSR 1.0 package’s col_add function was used to combine the AAC and DPC profiles to create a hybrid profile (https://webs.iiitd.edu.in/raghava/gpsr/).
Support vector
A SVM is a supervised machine learning technique (MLT) used for classification and regression analysis. For SVM implementation, predictive models were developed by converting the various sequence length into fixed length vectors by implementing several sequence properties. We have used SVMlight v6.02 to predict the various types of microbial rhodopsin proteins. While the performance was optimized using RBF kernel on diverse g and c values [32].
Random forest
Random forest (RF) is an ensemble-learning method based on decision tree model having bootstrapping algorithm. Firstly, decision tree was developed from training data sets and the classes of unknown sample is assigned either according to the mode of classes either in the classification or regression based data sets. We have used RF through Waikato Environment for Knowledge Analysis (WEKA) package for developing a prediction model [33].
Cross validation
We have used 5-fold and 10-fold cross validation method to evaluate the performance of all the module. For 10-fold cross validation, the data set is randomly divided into 10-equally sized sets [34]. From the 10 sets, one set is used for testing while the remaining nine sets are considered for training. This process is repeated ten times and each set will get the chance to be the testing data set. Likewise, in 5-fold cross validation, data set is divided into 5-sets, where 1 set is tested by the model developed on the remaining 4 sets. This process is also iterated 5 times.
Performance measures
The performance of the predictive models was evaluated by calculating specificity (SP), sensitivity (SN), accuracy (ACC) and Mathew's correlation coefficient (MCC) using the following equations [35]:
Webserver
Rhodopred webserver is developed using LAMPP software. The front-end was developed using PHP, HTML, CSS, JavaScript and PERL. The backend was linked to the apache server using linux platform. The webserver is freely accessible at https://bioinfo.imtech.res.in/servers/rhodopred. We have also provided the general information of webserver in the “About” section. Rhodopred webserver is a machine learning based classification method for predicting various microbial rhodopsin proteins. Rhodopsin protein modeling was done using support vector machines (SVM) and their classes, viz. actinorhodopsin, bacteriorhodopsin, halorhodopsin, proteorhodopsin, sensoryrhodopsin and xanthorhodopsin. On the home page, the user can paste/upload the protein sequence (fasta or multiple fasta) in the textbox. This will predict the input protein sequence as rhodopsin (YES) or non-rhodopsin (NO) proteins based on SVM score for amino acid composition (AAC), dipeptide composition (DPC) and hybrid (AAC+DPC). Users can also predict rhodopsin protein for each class by selecting each rhodopsin protein in the “Class” section of the webserver. It will also provide score and predict whether the sequence belongs to a particular rhodopsin protein or not.
Results
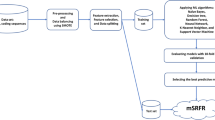
Many computational approaches are currently available for predicting diverse functional proteins utilizing a machine learning methodology. This work is concerned with predicting and analyzing various microbial rhodopsins and analysing our recently isolated Haloarchaeal strains of whole genome data available in NCBI database (https://www.ncbi.nlm.nih.gov/genome/). The developed SVM approaches were also evaluated against the annotated whole genome sequence of PWS Haloarchaeal isolates. According to our findings, the established approach accurately identifies the rhodopsin sequences and various types of Type-I microbial rhodopsins (Fig. 1).
Analyze the aminoacid profile of microbial rhodopsin
The average amino acids for various rhodopsin proteins were computed, and residues “L” and “A” are present in more than 10% of all rhodopsins. A high abundance of these non-polar amino acids like Leucine and Alanine are signature amino acids for integral membrane proteins like Microbial Type-I rhodopsins. Compared to other rhodopsins, bacteriorhodopsin and sensory rhodopsin make up almost 20% of the total residues “G” and “V” in excess of 5%. The residues “C” and "H" are mostly missing. The remaining residues are found in all rhodopsins in similar amounts. Figure 2a depicts the aminoacid composition of all rhodopsins.
We also computed the sequence length profile of several rhodopsins and found that the majority of the sequences were between 200 and 399 amino acids long. Interestingly, the majority of the bacteriorhodopsin sequences are in the 200–299 ranges. Furthermore, most xanthorhodopsin and Proteo rhodopsin sequences are found in the 300–399 ranges. Other rhodopsins, such as Sensory, Halo, and Actino rhodopsins, are found in various lengths 200–299, 300–399. The details of the results are shown in Fig. 2b.
Performance of AAC-SVM based classification
The entire classes of rhodopsin performed equally well during 10-fold cross validation. For AAC, the maximum accuracy and MCC has been achieved for actinorhodopsin followed by halorhodopsin, sensoryrhodopsin, xanthorhodopsin, proteorhodopsin, bacteriorhodopsin and overall with 99.88%, 1; 99.75%, 0.95; 99.38%, 0.80; 98.65%, 0.96; 98.27%, 0.95; 98.15%, 0.96 and 97.78%, 0.96 respectively during 10-fold cross validation. These models showed equal performance on independent data set on all classes of rhodopsin as shown in Table 1. Further, rhodopsin classes also performed well during 5-fold cross validation as given in Additional file 2: Fig. S1, Additional file 1: Table S1.
Performance of DPC-SVM based classification
For DPC, actinorhodopsin achieved the highest accuracy and MCC followed by bacteriorhodopsin, halorhodopsin, sensoryrhodopsin, xanthorhodopsin, proteorhodopsin, and overall with 99.88%, 1; 99.75%, 0.99; 99.75%, 0.95; 99.38%, 0.81; 99.02%, 0.97; 98.27%, 0.95; and 97.84%, 0.96 correspondingly during 10-fold cross validation. Similarly all models performed equally well on independent data set of all classes of rhodopsin (Table 1). Likewise, rhodopsin classes also showed good performance on 5-fold cross validation (Additional file 2: Fig. S1, Additional file 1: Table S1).
Performance of HYB-SVM based classification
In case of HYB, bacteriorhodopsin got the maximum accuracy and MCC of 99.75% and 0.99 followed by halorhodopsin, actinorhodopsin, sensoryrhodopsin, xanthorhodopsin, proteorhodopsin, and overall with 99.75%, 0.95; 99.63%, 0.99; 99.51%, 0.85; 99.02%, 0.97; 98.77%, 0.96 and 97.60%, 0.95 respectively during 10-fold cross validation. Similalrly, predictive models also performed equally well on the independent data set (Additional file 2: Fig. S1) (Table 1). Likewise, rhodopsin classes also showed good performance on 5-fold cross validation (Additional file 2: Fig. S1, Additional file 1: Table S1).
Performance of random forest (RF) based classification
Using RF based algorithm for 10-fold cross validation, we achieved the maximum MCC for actinorhodopsin with 0.99 followed by bacteriorhodopsin, overall, xanthorhodopsin, proteorhodopsin, halorhodopsin and sensoryrhodopsin with 0.98, 0.97, 0.94, 0.93, 0.84, and 0.53 respectively for AAC on the training data set. For DPC, actinorhodopsin also has the highest MCC of 1followed by bacteriorhodopsin, overall, xanthorhodopsin, proteorhodopsin, halorhodopsin and sensoryrhodopsin with 0.99, 0.98, 0.95, 0.95, 0.90, and 0.38 respectively. Likewise in HYB approach, actinorhodopsin has the MCC of 1 followed by bacteriorhodopsin, overall, xanthorhodopsin, proteorhodopsin, halorhodopsin and sensoryrhodopsin with 0.99, 0.98, 0.95, 0.95, 0.87, and 0.46 respectively (Additional file 2: Fig. S1, Additional file 1: Table S2). While, the complete result of rhodopsin classes during 5-fold cross validation on RF algorithm is given in Additional file 2: Fig. S1, Additional file 1: Table S3. Further these models showed equal performance on independent data set as shown in Additional file 2: Fig. S1, Additional file 1: Table S4.
Confusion matrix performance by prediction scoring graphs
The confusion matrix and prediction scoring graphs were also used to assess the performance of SVM modules. The prediction score for each unique sequence studied is depicted in the scoring graph, which shows how a threshold distinguishes the positive set's score from the negative set's score in order to distinguish between positive and negative predictions. However, not all positive or negative sequences can be accurately detected, resulting in false negative and positive predictions. In this analysis, we found that all models such as Amino acid composition, dipeptide composition, the SVM prediction scores for the Amino acid models are found to be positive scores for actinorhodopsin, bacteriorhodopsin, proteorhodopsin and xanthorhodopsin. This confirms the very distinct classification of Type I proton pumping among all Type I microbial rhodopsin. In this amino acid composition model the proton pumping rhodopsins were not confused with the other Type I microbial rhodopsin sequences (Fig. 3a–c).
BLAST dataset prediction and analysis
To validate of our developed methods microbial rhodopsin protein sequences was extracted from NCBI database to identify BLAST data using our developed models to analyse the performance of the developed models. In this investigation, a total of 500 sequences from each family were employed, with five sequences from our dataset running BLAST and collecting 100 each from a sequence. The output findings demonstrate that on an average 54% of actinorhodopsin BLAST sequences were recognized by its own models, 91% sequences on an average were recognized by bacteriorhodopsin models overlapping with halorhodopsin sequences suggest that bacteriorhodopsin and halorhodopsin sequences were over lapping each other which shows close sequences similarity in rhodopsin amino acid sequences. All models recognise BLAST data sequences. In the other classes, the BLAST sequences were recognised by its own all models as 53.4%, 97.4%, 97.6%, 99.4%, 45.2 %, and 99.6% in actinorhodopsin, (Table 2) bacteriorhodopsin, halorhodopsin, proteorhodopsin, sensory rhodopsin and xanthorhodopsin respectively. Actinorhodopsin and sensory rhodopsin BLAST data prediction percentage scores showing less percentage because of presence of rhodopsin like hypothetical sequences in the NCBI Database. Some sequences were predicted by other models rather than by their own, while a few sequences were recognized by both their own and other class models. Table 3 summarises the findings of this investigation.
Rhodopsin genes extraction from annotated whole genome sequence analysis
In this study, we used SVM_light to predict the various type-I Ion pumping Microbial Rhodopsin proteins. Whole genome sequencing data of our PWS1,5, SL3 and 11 isolates for identifying Type I microbial rhodopsin genes were analysed from the NCBI genome database (Table 3). Extracting the microbial rhodopsin gene sequences consist of following steps (1) Enter the accession number in the NCBI Database, (2) go to nucleotide sequence, (3) Enter Gen bank number and WGS : WOYG00000000.1, (4) Search rhodopsin in scaffolds. In addition to the microbial Rhodopsin classification, our group recently published and deposited whole-genome sequencing of PWS isolates PWS1,5,11 identified from Pondicherry Solar Salterns. (Pondicherry salterns located in the east coast road of Tamil nadu, India). These extreme haloarcheal isolates (PWS1, PWS5, PWS11) where subjected for whole-genome sequencing yielded 3.39 Mb, 4.0 Mb, 3.67 Mb, and SL3 is reference Haloarcula genome. The GC Content was found to be 65.7%, 61.3%, 62.0% and 66.1% for pws1, pws5, SL3, and pws11 respectively. The accession number for PWS1, PWS5, SL3, PWS11 was reported to be WOYG00000000.1, NZ_WOWA00000000.1, LIUF00000000.1, WOWC00000000.1 (Table 4, Fig. 4). The support vector machine classifier clearly distinguished the presence of rhodopsin proteins and Non rhodopsin proteins. In addition the SVM model identified the type of Type-I microbial rhodopsin A single proton pumping Bacteriorhodopsin expression in Halobacterium salianrium requires bop, Blp, brp, crtb, blh genes (Table 5) [36]. Presence of brp, blh, blp, bat, Crtb1 essential genes and structural rhodopsin genes in the reference Halobacterium salianrium NRC1 and R1 whole genome annotated sequence indicates that these two wild type Halobacterium strains capable to express milligram per liter scale of native bacteriorhodopsin protein. A total of 17 rhodopsin sequences were employed, with the majority of them recognized as bacteriorhodopsin, halorhodopsin, and sensory rhodopsins as per the whole genome sequence analysis (Fig. 4). Out of 17 microbial rhodopsin sequences extracted from NCBI whole genome database PWS1,5,11 were experimentally verified Haloarchaeal whole genome analysed rhodopsin sequences. Actinorhodopsin, Proteorhodopsin and xanthorhodopsin models showing negative histograms which shows the absence of rhodopsin proteins in the PWS Haloarchaeal isolates. This indicates that bacteriorhodopsin harboring PWS isolates such as PWS12 rhodopsin, PWS13 Sensory rhodopsin, PWS5 Cruxrhodopsin Cop3, SL3 Rhodopsin2, R1 Bacteriorhodopsin and NRC1 Bacteriorhodopsin bop were identified by all models of bacteriorhodopsin. R1 Halorhodopsin and NRC1 [27, 37] Halorhodopsin were rightly differentiated between other Type-I microbial rhodopsins (Fig. 4). Actinorhodopsin, Proteorhodopsin and Xanthorhodopsin protein models were not identified in the PWS-Isolates confirms our finding these whole genome sequenced rhodopsin sequences originates from extreme haloarchaea not from prokaryotic rhodopsin harboring microorganisms.
Rhodopred webserver performance using PWS Isolates rhodopsin sequences
Seventeen microbial rhodopsin sequences retrieved from PWS1,5, SL3 and reference genomes from Haloarchael NRC1 and R1 isolates were fed to rhodopred web server. The rhodopred webserver clearly identifies bacteriorhodopsin and cruxrhodopsin like Bacteriorhodopsin proteins and sensory rhodopsin I and II proteins from PWS1 and PWS5 whole-genome rhodopsin sequences. Absence of bacteriorhodopsin in PWS11 Haloarchael isolates indicates the presence of non-bacteriorhodopsin expressing genes. Bacteriorhodopsin, Halorhodopsin, sensory rhodopsin proteins present in the reference genome of haloarchaeal isolates like Halobacterium salianirum NRC-1 and R1 confirm that our developed webserver rhodopred accurately predicts the sub types of haloarchaeal rhodopsin proteins. Absence of actinorhodopsins and proteorhodopsin proteins in the respective models of rhodopred webserver indicates the presence of haloarchaeal whole genome rhodopsin sequences and absence of Prokaryotes microbial rhodopsins. Among the bacteriorhodopsin proteins identified through rhodopred webserver were further analysed for bacteriorhodopsin synthesizing genes in the NCBI Genome database. Absence of these bacteriorhodopsin genes in the Haloarchaeal genomes will express more red pigmented carotenoids which masks the bacteriorhodopsin protein expression in PWS1,5, SL3 isolates.
Discussion
In halophilic archaea, rhodopsin is a retinal binding protein that provides light-sensitive ion transport and sensory function. Marine and Prokaryotic organisms. It is difficult to express the rhodopsin proteins by culturable methods when all the bacteriorhodopsin synthesizing genes were absent in the genome [38]. The culturable methods for wild type and recombinant rhodopsin protein expression will be expensive and time consuming. Therefore, low-cost computational methods are required to identify the microbial rhodopsins proteins and their related subclasses. This study established a very reliable approach for recognizing several Ion pumping Type-I microbial rhodopsins. The first step is to predict Type-I Microbial rhodopsin and non-Type-I Microbial rhodopsin. The second step is to classify Type-I microbial rhodopsin classifications, such as actinorhodopsin, bacteriorhodopsin, haloarhodopsin, proteorhodopsin, sensory rhodopsin, and xanthorhodopsin. The overall prediction accuracy was achieved above 95% in all approaches except AAC, DPC and Hybrid approaches of actinorhodopsin and sensory rhodopsin. According to the results of the BLAST dataset, the developed methods are performing well in all approaches identifying microbial rhodopsins. In the confusion matrix analysis, the 233 sequences of actinothodopsin were identified by xanthorhodopsin, the results suggest that these two proteins sequences may have a close similarity or it may have an evolutionary relationship with one another. Also the results suggest that some sensory rhodopsin sequences have been identified as bacteriorhodopsin. Overall, according to BLAST data, the related sequences were not identified by the own class models, rather identified by other class protein models. As a result, when running, BLAST is unable to recognize the proper sequences; instead, it retrieves comparable proteins that are not the genuine proteins. So our developed method is successfully identifies the different types of Type-I microbial rhodopsins. SVM light and Rhodopred webserver based prediction accurately identifies the Type-I microbial rhodopsin protein sequences from annotated whole genome rhodopsin sequences.
We developed a very accurate method, for identifying various microbial rhodopsins using SVM light and rhodopred webserver with different amino acid approaches. As a result, all the developed models accurately detect the different subtypes of Type-I microbial rhodopsin. All our findings indicate that it is better than the BLAST search in identifying microbial rhodopsin, because the BLAST search did not accurately extract the genuine rhodopsin proteins and instead collected other than microbial rhodopsin. We anticipate that this work will aid researchers in finding new or undiscovered microbial rhodopsins having Ion pumping properties. These models accurately predicted the sub type of Type-I Microbial rhodopsin. The general blast search of microbial rhodopsin brings non specific microbial rhodopsin proteins in large numbers. Reference Halobacterium salinarium NRC1, R1 whole genome annotated data indicates the presence of Bacteriorhodopsin, Halorhodopsin, sensory rhodopsin I, II like genes in the genome [39]. Single bacteriorhodopsin protein in the NRC1 and R1 Halobacterium salinarium consist of bacteriorhodopsin structural and supporting genes like bop, brp, bat, blp, and Ctb1 [40]. Among these five genes expect bop gene four supporting genes were absent in the PWS1,5, SL3 isolates. Further it will explores the possibility for the recombinant rhodopsin protein expression in E-coli in functional form by adding all trans retinal chromophore invitro. Our group has recently published our findings on Initial 17 amino acids near the N-terminal rhodopsin sequences helps in the proper expression and folding of proton pumping rhodopsin [41]. Another published report on recombinant PWS-5 BR protein was expressed in E. coli with light driven proto pumping property by adding all trans retinal invitro [42]. This is the first detailed studied of Support vector machine based Proton pumping the recombinant bacteriorhodopsin protein expression by fishing it out bop gene using specific primers from these PWS isolates by choosing proper vector and host to demonstrate the light driven proton pumping property [43]. The From these two reported research work from our group and our current developed models by SVM light and Rhodopred webserver would be useful for designing rhodopsin genes primers for heterologous expression of rhodopsin proteins in E-coli and other host system for Optogentics and Microbial rhodopsin applications.
APC, DPC and HYB performance were good in recognizing the rhodopsin related proteins. We observed that the developed all approaches were equal performance on the independent dataset. The complete analysis results are shown in the Additional file 2: Fig. S1. The similar performance were observed in 10 and 5-fold cross validation. The SVM and random forest techniques performance were also similar in identification of microbial rhodopsins. Since there is no webserver or methods available for microbial rhodopsin, hence we cannot compare the performance with any other methods.
Conclusion
There is no separate method is available for predicting the various microbial rhodopsin. A method has been developed (Rhodopred) which accurately identify the rhodopsins. This method is developed with 10-fold and fivefold cross-validation techniques with the approaches of AAC, DPC and HYB. All the developed models are validated with the known and the unknown datasets. We also interested to use a deep learning method for our future studies [44,45,46]. The developed method will be useful for researches working on microbial rhodopsin proteins.
Availability of data and materials
The datasets were generated and analyzed for this study, and it is available publicly at the following link, https://bioinfo.imtech.res.in/servers/rhodopred/download.php. The individual class datasets are also available at the following links, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Actinorhodopsin.txt, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Bacteriorhodopsin.txt, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Halorhodopsin.txt, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Proteorhodopsin.txt, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Sensory-rhodopsin.txt, https://bioinfo.imtech.res.in/servers/rhodopred/dataset/Xanthorhodopsin.txt. Webserver http://bioinfo.imtech.res.in/servers/rhodopred, Databases/weblinks, https://www.haloweb.org/, https://www.ncbi.nlm.nih.gov/genome/, https://www.uniprot.org/. The NCBI Nucleotide accession number for PWS1, PWS5, SL3 and PWS11 is WOYG00000000.1, NZ_WOWA00000000.1, LIUF00000000.1, and WOWC00000000.1 respectively. Using these accession numbers in the NCBI Nucleotide Database “rhodopsin sequences” were extracted using below NCBI database weblink. PWS1 whole genome sequence weblink: (use “Open Hyperlink” to see the below weblink) https://www.ncbi.nlm.nih.gov/nuccore/WOYG00000000.1, https://www.ncbi.nlm.nih.gov/Traces/wgs/WOYG01?display=contigs. PWS5 whole genome sequence weblink: https://www.ncbi.nlm.nih.gov/nuccore/NZ_WOWA00000000.1, https://www.ncbi.nlm.nih.gov/Traces/wgs/WOWA01?display=contigs. SLR 3 whole genome sequence weblink: https://www.ncbi.nlm.nih.gov/nuccore/LIUF00000000.1, https://www.ncbi.nlm.nih.gov/Traces/wgs/LIUF01?display=contigs. PWS11 whole genome sequence weblink: https://www.ncbi.nlm.nih.gov/nuccore/WOWC00000000.1, https://www.ncbi.nlm.nih.gov/Traces/wgs/WOWC01?display=contigs.
References
Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev. 2014;114(1):126–63.
Kurihara M, Sudo Y. Microbial rhodopsins: wide distribution, rich diversity and great potential. Biophys Physicobiol. 2015;12:121–9.
Grote M, O’Malley MA. Enlightening the life sciences: the history of halobacterial and microbial rhodopsin research. FEMS Microbiol Rev. 2011;35(6):1082–99.
Oesterhelt D, Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. In: Methods in enzymology, vol. 31. Academic Press; 1974. p. 667–678.
Gazalah S, Alexander L, Kwang-Hwan J, Ranga P, Tal I, Joseph H, Michael W, Oded B. New insights into metabolic properties of marine bacteria encoding proteorhodopsins. PLoS Biol. 2005;3(8):e273.
Pushkarev A, Hevroni G, Roitman S, Shim JG, Choi A, Jung KH, Béjà O. The use of a chimeric rhodopsin vector for the detection of new proteorhodopsins based on color. Front Microbiol. 2018;2018(9):439.
Engelhard C, Chizhov I, Siebert F, Engelhard M. Microbial halorhodopsins: light-driven chloride pumps. Chem Rev. 2018;118(21):10629–45.
Balashov SP, Lanyi JK. Xanthorhodopsin: proton pump with a carotenoid antenna. Cell Mol Life Sci. 2007;64(18):2323–8.
Sharma AK, Zhaxybayeva O, Papke RT, Doolittle WF. Actinorhodopsins: proteorhodopsin-like gene sequences found predominantly in non-marine environments. Environ Microbiol. 2008;10(4):1039–56.
Sharma AK, Sommerfeld K, Bullerjahn GS, Matteson AR, Wilhelm SW, Jezbera J, Brandt U, Doolittle WF, Hahn MW. Actinorhodopsin genes discovered in diverse freshwater habitats and among cultivated freshwater Actinobacteria. ISME J. 2009;3(6):726–37.
Furutani Y, Takahashi H, Sasaki J, Sudo Y, Spudich JL, Kandori H. Structural changes of sensory rhodopsin I and its transducer protein are dependent on the protonated state of Asp76. Biochemistry. 2008;47(9):2875–83.
Kamo N, Shimono K, Iwamoto M, Sudo Y. Photochemistry and photoinduced proton-transfer by pharaonis phoborhodopsin. Biochem Mosc. 2001;66(11):1277–82.
Hendrix SG, Chang KY, Ryu Z, Xie ZR. DeepDISE: DNA binding site prediction using a deep learning method. Int J Mol Sci. 2021;22(11):5510. https://doi.org/10.3390/ijms22115510.PMID:34073705;PMCID:PMC8197219.
Pugalenthi G, Nithya V, Chou KC, Archunan G. Nglyc: a random forest method for prediction of N-glycosylation sites in eukaryotic protein sequence. Protein Pept Lett. 2020;27(3):178–86. https://doi.org/10.2174/0929866526666191002111404.
Sahu SS, Loaiza CD, Kaundal R. Plant-mSubP: a computational framework for the prediction of single- and multi-target protein subcellular localization using integrated machine-learning approaches. AoB Plants. 2019;12(3):plz068. https://doi.org/10.1093/aobpla/plz068.
Boeuf D, Audic S, Brillet-Guéguen L, Caron C, Jeanthon C. MicRhoDE: a curated database for the analysis of microbial rhodopsin diversity and evolution. Database. 2015;2015:1–8.
Karasuyama M, Inoue K, Nakamura R, Kandori H, Takeuchi I. Understanding colour tuning rules and predicting absorption wavelengths of microbial rhodopsins by data-driven machine-learning approach. Sci Rep. 2018;8(1):1–11.
Bhasin M, Raghava GPS. GPCRpred: an SVM-based method for prediction of families and subfamilies of G-protein coupled receptors. Nucleic Acids Res. 2004;32(suppl_2):W383–9.
Li Z, Zhou X, Dai Z, Zou X. Classification of G-protein coupled receptors based on support vector machine with maximum relevance minimum redundancy and genetic algorithm. BMC Bioinform. 2010;11(1):1–15.
Begum K, Mohl JE, Ayivor F, Perez EE, Leung MY. GPCR-PEnDB: a database of protein sequences and derived features to facilitate prediction and classification of G protein-coupled receptors. Database. 2020;2020:baaa087.
Peng ZL, Yang JY, Chen X. An improved classification of G-protein-coupled receptors using sequence-derived features. BMC Bioinform. 2010;11(1):1–13.
Muthukrishnan S, Puri M, Lefevre C. Support vector machine (SVM) based multiclass prediction with basic statistical analysis of plasminogen activators. BMC Res Notes. 2014;7(1):1–10.
Krishnan SM. Using Chou’s general PseAAC to analyze the evolutionary relationship of receptor associated proteins (RAP) with various folding patterns of protein domains. J Theor Biol. 2018;445:62–74.
Muthu KS. Classify vertebrate hemoglobin proteins by incorporating the evolutionary information into the general PseAAC with the hybrid approach. J Theor Biol. 2016;21(409):27–37. https://doi.org/10.1016/j.jtbi.2016.08.027.
Muthukrishnan S, Puri M. Harnessing the evolutionary information on oxygen binding proteins through support vector machines based modules. BMC Res Notes. 2018;11(1):1–8.
Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE. 2015;10(3):e0118432.
DasSarma SL, Capes MD, DasSarma P, DasSarma S. HaloWeb: the haloarchaeal genomes database. Saline Syst. 2010;6(1):1–4.
Govorunova EG, Sineshchekov OA, Li H, Spudich JL. Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu Rev Biochem. 2017;86:845–72. https://doi.org/10.1146/annurev-biochem-101910-144233.
Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. https://doi.org/10.1093/bioinformatics/btl158.
Shamim MTA, Anwaruddin M, Nagarajaram HA. Support vector machine-based classification of protein folds using the structural properties of amino acid residues and amino acid residue pairs. Bioinformatics. 2007;23(24):3320–7.
Selvaraj M, Puri M, Dikshit KL, Lefevre C. BacHbpred: support vector machine methods for the prediction of bacterial hemoglobin-like proteins. Adv Bioinform. 2016;2016:1–11.
Thakur A, Rajput A, Kumar M. MSLVP: prediction of multiple subcellular localization of viral proteins using a support vector machine. Mol Biosyst. 2016;12(8):2572–86. https://doi.org/10.1039/c6mb00241b.
Frank E, Hall M, Trigg L, Holmes G, Witten IH. Data mining in bioinformatics using Weka. Bioinformatics. 2004;20(15):2479–81. https://doi.org/10.1093/bioinformatics/bth261.
Kamboj S, Rajput A, Rastogi A, Thakur A, Kumar M. Targeting non-structural proteins of Hepatitis C virus for predicting repurposed drugs using QSAR and machine learning approaches. Comput Struct Biotechnol J. 2022;30(20):3422–38. https://doi.org/10.1016/j.csbj.2022.06.060.
Thakur N, Qureshi A, Kumar M. AVPpred: collection and prediction of highly effective antiviral peptides. Nucleic Acids Res. 2012;40(Web Server issue):W199-204. https://doi.org/10.1093/nar/gks450.
Shand RF, Betlach MC. bop gene cluster expression in bacteriorhodopsin-overproducing mutants of Halobacterium halobium. J Bacteriol. 1994;176:1655–60.
Pfeiffer F, Losensky G, Marchfelder A, Habermann B, Dyall-Smith M. Whole-genome comparison between the type strain of Halobacterium salinarum (DSM 3754T) and the laboratory strains R1 and NRC-1. Microbiol Open. 2020;9(2):e974.
Tarasov VY, Besir H, Schwaiger R, Klee K, Furtwängler K, Pfeiffer F, Oesterhelt D. A small protein from the bop–brp intergenic region of Halobacterium salinarum contains a zinc finger motif and regulates bop and crtB1 transcription. Mol Microbiol. 2008;67(4):772–80.
Pfeiffer F, Schuster SC, Broicher A, Falb M, Palm P, Rodewald K, Ruepp A, Soppa J, Tittor J, Oesterhelt D. Evolution in the laboratory: the genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1. Genomics. 2008;91(4):335–46.
Shand RF, Batlach MC. Expression of bop gene cluster of Halobacterium halobium is induced by low oxygen tension and by light. J Bacteriol. 1991;173:4692–9.
Verma DK, Baral I, Kumar A, Prasad SE, Thakur KG. Discovery of bacteriorhodopsins in Haloarchaeal species isolated from Indian solar salterns: deciphering the role of the N-terminal residues in protein folding and functional expression. Microb Biotechnol. 2019;12(3):434–46.
Verma DK, Chaudhary C, Singh L, Sidhu C, Siddhardha B, Prasad SE, Thakur KG. Isolation and taxonomic characterization of novel haloarchaeal isolates from Indian Solar Saltern: a brief review on distribution of bacteriorhodopsins and V-type ATPases in haloarchaea. Front Microbiol. 2020;2020:3130.
Hsu MF, Yu TF, Chou CC, Fu HY, Yang CS, Wang AH. Using Haloarcula marismortui bacteriorhodopsin as a fusion tag for enhancing and visible expression of integral membrane proteins in Escherichia coli. PLoS ONE. 2013;8(2):e56363.
Zhao BW, You ZH, Hu L, Guo ZH, Wang L, Chen ZH, Wong L. A novel method to predict drug-target interactions based on large-scale graph representation learning. Cancers (Basel). 2021;13(9):2111.
Zhao B-W, Hu L, You Z-H, Wang L, Su X-R. HINGRL: predicting drug–disease associations with graph representation learning on heterogeneous information networks. Brief Bioinform. 2022;23(1):bbab515.
Lun Hu, Zhang J, Pan X, Yan H, You Z-H. HiSCF: leveraging higher-order structures for clustering analysis in biological networks. Bioinformatics. 2021;37(4):542–50.
Acknowledgements
CSIR-IMTECH Director Dr.Sanjeev Koshla has been a great support to us. I am thankful to my collaborator (OLP 177) Dr. Kishan Gopal and Dr. Dipesh Kumar and his team for their support in recombinant bacteriorhodopsin protein expression and whole genome sequencing studies of Haloarchaeal strains and submitted in NCBI database. The Manuscript communication number is 026/2022.
Funding
This research work was funded by OLP 177 project.
Author information
Authors and Affiliations
Contributions
MK: Data collection, data analysis, and manuscript drafting, editing; AT: SVM models development and webserver development; MK: SVM models and webserver development supervision; AKP: rhodopsin genes analysis from whole genome sequence; CRS: conceptualization, manuscript editing; BS: conceptualization, work plan, manuscript editing; SP: data collection, data analysis, manuscript drafting, editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Performance results of SVM model in 5-fold cross validation.
Additional file 2.
Performance of SVM models in 10-fold and 5-fold.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Selvaraj, M.K., Thakur, A., Kumar, M. et al. Ion-pumping microbial rhodopsin protein classification by machine learning approach. BMC Bioinformatics 24, 29 (2023). https://doi.org/10.1186/s12859-023-05138-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-023-05138-x