Abstract
Background
Acephalic spermatozoa syndrome is a rare type of teratozoospermia causing male infertility due to detachment of the sperm head and flagellum, which precludes fertilization potential. Although loss-of-function variations in several genes, including TSGA10, have been associated with acephalic spermatozoa syndrome, the genetic cause of many cases remains unclear.
Results
We recruited a Pakistani family with two infertile brothers who suffered from acephalic spermatozoa syndrome. Through whole-exome sequencing (WES) followed by Sanger sequencing, we identified a novel missense variant in TSGA10 (c.1112T > C, p. Leu371Pro), which recessively co-segregated with the acephalic spermatozoa syndrome within this family. Ultrastructural analyses of spermatozoa from the patient revealed that 98% of flagellar cross-sections displayed abnormal axonemal ultrastructure, in addition to the head-flagellum detachment. Real-time quantitative PCR analysis revealed almost no detectable TSAG10 mRNA and western blot analysis also failed to detect TSAG10 protein in patient's sperm samples while TSGA10 expression was clearly detected in control samples. Consistently, immunofluorescence analysis demonstrated the presence of TSGA10 signal in the midpiece of sperm from the control but a complete absence of TSGA10 signal in sperm from the patient.
Conclusion
Altogether, our study identifies a novel TSGA10 pathogenic variant as a cause of acephalic spermatozoa syndrome in this family and provides information regarding the clinical manifestations associated with TSGA10 variants in human.
Résumé
Contexte
Le syndrome des spermatozoïdes acéphaliques est un type rare de tératozoospermie provoquant une infertilité masculine en raison du détachement de la tête et du flagelle des spermatozoïdes, ce qui exclut une potentielle fécondation. Bien que des variations de perte de fonction dans plusieurs gènes, y compris TSGA10, aient été associées au syndrome des spermatozoïdes acéphaliques, la cause génétique de nombreux cas reste incertaine.
Résultats
Nous avons recruté une famille pakistanaise avec deux frères infertiles qui souffraient du syndrome des spermatozoïdes acéphaliques. Grâce au séquençage de l’exome entier (WES) suivi du séquençage Sanger, nous avons identifié un nouveau variant faux-sens dans TSGA10 (c.1112T > C, p. Leu371Pro), qui co-ségréguait de manière récessive avec le syndrome des spermatozoïdes acéphaliques au sein de cette famille. Les analyses ultrastructurales des spermatozoïdes des patients ont révélé que 98% des coupes transversales flagellaires présentaient une ultrastructure axonémiques anormales, en plus du décollement tête-flagelle. L’analyse quantitative par PCR en temps réel n’a révélé presque aucun ARNm TSAG10 détectable; l’analyse par transfert Western n’a pas non plus réussi à détecter la protéine TSAG10 dans les échantillons de sperme des patients, tandis que l’expression de TSGA10 a été clairement détectée dans les échantillons du témoin. De manière cohérente, l’analyse par immunofluorescence a démontré la présence du signal TSGA10 dans la partie médiane des spermatozoïdes du témoin, mais une absence totale de signal TSGA10 chez ceux des patients.
Conclusion
Dans l’ensemble, notre étude identifie un nouveau variant pathogène de TSGA10 comme cause du syndrome des spermatozoïdes acéphaliques dans cette famille et fournit des informations concernant les manifestations cliniques associées aux variants de TSGA10 chez l’homme.
Mots-clés
Infertilité, TSGA10, Spermatozoïdes acéphaliques, Variations faux-sens.
Similar content being viewed by others
Introduction
Infertility is the third major disease impacting human health in the twenty-first century according to the World Health Organization [1]. Males are responsible for almost 50% of infertility [2], whereas acephalic spermatozoa syndrome (ASS) is one of the severest disorders in male infertility. ASS is characterized by the presence of a lot of headless sperm tails, a few tailless sperm heads and sperm with aberrant head–tail link [3]. Occasionally, acephalic spermatozoa have been found to be familial, implying that it is a genetic condition [4,5,6,7]. However, the specific pathophysiology is still unknown.
Several genes have been shown to play an essential role in development of acephalic spermatozoa [8]. SUN5 mutations were reported to account for acephalic spermatozoa in one-third to one-half of reported patients with ASS [9,10,11,12]. Another study reported that the homozygous p.G928D mutation in BRDT (Bromodomain Testis Associated gene) leads to acephalic spermatozoa in a consanguineous family [5]. In addition, PMFBP1 mutations were also associated with acephalic spermatozoa in infertile human patients, which was functionally validated by using a knockout mouse model [11, 13]. However, the causes of ASS in many patients remain uncharacterized.
TSGA10 (Testis-specific gene 10) is a testis-specifically expressed gene, encoding a protein highly expressed in the sperm. The TSGA10 protein can be cleaved into two parts: a 27-kDa N-terminus found in the principal component, and a 55-kDa C-terminus found in the centrosome and basal body [14,15,16,17], which is a centrosome scaffold component associated with mother centrioles. The 55-kDa C-terminus of TSGA10 can interact with ODF2 [16], implying that the TSGA10 C-terminus is involved in centriole assembly and function, particularly in the head–tail link. Previously, loss of function mutation in TSGA10 was reported to be associated with ASS in infertile patients [18]. Additionally, male mice heterozygous for Tsga10 deletion were also found to be infertile and presented significantly reduced sperm motility because of disordered mitochondrial sheath formation [19]. Hence, the genetic and phenotype correlation between the TSGA10 mutation and ASS are still need to be confirmed.
In the present study, a Pakistani family who suffered from ASS was enrolled in this research, and a novel TSGA10 variant was identified by using whole exome sequencing (WES) and Sanger sequencing analysis. Further, TEM examination of spermatozoa from TSGA10 mutant patient revealed headless spermatozoa, disrupted ODF and disorganized axonemal structure, which is in consistent with the reported phenotype of TSGA10 mutant patient [18]. Overall, all these findings provide the genetic evidence that the homozygous missense variant in TSGA10 is pathogenic for acephalic spermatozoa syndrome.
Materials and methods
Ethical statement
This family was recruited from the local hospital in Khyber Pakhtunkhwa, Pakistan, and recorded in the Human Reproductive Disease Resource Bank at the University of Science and Technology of China’s (USTC). At the beginning of this study a detailed written consent forms from the patients and controls were signed. The Gomal University Pakistan and USTC institutional ethics committees approved this study.
Patients and medical examinations
In this study we recruited a Pakistani family with two infertile brothers (35- and 29- years-old respectively). The patients had no previous exposure to hazardous chemicals and did not drink alcohol or smoke. They had no history of urogenital or other reproductive diseases. The patients had normal erection and ejaculation according to the clinical examination. Sperm concentration and motility were conducted from patient, and sperm smear slides were prepared according to the instructions of World Health Organization [20].
HE staining of TSGA10 patient’s spermatozoa
In accordance with WHO standards [20], the patient III:2 had undergone routine semen analysis twice. Semen smears slides were prepared and sequentially immersed in 4% paraformaldehyde (PFA) for 5 min, washed with 1X phosphate buffered saline (PBS) twice for 5 min each, stained in hematoxylin (Solarbio, Beijing, China) for 30 min, dipped in purified water three times, immersed in 50% acidic ethanol, and kept in tap water for 2 min. The slides were then dehydrated in 50% and 80% ethanol for 5 min each, stained for 5 min with Eosin Azure (Solar bio), serially dehydrated twice in 100% ethanol for 5 min each and in xylene for 5 min, and eventually covered with coverslips and natural balsam. At least 200 spermatozoa were captured and analyzed under the optical microscope (Nikon, Tokyo, Japan).
WES and variant filtration
Blood samples were taken from the patients (III: 1 and III: 2), their fertile brother (III: 3), and their mother (II: 2). WES was carried out as described previously [21]. Variants were filtered using the following criteria: (a) variants with autosomal recessive inheritance pattern were included; (b) variants in linkage regions with logarithm odds scores > 0.01 were included. (c) variants having minor allele frequencies less than 0.05 [22] in any public database, including the 1000 Genomes Project, ESP6500, or gnomAD database, as well as homozygous variants in our in-house WES database compiled from 578 fertile men (41 Pakistani, 254 Chinese, and 283 European) were included; (d) protein sequence-altering variants (nonsense, missense, splice-site, and coding indels) were included; (f) variants in genes expressed in the testis were included; (g) variants predicted to be deleterious by more than half of the software (provided by ANNOVAR) covering them were included [23], and (h) variants within genes dispensable for spermatogenesis in mice were excluded based on spermatogenesis online 1.0 or literature searches [24]. Sanger sequencing was further used to confirm the filtered variants in all of the available family members (Supplementary Fig. 1). Lists of the primer sequences used for Sanger sequencing are given in Supplementary Table 2.
Real time quantitative PCR
Total RNA for qPCR was extracted from semen samples and stored in Trizol reagents (TakaRa Bio) for the patient and control samples. For cDNA synthesis, 1 μg RNA was reversely transcribed using PrimSript RT Reagent Kit (Takara) according to the manufacturer’s instructions as previously described [25]. The relative expression level of TSGA10 mRNA was calculated by normalization of the cycle threshold (Ct) value of samples to the corresponding Ct values of ACTB. The primer sequences are given in Supplementary Table 1.
Transmission electron microscopy
TEM was performed as previously described [25, 26]. Spermatozoa from the patient and control (fertile) were collected, fixed in 0.1 mol/L phosphate buffer (PB; pH 7.4), which contained 0.2% picric acid, 8% glutaraldehyde, and 4% paraformaldehyde, and then kept overnight at 4°C. Next day after washing with 0.1 mol/L PB, the samples were fixed with 1% osmium tetroxide. Dehydration of spermatozoa was done using different graded alcohol solutions (30%, 60%, 90% and 100%; 10 min for each bath), followed by inclusion in epon resin and acetone mixture. Then the samples were sliced into ultra-thin (70 nm) sections and stained with lead and uranyl acetate. The ultrastructure of spermatozoa cross-sections was captured and examined using Tecnai 10 or 12 Microscopes (Philps CM10, Philips Electronics, Eindhoven, and the Netherlands) at 120 kV or 100 kV.
In silico analysis of TSGA10
The genomic sequence of TSAG10 was retrieved from the NCBI (http//ncbi.nlm.nih.gov). To assess the deleterious effect of the variant, we used several prediction tools, including SIFT, PANTHER, PredictSNP, MAPP, SNAP, Polyphen-1 and Polyphen-2 [27,28,29,30,31,32,33,34]. In addition, we conducted multiple sequence alignment of the TSGA10 protein and analyzed the evolutionary conservation of the mutated residue across various species using Mega-X and Jalview [35,36,37].
Immunofluorescence staining
Immunofluorescence staining was conducted on the spermatozoa from the patients and control individuals, as previously reported [25, 38]. Briefly, patient sperm samples were smeared onto clean slides and fixed with 4% paraformaldehyde. The slides were then washed three times with PBS. The slides were permeabilized with 0.5% Triton X-100 for 30 min and blocked with 1% BSA. Primary antibodies, namely anti-α-tubulin (Sigma, F2168), anti-TSGA10 (12,593–1-AP, Proteintech Group) were incubated with the slides overnight at 4°C. On the next day, the slides were washed with PBST (PBS containing 0.1% Triton X-100) and subsequently incubated with secondary antibodies DAR555 (Molecular Probes, A31572) and GAM488 (Molecular Probes, A21121) for 1 h at 37°C. After three washes with PBST, the slides were sealed with Hoechst and Vectashield. Image acquisition was performed using a microscope (Olympus). The information of primary and secondary antibodies used and their dilutions are provided in Supplementary Table 2.
Western blotting
To obtain protein lysate, the semen sample was subjected to lysis using a lysis buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 2.5 mM EDTA, and 0.5% Triton X-100). The mixture was then centrifuged at 4°C for 15 min, and the resulting supernatant was collected as previously described [39]. For denaturation, the supernatant was incubated in protein loading buffer (100 mM Tris–HCl (pH 7.4), 2% SDS, 15% glycerol, 0.1% bromophenol blue, and 5 mM dithiothreitol, DTT) for 10 min. Subsequently, the protein was separated through SDS-PAGE electrophoresis and transferred to a nitrocellulose filter membrane (GE Healthcare, 10,600,002, CT, USA). The membrane was blocked using a TBST solution (50 mM tris (pH 7.4), 150 mM NaCl, and 0.1% Tween-20), containing5% skimmed milk for 1 h. Primary antibodies were then incubated with the membrane overnight at 4°C. The membrane was subsequently washed three times with TBST solution for 10 min each. Following a one-hour incubation with horseradish peroxidase-coupled secondary antibodies, the membrane was developed using a chemiluminescent substrate (Thermo Fisher Scientific, 34,580) and imaged using the ImageQuant LAS 4000 Imaging System (GE Healthcare). The information of primary and secondary antibodies used and their dilutions are provided in Supplementary Table 2.
Results
Physical and semen characteristics of patients
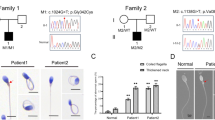
In this study, a Pakistani family with two infertile brothers was recruited (Fig. 1A). Both affected individuals had normal positions of abdominal viscera. The physical characteristic of patient (III: 1 and III: 2) are summarized in Table 1. III: 1 refused to provide semen samples and only shared his previous medical records. Patients (III: 1 and III: 2) had very low sperm concentrations (1 million/ml and 0.5 million/ml, respectively). Their sperm progressive motility were 4.0% and 7.0% respectively, which were below the reference range (32%) suggested by WHO [1]. Only 5% of spermatozoa were morphologically normal. We carried out the H&E staining of semen smear slides from patient (III: 2), to find out the morphological abnormalities of the spermatozoa. Among the sperm with abnormal morphology, almost no sperm were found with normal sperm head. The most prominent defect is absence of sperm head, accounting for 31% of total sperm. Multiple morphological defects of the sperm head were also identified, such as tapered, pyriform, round, and amorphous heads (Fig. 1B) and (Table 1). These results indicate that patient (III: 2) suffered from ASS.
A TSGA10 variant identified in a non-consanguineous Pakistani family with acephalic spermatozoa. A Pedigree of the Pakistani family with two infertile male patients, P1 (III: 1) and P2 (III: 2). Arrowheads point to the four individuals for which whole-exome sequencing (WES) was performed. Slashes denotes deceased family members. Squares represent males and circles represent females. Filled squares or circle represent male and female patient respectively. Clear symbols signify normal individuals. Diamonds represent multiple individuals without sex information. WT, wild-type. MT, (c.1112T > C, p. Leu371Pro). B Hematoxylin staining of the patient’s sperm. The sperm morphology was primarily acephalic. The semen smear slides were stained with the H&E staining, showing only the tails (ii-v). The red arrows indicate the acephalic spermatozoa. Scale bar: 10 μm, 5 μm respectively (C) Verification of the TSGA10 variant, c.1112T > C, in genomic DNA from all available family members
WES identified a homozygous missense variant in TSGA10
To determine the origin of the pathogenesis in this family, genomic DNA was extracted from the whole blood samples of the patients (III: 1) and (III: 2), their mother (II: 2), and fertile brother (III: 3), followed by WES analysis. Further bioinformatics analysis was performed for screening candidate pathogenic variations. Through a series of variant filtration methods, a TSGA10 homozygous missense variant (ENST00000393483, (c.1112T > C, p. Leu371Pro) was found (Supplementary Fig. 1). Later, Sanger sequencing confirmed that this variant was co-inherited with the ASS in this family in a recessive manner (Fig. 1C). Noticeably, we did not find any known genes related to acephalic spermatozoa except TSGA10 in these two patients. Therefore, we focused on this TSGA10 variant and hypothesized that this variant was potentially the cause of ASS in patients.
In silico analysis of the TSGA10 variant
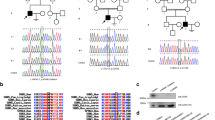
To evaluate the deleteriousness of this homozygous missense variant, we first performed several in silico analyses. This variant was identified in exon 15 of the TSGA10 and the affected amino acid was located in the functional domain of phosphodiesterase, as illustrated in Fig. 2A. Specifically the missense variant resulted in the replacement of the residue Leucine (L) at position 371 of the TSAG10 with Proline (P). The affected leucine is highly conserved in different species, indicating its functional significance (Fig. 2B). Figure 2C illustrates the position of the leucine residue within an α-helix of the wild-type TSGA10 protein. A mutation introducing proline was predicted to disrupt this α-helix and significantly alter the protein's structure. Furthermore, a comparative analysis of the properties of the wild-type and mutant amino acids reveals a discrepancy in their size. The mutant residue, being smaller, may result in a reduction of interactions within the protein structure. This size difference could lead to the loss of stabilizing interactions, such as hydrogen bonds or van der Waals forces, that are crucial for maintaining the structural integrity and function of the protein. Therefore, this mutation could potentially lead to a destabilized protein structure, further emphasizing the potential pathogenicity of this mutation. We evaluated the pathogenicity of the TSAG10 variant using deleteriousness- predicting software and the results are presented in Table 2. All seven-software predicted that this missense variant is deleterious. These findings provide further evidence to support the hypothesis that the p. Leu371Pro variant in TSGA10 is likely to be pathogenic and responsible for the infertility observed in patients (III: 1) and (III: 2).
Position of the identified TSGA10 variant at genomic, transcript and protein levels. A TSGA10 is located on chromosome (chr) 2, comprises 21 exons, and encodes a predicted 698-amino-acid protein (NCBI: ENST00000393483.8; UniProt KB: Q9BZW7-1). The TSGA10 variant (c.1112T > C, p. Leu371Pro), is located on exon 15. B Conservative analysis of the missense variant site in different species. C Effect of the variant on the helical structure of protein, predicted by Reprof software. Proline disrupts an α-helix. Due to this TSAG10 p. Leu371Pro variant, the α-helix is disrupted, which could have severe effects on the structure of the protein
TEM analysis of spermatozoa
TEM was carried out to check the ultrastructure of the sperm flagella of III: 2. Semen samples from a fertile male were used as a control. In contrast to the typical axoneme ultrastructure observed in sperm flagella from a control, which is composed of nine doublets of microtubules circularly arranged in an organized way (9 + 2 organization), disorganized axonemal structures with most of the cross-sections lacking the central pair (CP) and some doublets of microtubules were observed in patient (III: 2), which accounts for 98% of sperm flagella total of 30 cross-sections of mid-piece, principal and end piece were analyzed (Fig. 3). Hence, these findings indicated that patient with the missense variant in TSGA10 displayed defects in axonemal ultrastructure with the (CP) missing.
Ultrastructural defects in spermatozoa from the patient carrying homozygous TSGA10 variant. Transmission electron microscopic morphology of the cross-sections of the midpiece, principal and end-piece in patient and normal control. Scale bar: 500 nm. Abbreviations: MS, mitochondrial sheath; CP, central pair; ODF, outer dense fiber, MTDs; microtubule doublets
Level of the TSGA10 mutant mRNA in the sperm of the mutated patient
In order to verify the effect of this novel variant on TSGA10 expression. We performed qPCR to detect the expression level of TSGA10 mRNA in the patient and compared to that in control samples. The qPCR analysis clearly demonstrated the presence of TSGA10 mRNA in the control sample, while did not reveal any detectable expression of TSGA10 in the patient semen samples (Fig. 4A), indicating that the TSGA10 variant induce a drastic reduction of TSGA10 mRNA levels in the sperm of the mutated patient.
Expression of TSGA10 in the patients’ spermatozoa. A Real-time quantitative PCR analysis of TSGA10 mRNA expression in semen samples from the patient and control. β-Actin was used as an internal control. B Western blotting analysis of the protein level of TSGA10 in the patient and normal control. α-tubulin was used as a loading control (C) Representative image of spermatozoa from fertile control and patients carrying the TSGA10 variant stained with an anti-TSGA10 antibody (red), an anti-α-tubulin antibody (green), and Hoechst (blue, nuclear marker). The TSGA10 signal was not detected in the sperm midpiece from the patient. Scale bar: 10 μm
Level of the TSGA10 mutant protein in the sperm of the mutated patient’s sperm
To validate the impact of the TSGA10 mutation on its protein expression, we performed western blot and immunofluorescence staining analysis to assess the level of the TSGA10 protein in the patient’s sperm. Western blot analysis clearly demonstrated the expression of TSGA10 in the control sample, while the TSGA10 mutant protein was hardly detectable in the patient's sperm. Furthermore, immunostaining of spermatozoa from a healthy control confirmed the localization of TSGA10 in the midpiece. In contrast, no detectable TSGA10 signals were seen in the patient’s sperm (Fig. 4B). These results further substantiated that the TSGA10 variant, which resulted in the loss of TSGA10 protein expression, as the cause of acephalic spermatozoa in the patients (Fig. 4C).
Discussion
TSGA10 is a testis-specifically-expressed protein, which is located to the midpiece of sperm, centrosome and basal body [14, 17]. Previous research indicated a vital function of TSGA10 in the formation of head–tail link of centrioles, the arrangement of mitochondrial sheath and embryonic development [7, 15, 18]. So far, the genetic etiology of TSGA10 variations have been associated with about 3.1% of reported cases suffered from acephalic spermatozoa syndrome [5, 7,8,9,10,11,12,13, 18, 19, 40,41,42,43,44,45].
Here we reported a homozygous missense variant in TSGA10 (c.1112T > C, p. Leu371Pro) identified in infertile patients with acephalic spermatozoa from a Pakistani family. This variant was further verified by Sanger sequencing which revealed that the TSGA10 variant in the mutated patients recessively co-segregating with the infertility phenotype in this family. Multiple sequence alignment interpretations suggest that the mutated Leucine amino acid is significantly conserved among species, thus predicting the deleteriousness for p. Leu371Pro variant. The TSAG10 variant was submitted to several prediction tools to assess its degree of pathogenicity and all predicted it to be deleterious. Subsequent qPCR and western blotting revealed that the variant caused an almost complete loss of TSGA10 mRNA and protein in the sperm of the mutated patient. Moreover, the sperm phenotype of the mutated patient also resembles those of TSGA10-mutated patients previously reported [7]. Altogether, the pathogenicity of this variant is supported by in silico analysis, expression experiments, and clinical manifestations. Also, our results justify the previous reports on TSGA10 mutant infertile patient and Tsga10 knockout mouse model [19, 46].
We, and previous studies, observed headless sperm and defects in sperm axonemal ultrastructure, suggesting that TSGA10 plays a critical role in head/flagellum attachment, as well as sperm tail assembly and function [7]. It was reported that the C-terminus of TSGA10 was located to the midpiece of mature spermatozoa in an association with centrosome and basal body by the interaction of ODF2 protein [15, 16]. Odf2 haploinsufficiency also caused neck-midpiece separation [19], which is similar to the Tsga10 mutant phenotype, suggesting that these two proteins might function together. In the developing axoneme, the basal body plays an important role as a nucleation site, suggesting that it could have potential roles in flagellar biogenesis during spermiogenesis. Moreover, TSGA10 was also found localizing to the developing sperm tail, suggesting that it may participating in flagellar structure directly [7]. Further investigation into the ultrastructural localizations, specific protein interactions and signaling pathways involving TSGA10 will be critical for elucidating its precise role in sperm tail assembly and function, as well as its potential contribution to male infertility.
Conclusion
Our study reports a novel homozygous missense variant of TSGA10, c.1112T > C, p. Leu371Pro, associated with ASS and male infertility in the mutated patients. This discovery represents the first identification of a missense variant in the TSGA10 gene within a Pakistani family. This particular variant of TSGA10 is strongly linked to the cause of acephalic spermatozoa. The findings of this study contribute new knowledge to researchers and clinicians in the field of genetics and reproduction, enhancing our understanding of the pathology and molecular mechanisms underlying ASS.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Additional data are available from the corresponding author upon reasonable request.
Abbreviations
- TSGA10 :
-
Testis specific gene 10
- ASS :
-
Acephalic spermatozoa syndrome
- WES:
-
Whole-exome sequencing
- qPCR:
-
Real-time quantitative polymerase chain reaction
- WHO:
-
World health organization
- SUN5 :
-
Sad1 and UNC84 domain containing 5
- BRDT :
-
Bromodomain testis associated gene
- PMFBP1 :
-
Polyamine modulated factor 1 binding protein 1
- ODF2:
-
Outer dense fiber of sperm tails 2
- USTC:
-
University of science and technology of China
- PFA:
-
Paraformaldehyde
- PBS:
-
Phosphate buffered saline
- MAF:
-
Minor allele frequencies
- CP:
-
Central pair
- WT:
-
Wild-type
- MT:
-
Mutant
References
WHO. WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva: World Health Organization; 2021.
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12): e1001356.
Chemes HE, Carizza C, Scarinci F, Brugo S, Neuspiller N, Schwarsztein L. Lack of a head in human spermatozoa from sterile patients: a syndrome associated with impaired fertilization. Fertil Steril. 1987;47(2):310–6.
Chemes H, Puigdomenech E, Carizza C, Olmedo SB, Zanchetti F, Hermes R. Acephalic spermatozoa and abnormal development of the head–neck attachment: a human syndrome of genetic origin. Hum Reprod. 1999;14(7):1811–8.
Li L, Sha Y, Wang X, Li P, Wang J, Kee K, et al. Whole-exome sequencing identified a homozygous BRDT mutation in a patient with acephalic spermatozoa. Oncotarget. 2017;8(12):19914.
Porcu G, Mercier G, Boyer P, Achard V, Banet J, Vasserot M, et al. Pregnancies after ICSI using sperm with abnormal head–tail junction from two brothers: case report. Hum Reprod. 2003;18(3):562–7.
Sha YW, Sha YK, Ji ZY, Mei LB, Ding L, Zhang Q, et al. TSGA10 is a novel candidate gene associated with acephalic spermatozoa. Clin Genet. 2018;93(4):776–83.
Elkhatib RA, Paci M, Longepied G, Saias-Magnan J, Courbiere B, Guichaoua M-R, et al. Homozygous deletion of SUN5 in three men with decapitated spermatozoa. Hum Mol Genet. 2017;26(16):3167–71.
Fang J, Zhang J, Zhu F, Yang X, Cui Y, Liu J. Patients with acephalic spermatozoa syndrome linked to SUN5 mutations have a favorable pregnancy outcome from ICSI. Hum Reprod. 2018;33(3):372–7.
Sha Y-W, Xu X, Ji Z-Y, Lin S-B, Wang X, Qiu P-P, et al. Genetic contribution of SUN5 mutations to acephalic spermatozoa in Fujian China. Gene. 2018;647:221–5.
Zhu F, Liu C, Wang F, Yang X, Zhang J, Wu H, et al. Mutations in PMFBP1 cause acephalic spermatozoa syndrome. Am J Human Genet. 2018;103(2):188–99.
Shang Y, Yan J, Tang W, Liu C, Xiao S, Guo Y, et al. Mechanistic insights into acephalic spermatozoa syndrome–associated mutations in the human SUN5 gene. J Biol Chem. 2018;293(7):2395–407.
Sha YW, Wang X, Xu X, Ding L, Liu WS, Li P, et al. Biallelic mutations in PMFBP1 cause acephalic spermatozoa. Clin Genet. 2019;95(2):277–86.
Modarressi MH, Behnam B, Cheng M, Taylor KE, Wolfe J, van der Hoorn FA. Tsga 10 encodes a 65-kilodalton protein that is processed to the 27-kilodalton fibrous sheath protein. Biol Reprod. 2004;70(3):608–15.
Behnam B, Modarressi MH, Conti V, Taylor KE, Puliti A, Wolfe J. Expression of Tsga10 sperm tail protein in embryogenesis and neural development: from cilium to cell division. Biochem Biophys Res Commun. 2006;344(4):1102–10.
Behnam B, Mobahat M, Fazilaty H, Wolfe J, Omran H. TSGA10 is a centrosomal protein, interacts with ODF2 and localizes to basal body. J Cell Sci Ther. 2015;6(4):1.
Modarressi MH, Cameron J, Taylor KE, Wolfe J. Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene. 2001;262(1–2):249–55.
Ye Y, Wei X, Sha Y, Li N, Yan X, Cheng L, et al. Loss-of-function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol Genet Genomic Med. 2020;8(7): e1284.
Luo G, Hou M, Wang B, Liu Z, Liu W, Han T, et al. Tsga10 is essential for arrangement of mitochondrial sheath and male fertility in mice. Andrology. 2021;9(1):368–75.
WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. New York: World Health Organization; 2010.
Yin H, Ma H, Hussain S, Zhang H, Xie X, Jiang L, et al. A homozygous FANCM frameshift pathogenic variant causes male infertility. Genet Med. 2019;21(1):62–70.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic acids research. 2010;38(16):e164-e.
Zhang Y, Zhong L, Xu B, Yang Y, Ban R, Zhu J, et al. SpermatogenesisOnline 1.0: a resource for spermatogenesis based on manual literature curation and genome-wide data mining. Nucleic acids research. 2013;41(D1):D1055-D62.
Zhang B, Ma H, Khan T, Ma A, Li T, Zhang H, et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. 2020;217(2):e20182365.
Dil S, Khan A, Unar A, Yang M-L, Ali I, Zeb A, et al. A novel homozygous frameshift variant in DNAH8 causes multiple morphological abnormalities of the sperm flagella in a consanguineous Pakistani family. Asian J Androl. 2023;25(3):350.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7.20.
Bao L, Zhou M, Cui Y. nsSNPAnalyzer: identifying disease-associated nonsynonymous single nucleotide polymorphisms. Nucleic Acids Res. 2005;33(Web Server issue):W480–2.
Bendl J, Stourac J, Salanda O, Pavelka A, Wieben ED, Zendulka J, et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol. 2014;10(1): e1003440.
Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35(11):3823–35.
Brunham LR, Singaraja RR, Pape TD, Kejariwal A, Thomas PD, Hayden MR. Accurate prediction of the functional significance of single nucleotide polymorphisms and mutations in the ABCA1 gene. PLoS Genet. 2005;1(6): e83.
Capriotti E, Calabrese R, Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics. 2006;22(22):2729–34.
Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–4.
Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30(17):3894–900.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–4.
Troshin PV, Procter JB, Barton GJ. Java bioinformatics analysis web services for multiple sequence alignment–JABAWS:MSA. Bioinformatics. 2011;27(14):2001–2.
Urban J, Vanek J, Soukup J, Stys D. Expertomica metabolite profiling: getting more information from LC-MS using the stochastic systems approach. Bioinformatics. 2009;25(20):2764–7.
Zubair M, Khan R, Ma A, Hameed U, Khan M, Abbas T, et al. A recurrent homozygous missense mutation in CCDC103 causes asthenoteratozoospermia due to disorganized dynein arms. Asian J Androl. 2022;24(3):255.
Ma A, Zhou J, Ali H, Abbas T, Ali I, Muhammad Z, et al. Loss-of-function mutations in CFAP57 cause multiple morphological abnormalities of the flagella in humans and mice. JCI Insight. 2023;8(3):e166869.
Zhu F, Wang F, Yang X, Zhang J, Wu H, Zhang Z, et al. Biallelic SUN5 mutations cause autosomal-recessive acephalic spermatozoa syndrome. Am J Human Genet. 2016;99(4):942–9.
Chen H, Zhu Y, Zhu Z, Zhi E, Lu K, Wang X, et al. Detection of heterozygous mutation in hook microtubule-tethering protein 1 in three patients with decapitated and decaudated spermatozoa syndrome. J Med Genet. 2018;55(3):150–7.
Liu G, Wang N, Zhang H, Yin S, Dai H, Lin G, et al. Novel mutations in PMFBP1, TSGA10 and SUN5: Expanding the spectrum of mutations that may cause acephalic spermatozoa. Clin Genet. 2020;97(6):938–9.
Lu M, Kong S, Xiang M, Wang Y, Zhang J, Duan Z, et al. A novel homozygous missense mutation of PMFBP1 causes acephalic spermatozoa syndrome. J Assist Reprod Genet. 2021;38(4):949–55.
Sha Y, Wang X, Yuan J, Zhu X, Su Z, Zhang X, et al. Loss-of-function mutations in centrosomal protein 112 is associated with human acephalic spermatozoa phenotype. Clin Genet. 2020;97(2):321–8.
Zhang D, Huang WJ, Chen GY, Dong LH, Tang Y, Zhang H, et al. Pathogenesis of acephalic spermatozoa syndrome caused by SUN5 variant. Mol Hum Reprod. 2021;27(5):gaab028.
Tajaddini Mahani S, Behnam B, Abbassi M, Asgari H, Nazmara Z, Shirinbayan P, et al. Tsga10 expression correlates with sperm profiles in the adult formalin-exposed mice. Andrologia. 2016;48(10):1092–9.
Acknowledgements
We are grateful to all the participants for their cooperation. We thank the Bioinformatics Center of the University of Science and Technology of China, School of Life Science, for providing supercomputing resources. We also thank Li Wang and Dandan Song in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for their technical assistance on TEM and SEM, respectively.
Funding
This work was supported by the National Key Research and Developmental Program of China (2019YFA0802600, 2022YFC2702601, and 2021YFC2700202) and the National Natural Science Foundation of China (81971446 and U21A20204). This work was also supported by the Global Select Project (DJK-LX-2022010) of Institute of Health and Medicine, Hefei Comprehensive National Science Center, Hefei, China, and the Joint Fund for New Medicine of USTC (YD9100002034).
Author information
Authors and Affiliations
Contributions
KK, ZXJ and YJW performed the experiments; KK and SD wrote the manuscript; KK, IK and AZ recruited the patients, performed semen analysis and collected patient samples; WS, MZ and AUU modified the manuscript; HZ performed the WES sequencing and WES data analysis; MAK, WLM, XB, ZW and MH reviewed and edited the manuscript; QS conceived and supervised the study, and article drafting. All authors contributed to the report, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Investigations were carried out in accordance with the Declaration of University of Science and Technology of China (USTC). All experiments and examination on laboratory animals were conducted according to the institutional rules of the Institutional Animal Care Committee of the University of Science and Technology of China with approval number 2019-KY-168.
Consent for publication
All authors have seen the manuscript and approved to submit to the journal, and the authors claim that none of the material in the paper has been published or is under consideration for publication elsewhere.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Pipeline of the variant filtration followed by whole exome sequencing.
Additional file 2: Supplementary Table 1.
List of primers used in this study.
Additional file 3: Supplementary Table 2.
Information of antibodies used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khan, K., Zhang, X., Dil, S. et al. A novel homozygous TSGA10 missense variant causes acephalic spermatozoa syndrome in a Pakistani family. Basic Clin. Androl. 34, 4 (2024). https://doi.org/10.1186/s12610-024-00220-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12610-024-00220-7