Abstract
Background
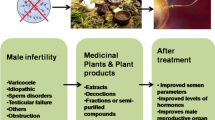
Red ginseng (RG) is a traditional herb commonly used in China, Korea, and other East Asian countries. Recently, it has demonstrated a better clinical value in men’s reproductive health (MRH). The present review aimed to examine the effects of RG treatment on MRH.
Results
Overall, 42 articles related to RG application in MRH were reviewed, of which 31 were animal experiments and 11 were clinical studies. Furthermore, this review analyzed the use of RG in some male reproductive diseases in clinical trials and determined the associated mechanisms of action. The mechanism of action of RG in MRH may be related to oxidative stress, regulation of sex hormones and spermatogenesis-related proteins, and anti-inflammation.
Conclusions
The application of RG for the treatment of male infertility, erectile dysfunction, and prostate diseases has the potential to contribute to MRH.
Résumé
Contexte
Le ginseng rouge est une herbe traditionnelle couramment utilisée en Chine, en Corée et dans d'autres pays d'Asie de l'Est. Récemment, il a démontré une valeur clinique en santé reproductive des hommes. La présente revue visait à examiner les effets du traitement par ginseng rouge en santé reproductive masculine.
Résultats
Au total, 42 articles liés à l’utilisation du traitement par ginseng rouge en situation d’altérations de la santé reproductive mâle ont été examinés, dont 31 étaient des expériences sur des animaux et 11 des études cliniques. De plus, cette revue a analysé l'utilisation du ginseng rouge dans certaines maladies de la reproduction masculine lors d’essais cliniques, et a déterminé les mécanismes d'action associés. Le mécanisme d'action du ginseng rouge en santé reproductive mâle peut être lié au stress oxydatif, à la régulation des hormones sexuelles et des protéines liées à la spermatogenèse, et à une action anti-inflammatoire.
Conclusions
L'application du ginseng rouge pour le traitement de l'infertilité masculine, de la dysfonction érectile et des maladies de la prostate a le potentiel de contribuer à la santé reproductive des hommes.
Similar content being viewed by others
Background
With a recent increase in the prevalence of male diseases, men’s reproductive health (MRH) has received an increasing attention [1]. A broad definition of MRH includes both pathophysiological and psychosocial issues [2]. MRH is related to the erectile function, testicular functions of steroidogenesis (testosterone synthesis), and spermatogenesis, and it is influenced by numerous factors [3, 4]. These factors include unhealthy eating habits, delayed marriage, exposure to environmental pollutants, psychological stress, drug abuse, and natural aging in humans [5,6,7,8]. Given the influence of traditional sexual concepts, many men are reluctant to admit that they have a reproductive disorder. Furthermore, some patients are concerned about the adverse effects of oral therapeutic drugs or have unrealistic perceptions regarding current treatments, hindering them from seeking healthcare [9]. Given this background, a series of food products focusing on MRH have emerged and are gradually being accepted by patients [10]. However, these functional food products differ from therapeutic drugs, and their exact therapeutic effects on male reproduction-related diseases remain uncertain.
Red ginseng (RG) is a cooked product of ginseng that undergoes several processes, such as infiltration, cleaning, sorting, steaming, and drying, and it is widely used as an herbal medicine in some East Asian countries. Many clinical studies and animal experiments have reported that Korean RG has beneficial effects on MRH, including the regulation of testicular, erectile, and prostate functions [11,12,13]. However, currently, there is a lack of systematic review of these studies, although a few reviews tend to report the efficacy and safety of Korean RG in male diseases by meta-analysis [14]. Moreover, to date, these studies have not been analyzed in detail from a holistic perspective. In the present study, we systematically reviewed the existing relevant literature, analyzed the efficacy of RG in MRH through clinical evidence, and elaborated its related mechanisms along with basic research to provide a reference for the future application of RG in MRH.
Materials and methods
Search strategy
The PubMed and Web of Science databases were searched for relevant studies (up to December 2022). We also reviewed the reference lists of the studies identified via our search strategy and selected those that seemed relevant according to our keywords. We set out relevant search terms by referring to previous systematic reviews on MRH [15, 16]. The following search terms were used in Table 1.
Eligibility criteria
Inclusion criteria
We comprehensively and systematically searched for studies in accordance with the criteria outlined in the Preferred Reporting Items for Systematic Reviews (PRISMA) statement. Further, the review process was organized using the PICO (Participant, Intervention, Comparison, and Outcomes) framework, as described below:
-
Participants: studies involving male patients or animals with reproductive system diseases or dysfunction.
-
Intervention: studies involving patients or animals receiving RG or its extracts.
-
Comparison: clinical studies involving conventional treatment, medication, placebo, and no treatment or animal models of reproductive system diseases or dysfunctions.
-
Outcomes: clinical studies assessing changes in sperm quality, International Index of Erectile Function 5th version (IIEF-5), sexual satisfaction, etc. or animal experiments assessing changes in testicular weight, sperm quality, intracavernous pressure, spermatogenesis-related genes, sex hormone, inflammatory cytokines, etc.
Exclusion criteria
The following studies were excluded: (1) nonclinical studies or studies not involving animal experiments (review articles, case reports, letters, comments, posters, book chapters, etc.); (2) duplicates and studies with incomplete data; (3) studies not primarily focusing on RG (such as those on panax ginseng, black ginseng, American ginseng, etc.); (4) studies not primarily focusing on male reproductive diseases or dysfunction (such as those on female sexual dysfunction, female reproductive health, etc.).
Data collection and analysis
Selection of studies
Two authors (Hao Wang and Bin Yan) searched for relevant articles according to the search items and then summarized the results. Original articles involving RG for MRH were included. Duplicate studies were eliminated. Some studies were excluded after analyzing the title, abstract, and the full text. The reference list of each study was also checked when necessary, to include relevant research that may have been missed in the initial search. Dissenting opinions were submitted to another author (Jiwei Zhang) for adjudication throughout the whole process (Fig. 1).
Data extraction
The two abovementioned authors independently extracted data in a standard format. Some of these data were the characteristics, intervention, and results. They also checked the extracted data for accuracy and completeness. Another author (Jiwei Zhang) participated in the discussions and helped resolve any disagreements.
Results
After removing duplicates, only 147 of 249 searched studies were included in the assessment, as shown in the selection flowchart (Fig. 1). Further, after reviewing the topics, abstracts, and full text of 147 studies, 105 were excluded because they were not primary RG, primary MRH, or original articles. Finally, we included 11 clinical studies involving 289 patients who received RG and 31 animal experiments, as shown in Tables 2 and 3, respectively.
Clinical studies on the effect of red ginseng on men’s reproductive health
Among 11 clinical studies included (Table 2), 10 were randomized controlled trials of RG in erectile dysfunction (ED) [11, 17,18,19,20,21,22,23,24,25,26] and 1 was a randomized controlled trial of RG in male infertility (MI) [11]. Varying doses of RG were used for treating male reproductive disorders. The daily dose of Korean RG ranged from 800 mg to 3000 mg, and the duration of treatment ranged from 4 weeks to 3 months. Five of these studies used the dose of 600 mg thrice daily, whereas two used the dose of 900 mg thrice daily. The results of the included studies revealed that 1500 mg of RG orally administered daily for 12 weeks significantly improved sperm concentration, viability, and morphology in the patients. Another 10 studies consistently reported that RG enhanced erectile function and alleviated male sexual dysfunction in patients. Although these clinical studies lacked consistency in the efficacy indicators and preferred subjective patient perceptions, they revealed that RG improved the IIEF-5 scores and sexual life satisfaction. Notably, in the study by Kim et al. [20], 21 patients received 900 mg of RG thrice daily, whereas 7 received 600 mg RG thrice daily. In their study, factors such as erectile hardness, sexual desire, and satisfaction with sex improved in 19 (67%) patients, of which 15 were from the 900 mg group and 4 from the 600 mg group. Given the large difference in the rates between the two groups, Kim et al. could not confirm which group was more effective. Further clinical studies are required to validate the optimal use of RG in male reproductive diseases. Nevertheless, the abovementioned 11 studies reported no significant serious adverse effects.
Animal experiments to verify the mechanisms of red ginseng on men’s reproductive health
The 31 studies in Table 3 showed that the effects of RG on MRH are mainly achieved by acting on target organs such as the testis, penis, and prostate [13, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. RG is beneficial to MRH through various mechanisms of action. The majority of these RG studies (20 articles) focused on spermatogenic dysfunction, particularly testicular function injury caused by RG improvement exposure factors. The choice of medicine was based more on RG extract. However, most studies did not specify the specific extract ingredients. RG is generally used as a health food because of the richness in ginsenosides, volatile oils, polysaccharides, amino acids, peptides, and other components with high nutritional values. Hence, our review results suggest the need for further exploration on the specific component of RG that is beneficial to male spermatogenic function. Nonetheless, our results also suggest that Korean RG has a restorative effect on ED caused by different diseases [51, 52]. Moreover, given that prostate was one of the target organs of RG in these studies, RG and its extracts were also being related to the regulation of prostate function.
Discussion
With the increasing prevalence of MI, ED, and prostate diseases in recent years [57,58,59], these diseases pose serious threats to patients’ quality of life, mental health, and family relations. Accordingly, more patients are actively seeking relevant treatment. Based on our clinical research evidence, RG—a safe functional food—can effectively improve erectile function in patients with ED, restore semen quality in patients with MI, and improve MRH. Further, based on the relevant evidence from animal experiments, RG’s potential mechanism of action on testis, penis, and prostate gland was clarified. To the best of our knowledge, no study in the relevant literature has analyzed various studies on RG utilization for MRH to date [60, 61]. The potential mechanisms of RG in the treatment of MI, ED, and prostate disease need to be summarized and analyzed to clarify the value of RG in MRH and to provide a reference for future clinical applications.
Red ginseng and male infertility
MI refers to the inability of the female partner to conceive naturally because of male factors after the couple has had regular sex for > 1 year without using any contraception [62, 63]. The causes of MI are complex, and they include aging [64], occupational exposure [65], drug injury [66], infection, and other self-inflicted diseases [67]. RG monotherapy somewhat reversed reproductive damage in animal models exposed to tetrachlorodibenzo-p-dioxin, zearalenone, and RG monotherapy in combination with numerous drugs to mitigate pharmacogenically induced reproductive toxicity [35, 36, 39]. For example, ciprofloxacin can lead to a decrease in testicular weight, sperm quality, and testosterone level in patients with testicular inflammation—an important factor in sperm arrest and testicular atrophy [68]. However, ciprofloxacin combined with RG has been reported to improve sperm quality and reduce apoptosis indicators in Wistar rats [28]. Animal experiments have reported that the combination of RG with antitumor drugs, busulfan and doxorubicin, also attenuates drug toxicity–induced damage to the reproductive system [37, 43, 44]. Although the exact mechanism is unclear, an increasing number of studies have tended to attribute RG’s mechanism of alleviation of pharmacogenic reproductive toxicity to antioxidant effects [43, 44].
Multifunctional oxidoreductases, such as glutathione peroxidase 4 (GPx4), glutathione S-transferase mu 5 (GSTm5), and peroxiredoxin 4 (PRx4), are involved in the spermatogenic pathway; when the expression of these proteins in testicular cells is downregulated, oxidative damage and cell death may occur [69,70,71]. RG—a natural antioxidant—increased the expression of PRx4, GSTm5, and GPx4 in animal models while decreasing the serum levels of reactive oxygen species (ROS), as confirmed by numerous relevant studies [30, 31, 41] (Fig. 2). In addition, RG normalized the recovery of antioxidants such as glutathione-S-transferase (GST), ascorbic acid, and α-tocopherol in the testes of aging rats and prevented oxidative damage to the testicular tissue by reducing the production of nicotinamide adenine dinucleotide phosphate oxidase and superoxide [29,30,31,32,33,34].
Mechanism of the therapeutic effect of red ginseng on male infertility. Abbreviations: FSH, follicle stimulating hormone; LH, luteinizing hormone; CREB, CAMP responsive element binding protein; PRx4, peroxiredoxin 4; GPx4, glutathione peroxidase 4; GSTm5, glutathione S-transferase mu 5, SIIS, subchronic intermittent immobilization stress. (Created with BioRender.com)
Key biomolecules such as inhibin-α, CAMP responsive element binding protein 1 (CREB-1), and nectin-2 are involved in testicular function and are considered to be sex hormone pathway molecules associated with spermatogenesis [72]. Inhibin-α, a gonadal glycoprotein, is essential in regulating the secretion of follicle stimulating hormone (FSH) [73]. CREB-1 is expressed during the mitotic phase of spermatogenesis and the differentiation phase of spermatogenesis, thereby playing an important role in spermatogenesis [74]. Furthermore, nectin-2 promotes support-support cells or support-mature germ cells in the seminiferous tubules of the testis in close contact and contributes to the development of mature spermatozoa in the seminal vesicle epithelium [74]. The impaired expression of these genes induced by doxorubicin, aging, subchronic intermittent immobilization stress, and heat stress can be reversed by RG, restoring spermatogenic function in rat testes (Fig. 2) [29,30,31,32,33,34, 42,43,44,45].
Moreover, RG may improve sperm quality by regulating the production of testosterone, FSH, and luteinizing hormone (LH) [29,30,31,32,33,34, 38, 43,44,45]. Testosterone is essential for the function and maintenance of the structure of the male secondary gonads and is related to spermatogenesis [75, 76]. Meanwhile, LH promotes testosterone synthesis by testicular mesenchymal cells, and FSH acts on the FSH receptor in supporting cells to aid spermatogenesis [77]. In animal experiments, RG and its extracts significantly attenuated spermatogenesis disorders caused by sex hormone alterations resulting from various factors (e.g., drug damage, aging, ethanol, and subchronic intermittent immobilization stress), elevated testosterone levels, and mitigated the aberrant increase in FSH and LH levels (Fig. 2) [29,30,31,32,33,34, 38, 43,44,45].
Red ginseng and erectile dysfunction
ED is a common sexual dysfunction, in which men cannot consistently obtain and maintain an erection sufficient to complete a satisfactory sexual intercourse [78, 79]. Its common risk factors include cardiovascular disease, including aging, diabetes, and metabolic syndrome [80,81,82]. Activation of the smooth muscle of the penile corpus cavernosum requires the action of endothelial cell relaxing factor or nitric oxide (NO) [83], which is an essential physiological signal for penile erection; diseases that reduce NO synthesis or release in the erectile tissue are usually associated with ED [84]. Nitric oxide synthase (NOS), which includes neuronal NOS and endothelial NOS, uses L-arginine as a substrate to generate NO from oxygen; acetylcholine may also facilitate NO production and release. The released NO activates guanylate cyclase in the smooth muscle cytoplasm to produce cyclic guanosine monophosphate (cGMP) [85]. RG may act by releasing or enhancing the endothelial cell relaxing factor and increasing acetylcholine-induced rabbit cavernous muscle relaxation, which may be mediated by NO and/or cGMP [49] (Fig. 3).
Mechanism of the therapeutic effect of red ginseng on erectile dysfunction. Abbreviations: NOS, nitric oxide synthase; NO, nitric oxide; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; GSH, glutathione; GSH-px, glutathione peroxidase; GSSG, glutathione oxidized; MDA, malondialdehyde; ROS, reactive oxygen species; TGF-β1, transforming growth factor-β 1. (Created with BioRender.com)
In the treatment of diabetes mellitus–associated ED, Ryu et al. used malondialdehyde (MDA) as an indicator of oxidative stress and measured the glutathione (GSH) levels to confirm the antioxidant effect of RG on the cavernous body of rats with diabetic mellitus–associated ED [51]. These rats had significantly higher MDA levels and significantly lower GSH levels than normal rats, but these outcomes were reversed after RG treatment [51]. Excessive ROS causes lipid peroxidation of unsaturated fatty acids on mitochondrial membranes to form lipid peroxides and then produce MDA, resulting in increased MDA content in mitochondria [86]. MDA can quantify free radical damage to cells. Glutathione peroxidase catalyzes the change in GSH, forming oxidized glutathione; it also promotes peroxide decomposition and protects the structure and function of cell membranes from interference and damage caused by peroxides (Fig. 3) [87]. Under long-term high glucose induction in diabetes, ROS is excessively produced in the organism and mitochondria, and the activity of free radical scavenging enzymes such as GSH peroxidase decreases as a result of non-enzymatic glycosylation; the scavenging of free radicals such as oxygen and hydrogen peroxide is also reduced, leading to further accumulation of ROS in the body and subsequently aggravating oxidative stress damage and organ dysfunction [87]. This mechanism supports an imbalance between free radical production and scavenging in the spongiosa of diabetic rats, and RG plays a role in the antioxidant activity [51].
Metabolic syndrome may cause vascular endothelial dysfunction through multiple pathways. With regard to improving erectile function in ED rats with metabolic syndrome, penile cavernous fibrosis progresses in vascular-derived ED. In addition, the expression of transforming growth factor-β 1 (TGF-β1), a molecular marker of fibrosis in fractionated vascular ED, induces damaged-tissue repair by aggregating fibroblasts in areas of ischemic-tissue damage and promoting the production of collagenous connective tissue [88]. In the study by Kim et al. [52], the proportion of penile smooth muscle cells in rats suffering from metabolic syndrome was significantly reduced, whereas that with RG treatment was close to that of normal controls and inhibited TGF-β1 expression in the penile corpus cavernosum (Fig. 3).
Red ginseng and prostate diseases
Prostate disease is a common disease in adult men, usually related to prostatitis, benign prostatic hyperplasia (BPH), and prostate cancer. The results of our study revealed that chronic prostatitis (CP) and BPH are closely related to RG. CP represents a group of syndromes caused by multiple factors, mainly pain or discomfort in the pelvic region and lower urinary tract symptoms; further, it is subdivided into bacterial and nonbacterial types [89]. Inflammatory diseases of the reproductive system, such as epididymitis and other accessory gonad infections caused by Escherichia coli, may cause prostate congestion and edema, inducing bacterial prostatitis [90]. RG can reduce prostate weight gain in rats with acute epididymitis and improve the low semen quality caused by ciprofloxacin administration for bacterial prostatitis [27, 28]. Various cytokines, including tumor necrosis factor-α, vascular endothelial growth factor, interleukin-6, interleukin-1β, and cyclooxygenase-2, are involved in the inflammatory response [91]. RG inhibited the expression of these inflammatory factors in rats and subsequently reversed the apoptosis caused by CP (Fig. 4) [13, 55].
BPH is a disease characterized by histological hyperplasia of the interstitial and glandular components of the prostate, affecting mostly middle-aged and older adult men; consequently, the prostate enlarges, causing urodynamic bladder outlet obstruction and clinical symptoms mainly in the lower urinary tract [92]. Testosterone increases the risk of BPH and worsens the lower urinary tract symptoms [93]. Through 5α-reductase (5AR) in the prostate, testosterone is converted into activated dihydrotestosterone, which then binds to the androgen receptor in prostate cells, inducing transcriptional activation of target genes and apoptosis and proliferative imbalance in prostate cells, leading to BPH [94]. Therefore, the inhibition of androgen production and blockade of androgen receptor signaling may be important strategies for BPH treatment [95]. In rats, daily injection of Korean RG water extract prevented testosterone-induced prostate overgrowth and epithelial cell thickening and inhibited androgen receptor activation [56]. In vivo studies have also shown an 18% reduction in prostate weight and downregulation of the expression of dihydrotestosterone, 5AR2, and 5AR1 in BPH rats receiving RG oil [54] (Fig. 4). In addition, increased apoptosis helps ameliorate the hyperproliferation of prostate cells. Both B-cell lymphoma 2 (Bcl-2, an anti-apoptotic protein) and Bcl-2-associated X protein (Bax, a pro-apoptotic protein that induces apoptosis) [96] aid in regulating the mitochondrion-mediated apoptotic pathway. Furthermore, changes in BPH were associated with increased Bcl-2 levels and decreased Bax levels in the same tissue [97], but these effects were reversed after RG extract treatment; hence, RG may inhibit prostate tissue overexpression by inducing apoptosis [54].
Conclusion
To the best of our knowledge, this study is the first to review the effects of RG on MRH, providing a new and unique perspective on the treatment of related diseases. For MI, the research on RG tends to be animal experiments, mainly concentrating on oxidative stress, sex hormones, and related indicators of spermatogenesis. Further studies are required to elucidate the upstream mechanisms and verify the mechanism of action of RG in regulating the related specific pathways. Although several clinical studies have analyzed the use of RG for ED, the data on the included participants and efficacy evaluation indexes significantly differ among the clinical trials. Thus, potential mechanisms to improve erectile function should be explored further. Moreover, given that the use of RG in prostate diseases remains unknown, the efficacy and specific mechanism involved in RG treatment for such diseases should be examined in the future.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- RG:
-
Red ginseng
- MRH:
-
Men’s reproductive health
- ED:
-
Erectile dysfunction
- MI:
-
Male infertility
- GPx4:
-
Glutathione peroxidase 4
- GSTm5:
-
Glutathione S-transferase mu 5
- PRx4:
-
Peroxiredoxin 4
- ROS:
-
Reactive oxygen species
- GST:
-
Glutathione-S-transferase
- CREB-1:
-
CAMP responsive element binding protein 1
- FSH:
-
Follicle stimulating hormone
- LH:
-
Luteinizing hormone
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- TGF-β 1:
-
Transforming growth factor-β 1
- BPH:
-
Benign prostatic hyperplasia
- CP:
-
Chronic prostatitis
- 5AR:
-
5α-reductase
- Bcl-2:
-
B-cell lymphoma 2
- Bax:
-
Bcl-2-associated X protein
References
Yu X, Zhang S, Wei Z, Zhang X, Wang Q. Prevalence of sexual dysfunction among the male populations who seeking medical care for infertility, pregnancy loss and preconception care: a cross-sectional study. Sci Rep. 2022;12:12969. https://doi.org/10.1038/s41598-022-17201-3.
Forrest KA. Men’s reproductive and sexual health. J Am Coll Health. 2001;49:253–66. https://doi.org/10.1080/07448480109596312.
Tseng CH. The Effect of Metformin on Male Reproductive function and prostate: an updated review. World J Mens Health. 2022;40:11–29. https://doi.org/10.5534/wjmh.210001.
Okonofua FE, Ntoimo LFC, Omonkhua A, Ayodeji O, Olafusi C, Unuabonah E, et al. Causes and risk factors for male infertility: a scoping review of published studies. Int J Gen Med. 2022;15:5985–97. https://doi.org/10.2147/ijgm.S363959.
Sharqawi M, Hantisteanu S, Bilgory A, Aslih N, Shibli Abu Raya Y, Atzmon Y, et al. The impact of lifestyle on sperm function, telomere length, and IVF outcomes. Am J Mens Health. 2022;16:15579883221119931. https://doi.org/10.1177/15579883221119931.
Demirci A, Hızlı F, Hamurcu HD, Başar H. Erectile dysfunction, anxiety, perceived stress, and insomnia are more common among acquired premature ejaculation patients compared to other premature ejaculation syndromes. Andrology. 2023;11:425–32. https://doi.org/10.1111/andr.13341.
Valeiro C, Matos C, Scholl J, van Hunsel F. Drug-Induced sexual dysfunction: an analysis of reports to a National Pharmacovigilance Database. Drug Saf. 2022;45:639–50. https://doi.org/10.1007/s40264-022-01174-3.
Dong S, Chen C, Zhang J, Gao Y, Zeng X, Zhang X. Testicular aging, male fertility and beyond. Front Endocrinol (Lausanne). 2022;13:1012119. https://doi.org/10.3389/fendo.2022.1012119.
Hearn J, Nordberg M, Andersson K, Balkmar D, Gottzén L, Klinth R, et al. Hegemonic masculinity and beyond:40 years of research in Sweden. Men and Masculinities. 2012;15:31–55. https://doi.org/10.1177/1097184X11432113.
Marini HR, Micali A, Squadrito G, Puzzolo D, Freni J, Antonuccio P, et al. Nutraceuticals: a new challenge against cadmium-induced testicular injury. Nutrients. 2022;14. https://doi.org/10.3390/nu14030663.
Park HJ, Choe S, Park NC. Effects of korean red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chin J Integr Med. 2016;22:490–5. https://doi.org/10.1007/s11655-015-2139-9.
Smith SJ, Lopresti AL, Teo SYM, Fairchild TJ. Examining the effects of herbs on testosterone concentrations in men: a systematic review. Adv Nutr. 2021;12:744–65. https://doi.org/10.1093/advances/nmaa134.
Kang SW, Park JH, Seok H, Park HJ, Chung JH, Kim CJ, et al. The effects of Korea red ginseng on inflammatory cytokines and apoptosis in rat model with chronic nonbacterial prostatitis. Biomed Res Int. 2019;2019:2462561. https://doi.org/10.1155/2019/2462561.
Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–50. https://doi.org/10.1111/j.1365-2125.2008.03236.x.
Wang Y, Xie Z. Exploring the role of gut microbiome in male reproduction. Andrology. 2022;10:441–50. https://doi.org/10.1111/andr.13143.
Gunes S, Hekim GN, Arslan MA, Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33:441–54. https://doi.org/10.1007/s10815-016-0663-y.
Hong B, Ji YH, Hong JH, Nam KY, Ahn TY. A double-blind crossover study evaluating the efficacy of korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol. 2002;168:2070–3. https://doi.org/10.1016/s0022-5347(05)64298-x.
Kim SW, Paick JS. Clinical efficacy of Korea red ginseng on vasculogenic impotent patients. Korean J Androl. 1999;17:23–8.
Choi HK, Seong DH, Rha KH. Clinical efficacy of korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7:181–6.
Kim YC, Hong YK, Shin JS, Kang MS, Sung DH, Choi HK. Effect of korean red ginseng on sexual dysfunction and serum lipid level in old aged men. J Ginseng Res. 1996;20:125–32.
Choi HK, Seong DH. Effectiveness for erectile dysfunction after the administration of korean red ginseng. J Ginseng Res. 1995;19:17–21.
Choi HK, Choi YD. Effectiveness of Korea red ginseng in erectile dysfunction-multi-national approach. J Ginseng Res. 1999;23:247–56.
Ham WS, Kim WT, Lee JS, Ju HJ, Kang SJ, Oh JH, et al. Efficacy and safety of red ginseng extract powder in patients with erectile dysfunction: multicenter, randomized, double-blind, placebo-controlled study. Korean J Urol. 2009;50:159–64. https://doi.org/10.4111/kju.2009.50.2.159.
Choi HK, Choi YJ. Evaluation of clinical efficacy of Korea red ginseng for erectile dysfunction by international index of erectile function (IIEF). J Ginseng Res. 2001;25:112–7.
de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, et al. Study of the efficacy of korean red ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–4. https://doi.org/10.1111/j.1745-7262.2007.00210.x.
Choi HK, Choi YJ, Kim JH. Penile blood change after oral medication of korean red ginseng in erectile dysfunction patients. J Ginseng Res. 2003;27:165–70. https://doi.org/10.5142/JGR.2003.27.4.165.
Eskandari M, Jani S, Kazemi M, Zeighami H, Yazdinezhad A, Mazloomi S, et al. Ameliorating effect of ginseng on epididymo-orchitis inducing alterations in sperm quality and spermatogenic cells apoptosis following infection by uropathogenic escherichia coli in rats. Cell J. 2016;18:446–57. https://doi.org/10.22074/cellj.2016.4573.
Eskandari M, Ghalyanchi Langeroudi A, Zeighami H, Rostami A, Kazemi M, Eyni H, et al. Co-administration of ginseng and ciprofloxacin ameliorates epididymo-orchitis induced alterations in sperm quality and spermatogenic cells apoptosis following infection in rats. Andrologia. 2017;49. https://doi.org/10.1111/and.12621.
Ku JY, Park MJ, Park HJ, Park NC, Joo BS. Combination of korean red ginseng extract and hydrogen-rich water improves spermatogenesis and sperm motility in male mice. Chin J Integr Med. 2020;26:361–9. https://doi.org/10.1007/s11655-019-3047-1.
Kopalli SR, Hwang SY, Won YJ, Kim SW, Cha KM, Han CK, et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol. 2015;69:94–102. https://doi.org/10.1016/j.exger.2015.05.004.
Kopalli SR, Cha KM, Ryu JH, Lee SH, Jeong MS, Hwang SY, et al. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis-related molecules. Exp Gerontol. 2017;90:26–33. https://doi.org/10.1016/j.exger.2017.01.020.
Kim IH, Kim SK, Kim EH, Kim SW, Sohn SH, Lee SC, et al. Korean red ginseng up-regulates C21-steroid hormone metabolism via cyp11a1 gene in senescent rat testes. J Ginseng Res. 2011;35:272–82. https://doi.org/10.5142/jgr.2011.35.3.272.
Hwang SY, Sohn SH, Wee JJ, Yang JB, Soo KJ, Kwak YS, et al. Panax ginseng improves senile testicular function in rats. J Ginseng Res. 2010;34:327–35. https://doi.org/10.5142/jgr.2010.34.4.327.
Kopalli SR, Cha KM, Ryu JH, Hwang SY, Kim SK. Specific activity of korean red ginseng saponin and non-saponin fractions in ageing-induced rat testicular dysfunction. J Funct Foods. 2017;29:226–37. https://doi.org/10.1016/j.jff.2016.12.031.
Hwang SY, Yang JB, Wee JJ, Kim OH, Kim SK. Crude saponin from korean red ginseng attenuates testicular toxicity of rats exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. J Ginseng Res. 2003;27:171–7. https://doi.org/10.5142/JGR.2003.27.4.171.
Hwang SY, Kim WJ, Wee JJ, Choi JS, Kim SK. Panax ginseng improves survival and sperm quality in guinea pigs exposed to 2,3,7,8-tetrachlorodibenzo- p-dioxin. BJU Int. 2004;94:663–8. https://doi.org/10.1111/j.1464-410X.2004.05019.x.
Jung SW, Kim HJ, Lee BH, Choi SH, Kim HS, Choi YK, et al. Effects of korean red ginseng extract on busulfan-induced dysfunction of the male reproductive system. J Ginseng Res. 2015;39:243–9. https://doi.org/10.1016/j.jgr.2015.01.002.
Jang M, Min JW, In JG, Yang DC. Effects of red ginseng extract on the epididymal sperm motility of mice exposed to ethanol. Int J Toxicol. 2011;30:435–42. https://doi.org/10.1177/1091581811405074.
Cho ES, Ryu SY, Jung JY, Park BK, Son HY. Effects of red ginseng extract on zearalenone induced spermatogenesis impairment in rat. J Ginseng Res. 2011;35:294–300. https://doi.org/10.5142/jgr.2011.35.3.294.
Lee J, Jeong JS, Cho KJ, Moon KN, Kim SY, Han B, et al. Developmental and reproductive toxicity assessment in rats with KGC-HJ3, korean Red Ginseng with Angelica gigas and deer antlers. J Ginseng Res. 2019;43:242–51. https://doi.org/10.1016/j.jgr.2017.12.004.
Kim YH, Kim GH, Shin JH, Kim KS, Lim JS. Effect of korean red ginseng on testicular tissue injury after torsion and detorsion. Korean J Urol. 2010;51:794–9. https://doi.org/10.4111/kju.2010.51.11.794.
Kopalli SR, Cha KM, Hwang SY, Jeong MS, Kim SK. Korean Red Ginseng (Panax ginseng Meyer) with enriched Rg3 ameliorates chronic intermittent heat stress-induced testicular damage in rats via multifunctional approach. J Ginseng Res. 2019;43:135–42. https://doi.org/10.1016/j.jgr.2018.06.004.
Cha KM, Kopalli SR, Han SY, Lee SH, Jeong MS, Cho JY, et al. Korean red ginseng attenuates doxorubicin-induced testicular dysfunction in rats by modulating inflammatory, oxidative, and autophagy responses. J Funct Foods. 2017;40:736–43. https://doi.org/10.1016/j.jff.2017.12.008.
Kopalli SR, Won YJ, Hwang SY, Cha KM, Kim SY, Han CK, et al. Korean red ginseng protects against doxorubicin-induced testicular damage: an experimental study in rats. J Funct Foods. 2016;20:96–107. https://doi.org/10.1016/j.jff.2015.10.020.
Lee SH, Choi KH, Cha KM, Hwang SY, Park UK, Jeong MS, et al. Protective effects of korean red ginseng against sub-acute immobilization stress-induced testicular damage in experimental rats. J Ginseng Res. 2019;43:125–34. https://doi.org/10.1016/j.jgr.2017.09.002.
Khouri NA, Daradka HM, Allouh MZ, Alkofahi AS. Comparative effects of orchis anatolica vs. the red korean panax ginseng treatments on testicular structure and function of adult male mice. J Complement Integr Med. 2015;12:33–41. https://doi.org/10.1515/jcim-2012-0035.
Choi YD, Xin ZC, Choi HK. Effect of korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res. 1998;10:37–43. https://doi.org/10.1038/sj.ijir.3900300.
Cho KS, Park CW, Kim CK, Jeon HY, Kim WG, Lee SJ, et al. Effects of korean ginseng berry extract (GB0710) on penile erection: evidence from in vitro and in vivo studies. Asian J Androl. 2013;15:503–7. https://doi.org/10.1038/aja.2013.49.
Choi YD, Rha KH, Choi HK. In vitro and in vivo experimental effect of korean red ginseng on erection. J Urol. 1999;162:1508–11. https://doi.org/10.1016/S0022-5347(05)68349-8.
Park SW, Lee CH, Shin DH, Bang NS, Lee SM. Effect of SA1, a herbal formulation, on sexual behavior and penile erection. Biol Pharm Bull. 2006;29:1383–6. https://doi.org/10.1248/bpb.29.1383.
Ryu JK, Lee T, Kim DJ, Park IS, Yoon SM, Lee HS, et al. Free radical-scavenging activity of korean red ginseng for erectile dysfunction in non-insulin-dependent diabetes mellitus rats. Urology. 2005;65:611–5. https://doi.org/10.1016/j.urology.2004.10.038.
Kim SD, Kim YJ, Huh JS, Kim SW, Sohn DW. Improvement of erectile function by korean red ginseng (Panax ginseng) in a male rat model of metabolic syndrome. Asian J Androl. 2013;15:395–9. https://doi.org/10.1038/aja.2012.159.
Yoshimura H, Kimura N, Sugiura K. Preventive effects of various ginseng saponins on the development of copulatory disorder induced by prolonged individual housing in male mice. Methods Find Exp Clin Pharmacol. 1998;20:59–64. https://doi.org/10.1358/mf.1998.20.1.485633.
Lee JY, Kim S, Kim S, Kim JH, Bae BS, Koo GB, et al. Effects of red ginseng oil(KGC11o) on testosterone-propionate-induced benign prostatic hyperplasia. J Ginseng Res. 2022;46:473–80. https://doi.org/10.1016/j.jgr.2021.11.005.
Miri M, Shokri S, Darabi S, Alipour Heidari M, Ghalyanchi A, Karimfar MH, et al. Efficacy of compound therapy by ginseng and ciprofloxacin on bacterial prostatitis. Cell J. 2016;18:103–11. https://doi.org/10.22074/cellj.2016.3993.
Bae JS, Park HS, Park JW, Li SH, Chun YS. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med. 2012;66:476–85. https://doi.org/10.1007/s11418-011-0609-8.
Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging. 2019;11:10952–91. https://doi.org/10.18632/aging.102497.
Zhang X, Yang B, Li N, Li H. Prevalence and risk factors for erectile dysfunction in chinese adult males. J Sex Med. 2017;14:1201–8. https://doi.org/10.1016/j.jsxm.2017.08.009.
Zhang Z, Li Z, Yu Q, Wu C, Lu Z, Zhu F, et al. The prevalence of and risk factors for prostatitis-like symptoms and its relation to erectile dysfunction in chinese men. Andrology. 2015;3:1119–24. https://doi.org/10.1111/andr.12104.
Lee HW, Lee MS, Kim TH, Alraek T, Zaslawski C, Kim JW, et al. Ginseng for erectile dysfunction: a Cochrane systematic review. World J Mens Health. 2022;40:264–9. https://doi.org/10.5534/wjmh.210071.
Lee HW, Kil KJ, Lee MS. Ginseng for improving semen quality parameters: a systematic review. World J Mens Health. 2020;38:377–84. https://doi.org/10.5534/wjmh.190125.
Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–20. https://doi.org/10.1016/j.eururo.2021.08.014.
Miller D, Vukina J. Recent advances in clinical diagnosis and treatment of male factor infertility. Postgrad Med. 2020;132:28–34. https://doi.org/10.1080/00325481.2020.1830589.
Mazur DJ, Lipshultz LI. Infertility in the aging male. Curr Urol Rep. 2018;19:54. https://doi.org/10.1007/s11934-018-0802-3.
Marić T, Fučić A, Aghayanian A. Environmental and occupational exposures associated with male infertility. Arh Hig Rada Toksikol. 2021;72:101–13. https://doi.org/10.2478/aiht-2021-72-3510.
Ajayi AF, Akhigbe RE. The physiology of male reproduction: impact of drugs and their abuse on male fertility. Andrologia. 2020;52:e13672. https://doi.org/10.1111/and.13672.
Farsimadan M, Motamedifar M. Bacterial infection of the male reproductive system causing infertility. J Reprod Immunol. 2020;142:103183. https://doi.org/10.1016/j.jri.2020.103183.
Drobnis EZ, Nangia AK. Antimicrobials and male reproduction. Adv Exp Med Biol. 2017;1034:131–61. https://doi.org/10.1007/978-3-319-69535-8_10.
Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, Ikawa M, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–58. https://doi.org/10.1042/bj20081526.
Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. https://doi.org/10.5534/wjmh.2014.32.1.1.
Barbieri ER, Hidalgo ME, Venegas A, Smith R, Lissi EA. Varicocele-associated decrease in antioxidant defenses. J Androl. 1999;20:713–7. https://doi.org/10.1002/j.1939-4640.1999.tb03375.x.
Cai K, Hua G, Ahmad S, Liang A, Han L, Wu C, et al. Action mechanism of inhibin α-subunit on the development of sertoli cells and first wave of spermatogenesis in mice. PLoS ONE. 2011;6:e25585. https://doi.org/10.1371/journal.pone.0025585.
Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, et al. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci U S A. 1987;84:5082–6. https://doi.org/10.1073/pnas.84.14.5082.
Lui WY, Sze KL, Lee WM. Nectin-2 expression in testicular cells is controlled via the functional cooperation between transcription factors of the Sp1, CREB, and AP-1 families. J Cell Physiol. 2006;207:144–57. https://doi.org/10.1002/jcp.20545.
Oduwole OO, Huhtaniemi IT, Misrahi M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms222312735.
Goto T, Hirabayashi M, Watanabe Y, Sanbo M, Tomita K, Inoue N, et al. Testosterone supplementation rescues spermatogenesis and in vitro fertilizing ability of sperm in kiss1 knockout mice. Endocrinology. 2020;161. https://doi.org/10.1210/endocr/bqaa092.
Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl. 2004;6:259–68.
Wang H, Zhao M, Zhang J, Yan B, Liu S, Zhao F, et al. The efficacy of acupuncture on patients with erectile dysfunction: a review. Evid Based Complement Alternat Med. 2022;2022:4807271. https://doi.org/10.1155/2022/4807271.
Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–41. https://doi.org/10.1016/j.juro.2018.05.004.
Defeudis G, Mazzilli R, Tenuta M, Rossini G, Zamponi V, Olana S, et al. Erectile dysfunction and diabetes: a melting pot of circumstances and treatments. Diabetes Metab Res Rev. 2022;38:e3494. https://doi.org/10.1002/dmrr.3494.
Salama MN, Eid AA, Hatem A, Swidan AK. Prevalence of erectile dysfunction in egyptian males with metabolic syndrome. Aging Male. 2020;23:257–63. https://doi.org/10.1080/13685538.2018.1479736.
Echeverri Tirado LC, Ferrer JE, Herrera AM. Aging and erectile dysfunction. Sex Med Rev. 2016;4:63–73. https://doi.org/10.1016/j.sxmr.2015.10.011.
Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005;106:233–66. https://doi.org/10.1016/j.pharmthera.2004.11.011.
Dutta S, Sengupta P. The role of nitric oxide on male and female reproduction. Malays J Med Sci. 2022;29:18–30. https://doi.org/10.21315/mjms2022.29.2.3.
Hiremath DS, Priviero FBM, Webb RC, Ko C, Narayan P. Constitutive LH receptor activity impairs NO-mediated penile smooth muscle relaxation. Reproduction. 2021;161:31–41. https://doi.org/10.1530/rep-20-0447.
Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011;650:184–94. https://doi.org/10.1016/j.ejphar.2010.09.033.
Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4:46–54. https://doi.org/10.2174/157339908783502398.
Siregar S, Adriansjah R, Sibarani J, Mustafa A. Effect of intracorporeal human adipose-derived stem cells (hADSCs) on corpora cavernosa transforming growth factor β(1) (TGFβ(1)) and collagen type I concentration in wistar rat priapism model. Res Rep Urol. 2020;12:21–7. https://doi.org/10.2147/rru.S232303.
Rees J, Abrahams M, Doble A, Cooper A. Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015;116:509–25. https://doi.org/10.1111/bju.13101.
Terai A, Yamamoto S, Mitsumori K, Okada Y, Kurazono H, Takeda Y, et al. Escherichia coli virulence factors and serotypes in acute bacterial prostatitis. Int J Urol. 1997;4:289–94. https://doi.org/10.1111/j.1442-2042.1997.tb00192.x.
Zang L, Tian F, Yao Y, Chen Y, Shen Y, Han M, et al. Qianliexin capsule exerts anti-inflammatory activity in chronic non-bacterial prostatitis and benign prostatic hyperplasia via NF-κB and inflammasome. J Cell Mol Med. 2021;25:5753–68. https://doi.org/10.1111/jcmm.16599.
Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I-Initial work-up and Medical Management. J Urol. 2021;206:806–17. https://doi.org/10.1097/ju.0000000000002183.
Rastrelli G, Vignozzi L, Corona G, Maggi M. Testosterone and benign prostatic hyperplasia. Sex Med Rev. 2019;7:259–71. https://doi.org/10.1016/j.sxmr.2018.10.006.
Vickman RE, Franco OE, Moline DC, Vander Griend DJ, Thumbikat P, Hayward SW. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020;7:191–202. https://doi.org/10.1016/j.ajur.2019.10.003.
Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. 2021;127:389–99. https://doi.org/10.1111/bju.15229.
Rho J, Seo CS, Park HS, Wijerathne CU, Jeong HY, Moon OS, et al. Ulmus macrocarpa hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J Ethnopharmacol. 2019;233:115–22. https://doi.org/10.1016/j.jep.2018.11.042.
Ub Wijerathne C, Park HS, Jeong HY, Song JW, Moon OS, Seo YW, et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull. 2017;40:2125–33. https://doi.org/10.1248/bpb.b17-00468.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82104674) and Science and Technology Innovation Project of China Academy of Chinese Medical Sciences(CI2021A02209).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception or design of the work (BY and FW) or the acquisition (DM), analysis (ZZ). Drafting of the work (HW) or revising it critically for important intellectual content (JZ). All authors approve the submitted final version to be published. All authors have agreed to take responsibility for all aspects of the work to ensure that any questions related to the accuracy or integrity of any part of the work are addressed.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Zhang, J., Ma, D. et al. The role of red ginseng in men’s reproductive health: a literature review. Basic Clin. Androl. 33, 27 (2023). https://doi.org/10.1186/s12610-023-00203-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12610-023-00203-0