Abstract
Background
The seminal virome and its implications for fertility remain poorly understood. To date, there are no defined panels for the detection of viruses of clinical interest in seminal samples.
Results
In this study, we characterized the human seminal virome based on more than 1,000 studies published over the last five years.
Conclusions
The number of studies investigating viruses that occur in human semen has increased, and to date, these studies have been mostly prospective or related to specific clinical findings. Through the joint analysis of all these studies, we have listed the viruses related to the worsening of seminal parameters and propose a new panel with the main viruses already described that possibly affect male fertility and health. This panel can assist in evaluating semen quality and serve as a tool for investigation in cases of infertility.
Résumé
Contexte
Le virome séminal et ses implications pour la fertilité restent mal compris. À ce jour, il n’existe pas de panels définis pour la détection des virus d’intérêt clinique dans les échantillons de sperme.
Résultats
Dans cette étude, nous avons caractérisé le virome séminal humain sur la base de plus de 1000 études publiées au cours des cinq dernières années.
Conclusions
Le nombre d’études portant sur les virus présents dans le sperme humain a augmenté et, à ce jour, ces études ont principalement été prospectives ou liées à des résultats cliniques spécifiques. Grâce à l’analyse conjointe de toutes ces études, nous avons répertorié les virus liés à l’aggravation des paramètres spermatiques, et nous proposons un nouveau panel avec les principaux virus déjà décrits qui peuvent affecter la fertilité et la santé masculines. Ce panel peut aider à évaluer la qualité du sperme et servir d’outil d’investigation dans les cas d’infertilité.
Similar content being viewed by others
Introduction
Infertility is a disease of the male or female reproductive system defined by the inability to establish a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse [1]. It is estimated that infertility affects 8–12% of couples of reproductive age worldwide, with the male factor being solely responsible for 20–30% of the cases, contributing to 50% of records in general [2].
Infections and inflammatory reactions in the male genital tract (MGT) are among the main causes of infertility, accounting for 6–10% of the cases [3]. These infections are mainly caused by sexually transmitted pathogens and can induce infertility through multiple pathophysiological mechanisms, including impairment of seminal parameters and sperm functions [4, 5].
Various microorganisms, including bacteria, viruses, and protozoa, can infect the male reproductive tract and impair fertility [6]. Viral infections usually correspond to complex conditions, as there are no therapeutic measures for their control, and they can be transmitted causing infertility or subfertility in men [5]. Many viral families have strong tropism for the male reproductive tract, especially for the testes [7]. Salam and Horby [8] reported that 27 viral species can be found in human semen. In addition, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been detected in human semen [9,10,11], raising concerns about the potential impact of this new coronavirus on male fertility.
In semen, viruses can infect sperm or sperm precursor cells, attach to molecules on the outside of sperm, present themselves as free virus particles, or reside in the seminal immune cells. Furthermore, viral infection of germ cells can result not only in changes in testicular function but also in the transmission of virus-induced mutations to subsequent generations [12].
Despite recent advances, there are no well-defined correlations regarding the effects of viral infections on fertility. This review aimed to identify and present the main recent findings about viruses in human semen, characterize the diversity of the seminal virome, identify the main viral species related to fertility, and discuss panels for viral identification that could have clinical applications and fertility research implications.
Methodology
To characterize the human seminal virome, all studies published until May 8, 2021 available in the PubMed database were initially identified without language restriction. The search was performed using the following parameters: (“virology”[MeSH Subheading] OR “virology”[All Fields] OR “viruses”[All Fields] OR “viruses”[MeSH Terms] OR “virus s” [All Fields] OR “viruse”[All Fields] OR “virus”[All Fields]) AND (“semen”[MeSH Terms] OR “semen”[All Fields] OR “semen s”[All Fields] OR “semens “[All Fields] OR (“sperm s”[All Fields] OR “spermatozoa”[MeSH Terms] OR “spermatozoa”[All Fields] OR “sperm”[All Fields] OR “sperms”[All Fields]) OR “ seminal”[All Fields]).
To determine the main viruses that occur in semen, titles, abstracts, and full texts of articles published in the last 5 years were examined for discussion of the presence of the virus in semen by isolation or amplification and for the detection of nucleic acids or of specific antigens. Reviews, meta-analyses, and other publications that did not report the original clinical data were excluded. Studies conducted in vitro or in animal models and those with unavailable full texts were also not considered. Once the main viruses were identified, the search was expanded to include previously published articles and animal studies to deepen the discussion for each case.
Results
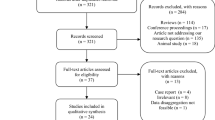
We identified 4,239 articles published until May 08, 2021 (Fig. 1). Based on the number of articles published per year, it was observed that the number of studies concerning viruses and semen increased over time, becoming more evident from 2016 (Fig. 2). Given the considerable increase in the number of studies published in the last 5 years, we chose to consider only the articles published from 2016 onwards in this review, resulting in a total of 1,030 articles. After screening by title and abstract, 257 articles were selected, 75 were discarded because they were not directly related to the topic, and seven were not available in full. Thus, 175 articles (17%) met the eligibility criteria established for full text analysis.
The analysis of selected articles revealed that 27 virus species were identified in human semen in the last 5 years, with the human immunodeficiency virus (HIV) being the most cited, followed by the Zika virus (ZIKV), Ebola virus (EBOV), human papillomavirus (HPV), and human cytomegalovirus (HCMV) (Fig. 3). Among the 27 viruses identified, 13 were associated with abnormalities in seminal parameters (Fig. 3). The main characteristics of each of the 27 viruses identified as well as a summary of the main effects already described on male reproductive health are presented in Table 1. The main information regarding the 13 viruses related to abnormalities in seminal parameters is described in the Discussion section.
Overview of viruses detected in semen in this review and their associations with seminal parameters. The X-axis corresponds to the virus detected in human semen in the last five years, and the Y-axis represents the number of papers published. Abbreviations: HIV: Human immunodeficiency virus; ZIKV: Zika virus; EBOV: Ebola virus; HPV: Human papillomavirus; HCMV: Human cytomegalovirus; HSV 1/2: Herpes simplex virus type 1 and 2; EBV: Epstein-Barr virus; HVV-6: Human herpesvirus-6; HBV: Hepatitis B virus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; LASV: Lassa virus; HHV-8: Human herpesvirus-8; VZV: Varicella zoster virus; HEV: Hepatitis E virus; HHV-7: Human herpesvirus-7; AVs: Anelloviruses; SFTSV: Severe fever with thrombocytopenia syndrome virus; NiV: Nipah virus; WNV: West Nile virus; AAV: Adeno-associated virus; ANDV: Andes virus; DENV: Dengue virus; YFV: Yellow Fever Virus; JCPyV: JC polyomavirus; CHIKV: Chikungunya virus; RVFV: Rift Valley fever virus; HCV: Hepatitis C virus.
Discussion
HIV
Semen is the vector for most sexual transmissions of HIV worldwide. HIV can contaminate semen during the stages of acute and chronic infection and acquired immunodeficiency syndrome (AIDS) [99, 100]. The human immunodeficiency virus type 1 (HIV-1) may present in semen as free virions, associated with sperm, or virions that have invaded leukocytes [101]. The expression of the β-chemokine receptor C-C chemokine receptor type 5 (CCR5) in the peri-acrosomal region of the sperm surface and the presence of C-C chemokine receptor type 3 (CCR3) in the post-acrosomal cap could be involved in HIV-1 adhesion to spermatozoa, enabling these cells to act as virion carriers during sexual transmission of HIV-1 [5]. Leukocytes, including lymphocytes, monocytes, and macrophages, are considered the main vectors of HIV-1 in the semen [6].
The HIV viral load in seminal fluid is generally lower than that in blood [7]. However, HIV shedding persists in the semen of a subset of individuals who receive antiretroviral therapy, indicating that MGT may constitute a viral reservoir [100, 102]. This persistence is associated with different factors, including sexually transmitted infections (STIs), viral load in the blood, co-infection with HCMV or Epstein-Barr virus (EBV), and seminal cytokine levels [103,104,105,106,107].
The effects of HIV infection on seminal parameters can be observed in both asymptomatic and symptomatic patients [108]. However, the sperm abnormalities found in HIV patients are poorly understood, as both viral and antiretroviral treatments can cause changes [108, 109]. Semen alterations in HIV-infected men include decreased motility, sperm concentration, total sperm count, and ejaculate volume. In addition to this, abnormal morphology and a high percentage of sperm with DNA damage indicates impaired spermatogenesis [13, 110, 111].. The hypothesis is that decreased motility is related to mitochondrial toxicity caused by the nucleotide reverse transcriptase inhibitors used in therapy [112].
Furthermore, testis morphology and spermatogenesis are affected by disease progression [113]. Likewise, AIDS patients may develop chronic orchitis and, consequently, progressive hypergonadotropic hypogonadism, suggesting impaired testicular steroidogenesis [7].
ZIKV
ZIKV can enter the testicular microenvironment, disrupt cellular metabolism, alter testicular physiology, and activate an intense immune response that can result in severe testicular damage and infertility [114].
Several studies have demonstrated that ZIKV is detected in the semen of infected men [115,116,117,118] up to six months after infection [119]. Although the reported persistence of ZIKV varies from days to months after the onset of symptoms, it is widely accepted that viral RNA persists longer and has a higher viral load in semen than in other bodily fluids. These observations suggest that ZIKV has tropism for the MGT, which may act as a viral reservoir, possibly due to the immunological privilege of the testes [6, 120]. Consequently, pregnant women should protect themselves against mosquito bites and also ensure safe sexual intercourse with their partner during pregnancy. Sexual transmission of ZIKV is possible and the most important condition associated with the infection is microcephaly, which forms in fetuses [121]. Ex vivo human tissue studies have revealed that several cell types, including germ cells, Sertoli cells, Leydig cells, and testicular resident macrophages, are permissive to ZIKV infection [122,123,124]. Likewise, it has been shown that, in mice, ZIKV preferentially infects spermatogonia, primary spermatocytes, and Sertoli cells in the testis, resulting in cell death and destruction of the seminiferous tubules [20]. In addition, there is evidence that ZIKV infection is associated with acute and chronic prostatitis in mouse and non-human primate models [125].
Sperm concentration and motility percentage may be significantly lower in the semen of ZIKV-positive individuals [21]. In addition to oligospermia, increased leukocytospermia, hematospermia, painful ejaculation, and penile secretion in patients with ZIKV infection, suggestive of local inflammation and tissue damage, have also been reported [22, 126]. Regarding strict Kruger morphology, a large percentage of sperm with head defects or various anomalies was also observed in ZIKV-positive samples [21, 23].
HPV
HPV can be found anywhere in the male genital system, such as the external genitalia, urethra, prostate, epididymis, vas deferens, testes, and semen [127,128,129,130,131,132]. HPV DNA can be detected in sperm, somatic cells, and seminal plasma [133].
Several studies have reported that semen infection by HPV can interfere with different sperm parameters, such as count, vitality, motility and morphology, pH, semen viscosity, and the number of leukocytes can increase DNA fragmentation and the level of anti-sperm antibodies in semen [29,30,31,32,33,34].
The prevalence of seminal HPV infection is significantly higher in infertile men than in the general population [35]. It has been reported that HPV16, HPV51, HPV52, and HPV45 are the most frequently found genotypes [127, 129, 134]. HPV 16 appears to be the most frequent type, with a prevalence of 5.9% in the infertile population and 4.7% in the general population [35]. However, the prevalence of HPV genotypes can vary depending on the geographic area or country and additional factors, such as lifestyle or number of partners [135]. In addition, different semen fractions can contain multiple HPV types in varying amounts, with different HPV genotypes in the same fraction [136].
Infected sperms also serve as vectors for HPV transfer [133]. The penetration of HPV-infected sperm cells into oocytes results in the intracellular delivery of the HPV genome, followed by active transcription of the HPV genes in the fertilized egg [137, 138]. Perino et al. [134] reported that when HPV was present in semen, assisted reproduction techniques resulted in a lower fertilization rate and an increased percentage of abortions. Tangal et al. [139] also observed that after in vitro fertilization (IVF) treatment, implantation and pregnancy rates were similar in infected and uninfected males, but lower numbers of good-quality embryos and increased abortion rates were found in the presence of HPV-positive sperm.
The European Society of Human Reproduction and Embryology (ESHRE) Guideline on Viral Infection/Disease [140] also points to other studies that demonstrate the impact of seminal HPV infection on the results of assisted reproduction techniques. Among these, we highlight the following:
(1) Depuydt et al. [141] when investigating the clinical pregnancy rate of 732 couples, observed that the clinical pregnancy rate was significantly lower in women inseminated with HPV-positive semen (2.9% per cycle) than in those inseminated with HPV-negative semen (11.1% per cycle). Furthermore, above a ratio of 0.66 HPV virions/sperm, no pregnancies were observed.
(2) Garolla et al. [142] also reported that the cumulative pregnancy rate by intrauterine insemination and intracytoplasmic sperm injection (ICSI) for HPV-positive men was 14.2% (5/54) compared to that of 38.4% (66/172) for HPV-negative men, whereas the miscarriage rate was significantly higher in HPV-positive than in HPV-negative men (62.5% vs. 16.7%).
(3) Depuydt et al. [143] also analyzed 514 sperm samples from donors from three different sperm banks for 18 different HPV types. Overall, 3.9% (20/514) of tested donor sperms were positive for HPV, with different prevalences among the three different sperm banks (3.6% bank A, 3.1% bank B, and 16.7% bank C). It was observed that when sperm from the HPV-positive donor was used, no clinical pregnancy resulted, whereas when HPV-negative donor sperm was used, the clinical pregnancy rate was 14.6%.
Emerging evidence indicates that HPV infection in men affects sperm parameters and can reduce pregnancy and increase abortion rates [140].
HCMV
HCMV has already been isolated from several secretions, including semen, indicating that this virus can infect the MGT and that semen can act as a vector for viral propagation [5, 144].
Some studies have not found any association [39, 45, 79, 145] between the impact of HCMV on male fertility and seminal parameters, whereas others have observed a positive correlation [12, 36, 40].
Bezold et al. [29] observed that HCMV was the most frequently detected pathogen in the semen of patients with infertility. However, they did not observe a significant association between the presence of HCMV DNA and the sperm parameters. In contrast, Jahromi et al. [36], in a study carried out at the infertility center of the Ghadir Maternal Child Hospital, identified an estimated prevalence of HCMV in semen (18.6%). They observed a higher prevalence of HCMV in the semen of men with abnormal semen analysis and a significant reduction in sperm morphology and count in the presence of HCMV, which supports the hypothesis that HCMV has a negative impact on male fertility.
HCMV can attach to the sperm surface and infect immature germ cells, which then develop into mature HCMV-carrying sperm [5, 37]. Furthermore, Naumenko et al. [37] observed a considerable decrease in the number of immature germ cells, indicating that HCMV produces a direct gametotoxic effect that may contribute to male infertility.
Herpes simplex virus (HSV)
Herpes simplex virus type 1 (HSV-1) and herpes type 2 (HSV-2) have been widely detected in human semen with varying frequencies [29, 41, 42, 45, 60]. Bai et al. [146] observed that 2–50% of infertile men were positive for HSV-1/2.
The sources of HSV seminal DNA remain to be clarified, but it is known that HSV-2 can be internalized into healthy, motile sperm and is likely to cause direct damage to male germ cells [147].
HSV infections are associated with abnormal sperm parameters and male infertility, indirectly resulting in immune responses [42, 146, 148]. A strong association has been reported between HSV infection and problems with seminal parameters, including hematospermia, oligospermia, and increased apoptotic cells [39,40,41,42,43, 149, 150].
A reduction in seminal volume and abnormal viscosity has also been reported in men infected with HSV-2, which indicates prostate dysfunction [41]. Bezold et al. [29] reported significantly reduced sperm concentration and motility in addition to lower citrate and neutral α-glucosidase concentrations in HSV-infected men, suggesting compromised epididymal and prostate function.
Human herpesvirus-6 (HHV-6)
HHV-6 is frequently found in semen samples [29, 38, 45, 151], but its effect on male fertility is unclear. Specific binding of human herpesvirus 6 B (HHV-6B) to the sperm acrosome has been observed, suggesting the existence of a specific receptor for the virus [65]. Furthermore, a higher prevalence of HHV-6 in men with chronic inflammatory disease of the urogenital tract has been observed [38]. This suggests that HHV-6 may contribute to the etiology of these diseases, but it does not lead to infertility, as a correlation between the detection of HHV-6 and reduced sperm parameters has not been observed.
HHV-6 is the only human herpes virus that integrates into the germline [152, 153]. HHV-6 genomes are usually found on chromosomes close to the telomeric ends, probably facilitated by homologous recombination through repeat sequences that flank the viral genome [154, 155]. Thus, HHV-6 integrated into germ cells can be transmitted vertically from parents to children, leading to congenital HHV-6 infection [156]. Godet et al. [151] detected two semen samples with high HHV-6 viral loads, consistent with the presence of integrated HHV-6 chromosomes. In these samples, sperm parameters revealed abnormal sperm morphology and immobile sperms.
Hepatitis B virus (HBV)
Several studies have reported a reduction in sperm parameters attributed to HBV infection of semen [30, 47,48,49,50]. Lorusso et al. [49] observed that sperm concentration, motility, morphology, and viability were significantly impaired in HBV-seropositive patients. Karamolahi et al. [94] also reported similar results, whereby men infected with HBV and hepatitis C virus (HCV) showed a decrease in total sperm count, liquefaction time, and sperm motility, in addition to having an impaired morphology.
In addition to affecting seminal parameters, HBV infection can also cause serious damage to sperm DNA [157], as the HBV genome can integrate not only into host hepatocytes but also into human sperm chromosomes and induce chromosomal aberrations [158]. In this context, Huang et al. [159], using fluorescence in situ hybridization (FISH), showed that the HBV genome integrated into sperm chromosomes can be vertically transmissible through germ cells, producing heritable effects. Furthermore, HBV infection can have mutagenic effects on sperm chromosomes, leading to genomic instability and chromosomal aberrations.
Moreover, HBV induces the generation of reactive oxygen species (ROS) and reduces the antioxidant capacity of sperm cells, leading to an increase in oxidative stress [5, 160,161,162,163]. Thus, an increase in ROS concentration in spermatozoa can result in loss of membrane integrity, mitochondrial damage, genome damage, and apoptosis [5, 164,165,166]. In addition, HBV-induced oxidative stress can also affect male fertility, as observed by Qian et al. [163], who reported that oxidative stress can lead to a reduction in seminal parameters. They observed that the concentration of ROS in the semen of infertile men was negatively correlated with seminal volume, pH, sperm density, motility, morphology, activation rate, and sperm vitality.
Male HBV infection may result in a lower success rate in assisted reproduction procedures. Men with chronic HBV infection have been observed to have a significantly higher risk of low fertilization rate after IVF. This leads to a slight decrease in the total number of embryos fertilized [51]. Zhou et al. [50] also concluded that HBV infection in men is associated with impaired intracytoplasmic sperm injection and embryo transfer outcomes as well as reduced sperm quality.
HBV can exert a considerable impact on male fertility, as noted by Su et al. [167], who reported an increased incidence and risk of infertility among men with HBV infection compared to men without HBV infection.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Li et al. [9] identified SARS-CoV-2 in the semen of six hospitalized patients with coronavirus disease 2019 (COVID-19), including four patients who were in the acute phase of infection and two patients who were in clinical recovery. Additionally, Machado et al. [168] reported the presence of SARS-CoV-2 viral RNA in the semen of 15 asymptomatic and mildly symptomatic COVID-19 patients.
Gacci et al. [169] tested urine samples collected before and after ejaculation and semen from 43 sexually active men who recovered from SARS-CoV-2 infection. In the present study, sperm parameters and seminal interleukin-8 (IL-8) levels were evaluated. Only three patients tested positive in at least one sample. After recovery from COVID-19, 25% of the men studied were oligo-crypto-azoospermic, and among the 11 men with semen deficiency, eight were azoospermic, and three were oligospermic. Additionally, 33 patients had pathological levels of IL-8 in their semen. Likewise, Holtmann et al. [170] reported that the concentration, progressive motility, and total sperm count of patients with moderate infection were significantly lower than those of the controls.
In contrast, Ma et al. [24] reported the absence of SARS-COV-2 in semen samples from 12 patients with COVID-19. However, 33% of the population studied had altered seminal parameters and a greater number of sperms with DNA fragmentation. Thus, SARS-CoV-2 can activate cellular oxidative stress, leading to sperm DNA fragmentation, which is correlated with impaired embryonic development, lower implantation rates, and higher abortion rate [52].
Yang et al. [171] investigated whether SARS-COV-2 is present in 12 postmortem testis samples from COVID-19 patients. They reported the absence of SARS-COV-2 in the testis in 90% of cases. However, the testes of all patients exhibited significant seminiferous tubular injury, reduced number of Leydig cells, swelling, vacuolization, cytoplasmic rarefaction, detachment of the tubular basement membranes of Sertoli cells, and mild lymphocytic inflammation, corresponding to symptoms of orchitis. Likewise, Achua et al. [172] analyzed testicular specimens at the autopsies of six men with COVID-19 and relevant comorbidities. Three of the COVID-19 cases had impaired spermatogenesis, and only one case reported macrophage and lymphocyte infiltration into testicular tissues. Thus, orchitis and impaired spermatogenesis are possible complications of COVID-19 infection. Furthermore, other studies have reported that most male participants with COVID-19 have reduced testosterone levels, suggesting hypogonadism [173, 174].
Overall, these studies suggest that the occurrence of SARS-CoV-2 in semen is a rare event because of the small number of positive samples analyzed and the absence of viral RNA in semen [175,176,177,178,179]. However, previous studies have indicated that COVID-19 can have a negative impact on spermatogenesis and male fertility [53].
Varicella-zoster virus (VZV)
The number of reports on the influence of VZV on semen is small, and these studies are controversial. Behboudi et al. [79] reported that 2.8% of the semen samples were positive for VZV DNA, but no significant difference was found between the seminal parameters of positive and negative samples. Neofytou et al. [45] detected VZV DNA in four semen samples, one with normozoospermia and three with sperm alterations. They also reported that VZV could be identified in both the sperm fraction and seminal fluid. In addition, a statistically significant difference was observed between VZV infection and teratospermia. Likewise, Tavakolian et al. [60] detected VZV in two semen samples from men with teratozoospermia.
Hepatitis E virus (HEV)
The presence of HEV RNA in semen suggests that HEV can infect MGT and cause testicular damage [180]. Horvatits et al. [62] reported the presence of hepatitis E genotype 3 (HEV-3) viral particles in the ejaculate of immunocompromised men with chronic infection. In that study, HEV-3 was detected at much higher concentrations in the semen than in the blood, demonstrating HEV-3 replication in the male reproductive system. Furthermore, viral RNA concentrations were comparable in both fractions of the ejaculate, which may indicate that HEV-3 originates in the testes and prostate. In view of this, the authors concluded that MGT may be a niche for HEV-3 persistence in chronic infections.
HEV infection has also been described in the semen of infertile men. Huang et al. [63] reported a high prevalence of HEV RNA in the semen of infertile Chinese males. In this study, among patients with oligospermia, 53.57% were positive for HEV RNA, and more than 60% of sperm from patients infected with HEV were immotile. Additionally, changes in sperm morphology and vitality were observed. Thus, owing to oligospermia, asthenospermia, and necrozoospermia in HEV-infected men, the authors concluded that HEV infection impairs seminal quality.
Phylogenetic analyses indicated that all HEV isolates belonged to genotype 4 (HEV-4), the dominant strain in China. In contrast, El-Mokhtar et al. [181] reported discrepant results, in which HEV RNA was detected in the urine of patients with acute hepatitis E, but not in the serum. They also did not observe any changes in seminal quality. Horvatits et al. [182] reported no association between male infertility and HEV-3 infection.
Adeno-associated virus (AAV)
AAV DNA is detected significantly more frequently in semen samples from infertile men than in normal semen samples [30]. Rohde et al. [80] reported, for the first time, the presence of AAV DNA in semen in 30% of samples from infertile men and absence in fertile men, suggesting that the presence of AAV in semen can affect sperm motility. Likewise, Erles et al. [81] detected AAV DNA in 38% of ejaculates from men with alterations in seminal parameters (oligoasthenozoospermia or asthenzoospermia) and in 4.6% of semen samples without alterations. The same study also detected AAV DNA in 10 of 38 testicular tissue biopsies from infertile men and in two of eight orchidectomy samples.
In a study by Schlehofer et al. [82], in infertile couples, the presence of AAV DNA was observed in 14.9% of cervical smears and 19.9% of semen samples. However, no significant association with fertility was observed. In addition, there was no evidence of sexual transmission of AAV. Furthermore, Behboudi et al. [79] detected AAV DNA in 27.6% of semen samples, but no association was found between seminal AAV infection and semen quality.
JC polyomavirus (JCPyV)
The shedding of JCPyV in urine has been commonly reported and this virus has also been detected in prostate tissue [183,184,185,186]. Few studies report the presence of JCPyV in human semen [89, 90, 187].
Rotondo et al. [89] identified JCPyV DNA in semen samples with an overall prevalence of 27.6%. Likewise, Comar et al. [90] also reported a higher prevalence of JCPyV sequences in semen (24.5%) and urine (43.4%) samples from infertile men than in those form the control group. This study was the first to indicate an association between JCPyV and male infertility. A reduction in sperm motility was observed in 84.6% of the positive samples for JCPyV, whereas 76.9% had altered sperm morphology. However, further investigations are needed to better understand the possible role of JCPyV in male infertility, as well as its persistence in semen and its capacity for sexual transmission.
HCV
Several studies have demonstrated a negative impact of HCV on seminal quality [49, 95,96,97,98]. In a clinical evaluation of 82 HCV patients aged between 18 and 60 years, the mean total sperm count and the levels of normal motility and morphology were significantly lower than those of control subjects. Likewise, a significantly higher frequency of disomy for chromosomes 18, X, and Y was observed in men with chronic hepatitis C than in the control subjects. Baseline serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone were also significantly lower [97].
Karamolahi et al. [94] observed that men infected with HBV and HCV had reduced sperm counts, progressive motility, and normal sperm morphology. Furthermore, Moretti et al. [188] reported not only a lower fertility index but also higher sperm diploidy in individuals with seminal HCV infections, suggesting that apoptosis and sperm necrosis play important roles in these patients. Similarly, an analysis of 40 patients with chronic hepatitis C infection and primary infertility and another 20 patients with HCV infection and secondary infertility demonstrated that progressive motility and sperm morphology were significantly impaired in these patients compared to those in the controls. It was also observed that sperm mitochondrial membrane potential, chromatin compaction, and sperm DNA fragmentation were significantly altered. In addition, seminal levels of ROS and viral replication correlated with a worsening of seminal parameters [95].
Ebola virus (EBOV)
The testis is likely to be an anatomical reservoir for EBOV persistence in humans [7]. Although the exact mechanism of viral tropism has not yet been determined, it has been hypothesized that the persistence of EBOV is established in the interstitium of the male reproductive tract (seminal vesicle, epididymis, prostate gland, and testis) and is shuttled to the seminal fluid via infected tissue macrophages [189, 190].
To date, the longest time from acute Ebola virus disease (EVD) illness to the detection of viral RNA in a semen sample is 40 months [191]. Furthermore, sexual transmission from male survivors to female partners has been identified up to 470 days after the illness offset [192]. Therefore, the World Health Organization updated its guidelines for the prevention of EBOV transmission in 2016 to include practicing safe sex and hygiene for 12 months from the onset of symptoms or until two negative semen tests for EBOV were reported [193].
However, because of logistical challenges in affected countries and biosafety considerations related to laboratory manipulation of EVD, much of the pathophysiology of viral persistence in semen has been overlooked [194]. Sexual health complaints such as erectile dysfunction and decreased libido are commonly reported among EVD survivors [26, 27, 120]. Nevertheless, the causal mechanisms underlying these complaints remain unclear. Although physiologic conditions can play a role, it is also likely that psychosocial factors including residual stress, trauma, stigma, and grief contribute as well [120].
Panel for detection of viruses associated with male infertility
Based on the data available to date, we propose a panel that includes the main viruses affecting the quality of human semen (Fig. 4).
Panel of viruses of medical importance to be analyzed in infertile men. Abbreviations: HPV, human papillomavirus; HBV, hepatitis B virus; HSV1/2, herpes simplex virus type 1 and 2; HCMV, human cytomegalovirus; HVV-6, human herpesvirus-6; VZV, varicella-zoster virus; HIV, human immunodeficiency virus; ZIKV, zika virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HCV, hepatitis C virus; HEV, hepatitis E virus; EBOV, Ebola virus
HPV was the first virus included in this panel, as it is usually present in semen samples [34, 129, 195, 196]. Furthermore, according to Boeri et al. [197], accurate investigation of the presence of seminal HPV in the diagnostic analysis of infertile men is of paramount importance, not only for its potential negative pathophysiological impact on male fertility but also in terms of the general health of men.
HBV can integrate its DNA into the genome of male germ cells [5], which raises safety issues regarding paternal-fetal transmission in men with chronic hepatitis B, especially in assisted reproduction procedures, such as ICSI. Therefore, the Belgian–Dutch Association for Artificial Insemination advises against ICSI treatment for chronic patients with HBV [50]. Condijts et al. [198] suggested that strategies to select sperm cells without HBV incorporation are necessary to avoid excluding chronically infected men.
HCV can be transmitted during IVF [199]. Thus, sequential semen preparation with density gradient centrifugation followed by swim-up is recommended for HCV-positive men. Likewise, if one of the partners is chronically infected, therapy should be considered before fertility treatment to reduce viral load [5]. Thus, seminal detection of HCV is essential, both to verify the success of antiviral therapy in the seminal elimination of HCV in these patients and to minimize the risk of cross-transmission.
The Herpesviridae family, composed of eight members (HSV-1, HSV-2, VZV, EBV, HHV-6–8, and HCMV), is considered one of the main risk factors for infertility [60]. In this review, we observed that HSV-1, HSV-2, HCMV, and HHV-6 are herpesviruses that present a greater risk for male fertility and recommend the investigation of these four pathogens.
HIV-infected men, in addition to having alterations in seminal parameters, may also intermittently release HIV-1 RNA into the seminal plasma during antiretroviral therapy, even with undetectable RNA in blood plasma [102, 200, 201]. Thus, semen washing by density gradient centrifugation followed by sperm swim-up has been used as an option for serodiscordant couples who wish to become pregnant when the man is infected with HIV [5, 202, 203]. Therefore, molecular investigation of HIV in semen can be used to verify the success of seminal lavage and the safe use of clinical specimens, as well as to assess the efficiency of antiviral treatment.
Likewise, owing to the global spread and lack of knowledge about its potential effect on male fertility and embryonic and fetal development, investigation of the presence of SARS-CoV-2 in semen is necessary as a preventive measure to ensure greater protection of assisted reproductive technologies and assessment of male infertility.
ZIKV and EBOV are also of great importance for the assessment of fertility, as they can infect not only the testis but also several male genital organs that act as viral reservoirs [25]. However, these viruses can be included in this panel depending on the epidemiological scenario in each region. For example, frequent outbreaks of EBOV in the African continent have been reported, which, in this context, justify its investigation, due to the persistent elimination of EBOV in semen. Likewise, arboviruses have great epidemiological importance in Latin America, and as ZIKV is a virus that can reduce sperm quality and affect male fertility, in addition to having a potential impact on fetal development, its investigation is of great relevance.
Conclusions
Based on the data collected here, it is possible to propose a new panel of viruses that affect seminal quality. This panel is composed of HPV, HBV, HSV-1/2, HCMV, HHV-6, VZV, HIV, ZIKV, SARS-Cov-2, HCV, HEV, and EBOV. This set of viruses could be a starting point for the development of different methods for quality semen screening and diagnosis, contributing to the standardization of viral identification kits. In this way, the implementation of this panel will improve the quality control of semen, allowing a more accurate diagnosis for counseling infertile couples.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- AAV:
-
Adeno-associated virus
- AIDS:
-
Acquired immunodeficiency syndrome
- ANDV:
-
Andes virus
- AVs:
-
Anelloviruses
- CCR3:
-
C-C chemokine receptor type 3
- CCR5:
-
C-C chemokine receptor type 5
- CHIKV:
-
Chikungunya virus
- COVID-19:
-
Coronavirus disease 2019
- DENV:
-
Dengue virus
- EBOV:
-
Ebola virus
- EBV:
-
Epstein-Barr virus
- ESHRE:
-
European Society of Human Reproduction and Embryology
- EVD:
-
Ebola virus disease
- FISH:
-
Fluorescence in situ hybridization
- FSH:
-
Follicle-stimulating hormone
- HBV:
-
Hepatitis B virus
- HCMV:
-
Human cytomegalovirus
- HCV:
-
Hepatitis C virus
- HEV:
-
Hepatitis E virus
- HEV-3:
-
Hepatitis E virus genotype 3
- HEV-4:
-
Hepatitis E virus genotype 4
- HHV-6:
-
Human herpesvirus-6
- HHV-6B:
-
Human herpesviruses 6B
- HHV-7:
-
Human herpesvirus-7
- HHV-8:
-
Human herpesvirus-8
- HIV:
-
Human immunodeficiency virus
- HIV-1:
-
Human immunodeficiency virus type 1
- HPV:
-
Human papillomavirus
- HPV16:
-
Human papillomavirus 16
- HPV45:
-
Human papillomavirus 45
- HPV51:
-
Human papillomavirus 51
- HPV52:
-
Human papillomavirus 52
- HSV:
-
Herpes simplex virus
- HSV-1:
-
Herpes simplex virus type 1
- HSV-2:
-
Herpes simplex virus type 2
- ICSI:
-
Intracytoplasmic sperm injection
- IL-8:
-
Interleukin-8
- IVF:
-
In vitro fertilization
- JCPyV:
-
JC polyomavirus
- LASV:
-
Lassa virus
- LH:
-
Luteinizing hormone
- MGT:
-
Male genital tract
- NiV:
-
Nipah virus
- ROS:
-
Reactive oxygen species
- RVFV:
-
Rift Valley fever virus
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SFTSV:
-
Severe fever with thrombocytopenia 96 syndrome virus
- STIs:
-
Sexually transmitted infections
- VZV:
-
Varicella zoster virus
- WNV:
-
West Nile virus
- YFV:
-
Yellow Fever virus
- ZIKV:
-
Zika virus
References
World Health Organization (WHO). International Classification of Diseases, 11th Revision (ICD-11). Geneva: WHO; 2018. https://icd.who.int/en (
Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2–10. https://doi.org/10.1016/j.clinbiochem.2018.03.012.
Schuppe H-C, Pilatz A, Hossain H, Diemer T, Wagenlehner F, Weidner W. Urogenital Infection as a Risk Factor for Male Infertility. Dtsch Arzteblatt Int. 2017;114:339–46. https://doi.org/10.3238/arztebl.2017.0339.
Henkel R, Offor U, Fisher D. The role of infections and leukocytes in male infertility. Andrologia. 2021;53:e13743. https://doi.org/10.1111/and.13743.
Gimenes F, Souza RP, Bento JC, Teixeira JJV, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11:672–87. https://doi.org/10.1038/nrurol.2014.285.
Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50:e13140. https://doi.org/10.1111/and.13140.
Teixeira T, Oliveira Y, Bernardes F, Kallas E, Duarte-Neto A, Esteves S, et al. Viral infections and implications for male reproductive health. Asian J Androl. 2021;23:335. https://doi.org/10.4103/aja.aja_82_20.
Salam AP, Horby PW. The Breadth of Viruses in Human Semen. Emerg Infect Dis. 2017;23:1922–4. https://doi.org/10.3201/eid2311.171049.
Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3:e208292. https://doi.org/10.1001/jamanetworkopen.2020.8292.
Carpinello OJ. SARS-CoV-2 found in semen. Glob Reprod Health. 2020;5. https://doi.org/10.1097/GRH.0000000000000044.
Saylam B, Uguz M, Yarpuzlu M, Efesoy O, Akbay E, Çayan S. The presence of SARS-CoV-2 virus in semen samples of patients with COVID-19 pneumonia. Andrologia. 2021;53:e14145. https://doi.org/10.1111/and.14145.
Dejucq N, Jégou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev MMBR 2001;65:208-231; first and second pages, table of contents. https://doi.org/10.1128/MMBR.65.2.208-231.2001.
Dulioust E, Du AL, Costagliola D, Guibert J, Kunstmann J-M, Heard I, et al. Semen alterations in HIV-1 infected men. Hum Reprod Oxf Engl. 2002;17:2112–8. https://doi.org/10.1093/humrep/17.8.2112.
Nicopoullos JDM, Almeida PA, Ramsay JWA, Gilling-Smith C. The effect of human immunodeficiency virus on sperm parameters and the outcome of intrauterine insemination following sperm washing. Hum Reprod Oxf Engl. 2004;19:2289–97. https://doi.org/10.1093/humrep/deh426.
Bujan L, Hollander L, Coudert M, Gilling-Smith C, Vucetich A, Guibert J, et al. Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS Lond Engl. 2007;21:1909–14. https://doi.org/10.1097/QAD.0b013e3282703879.
Wong N, Levy M, Stephenson I. Hypogonadism in the HIV-Infected Man. Curr Treat Options Infect Dis. 2017;9:104–16. https://doi.org/10.1007/s40506-017-0110-3.
Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol. 1991;139:149–60.
Poretsky L, Can S, Zumoff B. Testicular dysfunction in human immunodeficiency virus-infected men. Metabolism. 1995;44:946–53. https://doi.org/10.1016/0026-0495(95)90250-3.
Shevchuk MM, Pigato JB, Khalife G, Armenakas NA, Fracchia JA. Changing testicular histology in AIDS: its implication for sexual transmission of HIV. Urology. 1999;53:203–8. https://doi.org/10.1016/s0090-4295(98)00463-4.
Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438–42. https://doi.org/10.1038/nature20556.
Vanegas H, González F, Reyes Y, Centeno E, Palacios J, Zepeda O, et al. Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects. Viruses. 2021;13:152. https://doi.org/10.3390/v13020152.
Huits R, De Smet B, Ariën KK, Van Esbroeck M, Bottieau E, Cnops L. Zika virus in semen: a prospective cohort study of symptomatic travellers returning to Belgium. Bull World Health Organ. 2017;95:802–9. https://doi.org/10.2471/BLT.17.181370.
Joguet G, Mansuy J-M, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200–8. https://doi.org/10.1016/S1473-3099(17)30444-9.
Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–62. https://doi.org/10.1002/jmv.26259.
Le Tortorec A, Matusali G, Mahé D, Aubry F, Mazaud-Guittot S, Houzet L, et al. From Ancient to Emerging Infections: The Odyssey of Viruses in the Male Genital Tract. Physiol Rev. 2020;100:1349–414. https://doi.org/10.1152/physrev.00021.2019.
Guetiya Wadoum RE, Samin A, Mafopa NG, Giovanetti M, Russo G, Turay P, et al. Mobile health clinic for the medical management of clinical sequelae experienced by survivors of the 2013-2016 Ebola virus disease outbreak in Sierra Leone, West Africa. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2017;36:2193–200. https://doi.org/10.1007/s10096-017-3045-1.
de St MA, Ervin E, Orone R, Choi M, Dokubo EK, Rollin PE, et al. Care of Ebola Survivors and Factors Associated With Clinical Sequelae-Monrovia, Liberia. Open Forum. Infect Dis. 2018;5:ofy239. https://doi.org/10.1093/ofid/ofy239.
Thorson AE, Deen GF, Bernstein KT, Liu WJ, Yamba F, Habib N, et al. Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: A cohort study of frequency, duration, and risk factors. PLoS Med. 2021;18:e1003273. https://doi.org/10.1371/journal.pmed.1003273.
Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87:1087–97. https://doi.org/10.1016/j.fertnstert.2006.08.109.
Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013;100:20–9. https://doi.org/10.1016/j.jri.2013.03.004.
Foresta C, Pizzol D, Moretti A, Barzon L, Palù G, Garolla A. Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertil Steril. 2010;94:1723–7. https://doi.org/10.1016/j.fertnstert.2009.11.012.
Connelly DA, Chan PJ, Patton WC, King A. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am J Obstet Gynecol. 2001;184:1068–70. https://doi.org/10.1067/mob.2001.115226.
Moghimi M, Zabihi-Mahmoodabadi S, Kheirkhah-Vakilabad A, Kargar Z. Significant Correlation between High-Risk HPV DNA in Semen and Impairment of Sperm Quality in Infertile Men. Int J Fertil Steril. 2019;12:306–9. https://doi.org/10.22074/ijfs.2019.5421.
Piroozmand A, Mousavi Nasab SD, Erami M, Hashemi SMA, Khodabakhsh E, Ahmadi N, et al. Distribution of Human Papillomavirus and Antisperm Antibody in Semen and Its Association with Semen Parameters Among Infertile Men. J Reprod Infertil. 2020;21:183–8.
Moreno-Sepulveda J, Rajmil O. Seminal human papillomavirus infection and reproduction: a systematic review and meta-analysis. Andrology. 2021;9:478–502. https://doi.org/10.1111/andr.12948.
Jahromi BN, Yaghobi R, Matlub N, Fazelzadeh A, Ramzi A, Anvar Z, et al. Prevalence of Cytomegalovirus in Semen of Male Partners of Infertile Couples and the Virus Impact on Sperm Parameters. J Reprod Infertil. 2020;21:124–9.
Naumenko VA, Tyulenev YA, Yakovenko SA, Kurilo LF, Shileyko LV, Segal AS, et al. Detection of human cytomegalovirus in motile spermatozoa and spermatogenic cells in testis organotypic culture. Herpesviridae. 2011;2:7. https://doi.org/10.1186/2042-4280-2-7.
Naumenko V, Tyulenev Y, Kurilo L, Shileiko L, Sorokina T, Evdokimov V, et al. Detection and quantification of human herpes viruses types 4-6 in sperm samples of patients with fertility disorders and chronic inflammatory urogenital tract diseases. Andrology. 2014;2:687–94. https://doi.org/10.1111/j.2047-2927.2014.00232.x.
Klimova RR, Chichev EV, Naumenko VA, Gadzhieva ZS, Tsibisov AS, Adieva AA, et al. Herpes simplex virus and cytomegalovirus in male ejaculate: herpes simplex virus is more frequently encountered in idiopathic infertility and correlates with the reduction in sperm parameters. Vopr Virusol. 2010;55:27–31.
Wu K-H, Zhou Q-K, Huang J-H, Lai R-Q, Lin F-H, Li B, et al. [Infection of cytomegalovirus and herpes simplex virus and morphology of the infected spermatogenic cells in infertile men]. Zhonghua Nan Ke Xue Natl. J Androl. 2007;13:1075–9.
Kurscheidt FA, Damke E, Bento JC, Balani VA, Takeda KI, Piva S, et al. Effects of Herpes Simplex Virus Infections on Seminal Parameters in Male Partners of Infertile Couples. Urology. 2018;113:52–8. https://doi.org/10.1016/j.urology.2017.11.050.
Monavari SH, Vaziri MS, Khalili M, Shamsi-Shahrabadi M, Keyvani H, Mollaei H, et al. Asymptomatic seminal infection of herpes simplex virus: impact on male infertility. J Biomed Res. 2013;27:56–61. https://doi.org/10.7555/JBR.27.20110139.
Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril. 2003;79(Suppl 3):1566–70. https://doi.org/10.1016/s0015-0282(03)00370-4.
Bradshaw CS, Tabrizi SN, Read TRH, Garland SM, Hopkins CA, Moss LM, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193:336–45. https://doi.org/10.1086/499434.
Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1-7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. 2009;91:2487–94. https://doi.org/10.1016/j.fertnstert.2008.03.074.
Bezold G, Schuster-Grusser A, Lange M, Gall H, Wolff H, Peter RU. Prevalence of human herpesvirus types 1-8 in the semen of infertility patients and correlation with semen parameters. Fertil Steril. 2001;76:416–8. https://doi.org/10.1016/s0015-0282(01)01920-3.
Vicari E, Arcoria D, Di Mauro C, Noto R, Noto Z, La Vignera S. Sperm output in patients with primary infertility and hepatitis B or C virus; negative influence of HBV infection during concomitant varicocele. Minerva Med. 2006;97:65–77.
Lee VCY, Ng EHY, Yeung WSB, Ho PC. Impact of positive hepatitis B surface antigen on the outcome of IVF treatment. Reprod Biomed Online. 2010;21:712–7. https://doi.org/10.1016/j.rbmo.2010.06.036.
Lorusso F, Palmisano M, Chironna M, Vacca M, Masciandaro P, Bassi E, et al. Impact of chronic viral diseases on semen parameters. Andrologia. 2010;42:121–6. https://doi.org/10.1111/j.1439-0272.2009.00970.x.
Zhou X-P, Hu X-L, Zhu Y-M, Qu F, Sun S-J, Qian Y-L. Comparison of semen quality and outcome of assisted reproductive techniques in Chinese men with and without hepatitis B. Asian J Androl. 2011;13:465–9. https://doi.org/10.1038/aja.2010.164.
Oger P, Yazbeck C, Gervais A, Dorphin B, Gout C, Jacquesson L, et al. Adverse effects of hepatitis B virus on sperm motility and fertilization ability during IVF. Reprod Biomed Online. 2011;23:207–12. https://doi.org/10.1016/j.rbmo.2011.04.008.
Guo J, Sheng K, Wu S, Chen H, Xu W. An Update on the Relationship of SARS-CoV-2 and Male Reproduction. Front Endocrinol. 2021;12:788321. https://doi.org/10.3389/fendo.2021.788321.
Sengupta P, Leisegang K, Agarwal A. The impact of COVID-19 on the male reproductive tract and fertility: A systematic review. Arab J Urol. 2021;19:423–36. https://doi.org/10.1080/2090598X.2021.1955554.
McElroy AK, Akondy RS, Harmon JR, Ellebedy AH, Cannon D, Klena JD, et al. A Case of Human Lassa Virus Infection With Robust Acute T-Cell Activation and Long-Term Virus-Specific T-Cell Responses. J Infect Dis. 2017;215:1862–72. https://doi.org/10.1093/infdis/jix201.
Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol. 2017;17:195–207. https://doi.org/10.1038/nri.2016.138.
Henning JD, Bonachea LA, Bunker CH, Patrick AL, Jenkins FJ. Human herpesvirus 8 infection contributes to a T helper 2 immune response in men from Tobago with prostate cancer. Int J Urol Off J Jpn Urol Assoc. 2017;24:64–8. https://doi.org/10.1111/iju.13243.
Henning JD, Karamchandani JM, Bonachea LA, Bunker CH, Patrick AL, Jenkins FJ. Elevated Serum PSA is Associated With Human Herpesvirus 8 Infection and Increased Circulating Cytokine Levels in Men From Tobago. The Prostate. 2017;77:617–24. https://doi.org/10.1002/pros.23308.
Bellocchi MC, Svicher V, Ceccherini-Silberstein F. HHV-8 Genetic Diversification and Its Impact on Severe Clinical Presentation of Associated Diseases. J Infect Dis. 2020;222:1250–3. https://doi.org/10.1093/infdis/jiaa182.
Bagasra O, Patel D, Bobroski L, Abbasi JA, Bagasra AU, Baidouri H, et al. Localization of human herpesvirus type 8 in human sperms by in situ PCR. J Mol Histol. 2005;36:401–12. https://doi.org/10.1007/s10735-005-9010-9.
Tavakolian S, Goudarzi H, Nazarian H, Raee P, Niakan S, Faghihloo E. The evaluation of Human papilloma virus and human herpes viruses (EBV, CMV, VZV HSV-1 and HSV-2) in semen samples. Andrologia. 2021;53:e14051. https://doi.org/10.1111/and.14051.
Ouwendijk WJD, Depledge DP, Rajbhandari L, Lenac Rovis T, Jonjic S, Breuer J, et al. Varicella-zoster virus VLT-ORF63 fusion transcript induces broad viral gene expression during reactivation from neuronal latency. Nat Commun. 2020;11:6324. https://doi.org/10.1038/s41467-020-20031-4.
Horvatits T, Wißmann J-E, Johne R, Groschup MH, Gadicherla AK, Schulze Zur Wiesch J, et al. Hepatitis E virus persists in the ejaculate of chronically infected men. J Hepatol. 2021;75:55–63. https://doi.org/10.1016/j.jhep.2020.12.030.
Huang F, Long F, Yu W, Situ J, Fu L, He Z, et al. High prevalence of hepatitis E virus in semen of infertile male and causes testis damage. Gut. 2018;67:1199–201. https://doi.org/10.1136/gutjnl-2017-314884.
Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, et al. Hepatitis E virus infection. Nat Rev Dis Primer. 2017;3:17086. https://doi.org/10.1038/nrdp.2017.86.
Kaspersen MD, Larsen PB, Kofod-Olsen E, Fedder J, Bonde J, Höllsberg P. Human herpesvirus-6A/B binds to spermatozoa acrosome and is the most prevalent herpesvirus in semen from sperm donors. PloS One. 2012;7:e48810. https://doi.org/10.1371/journal.pone.0048810.
Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42:227–40. https://doi.org/10.1038/bmt.2008.162.
Varsani A, Opriessnig T, Celer V, Maggi F, Okamoto H, Blomström A-L, et al. Taxonomic update for mammalian anelloviruses (family Anelloviridae). Arch Virol. 2021;166:2943–53. https://doi.org/10.1007/s00705-021-05192-x.
Li Y, Altan E, Pilcher C, Hartogensis W, Hecht FM, Deng X, et al. Semen virome of men with HIV on or off antiretroviral treatment. AIDS Lond Engl. 2020;34:827–32. https://doi.org/10.1097/QAD.0000000000002497.
Kaczorowska J, van der Hoek L. Human anelloviruses: diverse, omnipresent and commensal members of the virome. FEMS Microbiol Rev. 2020;44:305–13. https://doi.org/10.1093/femsre/fuaa007.
Martínez NM, García F, García F, Alvarez M, Bernal MC, Piédrola G, et al. TT virus DNA in serum, peripheral blood mononuclear cells and semen of patients infected by HIV. AIDS Lond Engl. 2000;14:1464–6. https://doi.org/10.1097/00002030-200007070-00028.
Kwak J-E, Kim Y-I, Park S-J, Yu M-A, Kwon H-I, Eo S, et al. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat Commun. 2019;10:3836. https://doi.org/10.1038/s41467-019-11815-4.
Lee S-Y, Kang J-G, Jeong H-S, Kim W-M, Son K-D, Kim JS, et al. Complete Genome Sequences of Two Severe Fever with Thrombocytopenia Syndrome Virus Strains Isolated from a Human and a Dog in the Republic Of Korea. Microbiol Resour Announc. 2019;8:e01695–18. https://doi.org/10.1128/MRA.01695-18.
Koga S, Takazono T, Ando T, Hayasaka D, Tashiro M, Saijo T, et al. Severe Fever with Thrombocytopenia Syndrome Virus RNA in Semen, Japan. Emerg Infect Dis. 2019;25:2127–8. https://doi.org/10.3201/eid2511.190061.
Aditi null, Shariff M. Nipah virus infection: A review. Epidemiol Infect. 2019;147:e95. https://doi.org/10.1017/S0950268819000086.
Arunkumar G, Abdulmajeed J, Santhosha D, Aswathyraj S, Robin S, Jayaram A, et al. Persistence of Nipah Virus RNA in Semen of Survivor. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;69:377–8. https://doi.org/10.1093/cid/ciy1092.
Gorchakov R, Gulas-Wroblewski BE, Ronca SE, Ruff JC, Nolan MS, Berry R, et al. Optimizing PCR Detection of West Nile Virus from Body Fluid Specimens to Delineate Natural History in an Infected Human Cohort. Int J Mol Sci. 2019;20:E1934. https://doi.org/10.3390/ijms20081934.
Suthar MS, Diamond MS, Gale M. West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11:115–28. https://doi.org/10.1038/nrmicro2950.
Smith RD, Konoplev S, DeCourten-Myers G, Brown T. West Nile virus encephalitis with myositis and orchitis. Hum Pathol. 2004;35:254–8. https://doi.org/10.1016/j.humpath.2003.09.007.
Behboudi E, Mokhtari-Azad T, Yavarian J, Ghavami N, Seyed Khorrami SM, Rezaei F, et al. Molecular detection of HHV1-5, AAV and HPV in semen specimens and their impact on male fertility. Hum Fertil Camb Engl. 2019;22:133–8. https://doi.org/10.1080/14647273.2018.1463570.
Rohde V, Erles K, Sattler HP, Derouet H, Wullich B, Schlehofer JR. Detection of adeno-associated virus in human semen: does viral infection play a role in the pathogenesis of male infertility? Fertil Steril. 1999;72:814–6. https://doi.org/10.1016/s0015-0282(99)00363-5.
Erles K, Rohde V, Thaele M, Roth S, Edler L, Schlehofer JR. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum Reprod Oxf Engl. 2001;16:2333–7. https://doi.org/10.1093/humrep/16.11.2333.
Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W. Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod Oxf Engl. 2012;27:770–8. https://doi.org/10.1093/humrep/der427.
Kuenzli AB, Marschall J, Schefold JC, Schafer M, Engler OB, Ackermann-Gäumann R, et al. Hantavirus Cardiopulmonary Syndrome Due to Imported Andes Hantavirus Infection in Switzerland: A Multidisciplinary Challenge, Two Cases and a Literature Review. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:1788–95. https://doi.org/10.1093/cid/ciy443.
Lalle E, Colavita F, Iannetta M, Gebremeskel Teklè S, Carletti F, Scorzolini L, et al. Prolonged detection of dengue virus RNA in the semen of a man returning from Thailand to Italy, January 2018. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2018;23. https://doi.org/10.2807/1560-7917.ES.2018.23.18.18-00197.
Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–28. https://doi.org/10.1038/nrmicro1690.
Duarte-Neto AN, Cunha MDP, Marcilio I, Song ATW, de Martino RB, Ho Y-L, et al. Yellow fever and orthotopic liver transplantation: new insights from the autopsy room for an old but re-emerging disease. Histopathology. 2019;75:638–48. https://doi.org/10.1111/his.13904.
Barbosa CM, Di Paola N, Cunha MP, Rodrigues-Jesus MJ, Araujo DB, Silveira VB, et al. Yellow Fever Virus RNA in Urine and Semen of Convalescent Patient, Brazil. Emerg Infect Dis. 2018;24. https://doi.org/10.3201/eid2401.171310.
Couto-Lima D, Madec Y, Bersot MI, Campos SS, Motta MD, Santos FB, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7:4848. https://doi.org/10.1038/s41598-017-05186-3.
Rotondo JC, Candian T, Selvatici R, Mazzoni E, Bonaccorsi G, Greco P, et al. Tracing Males From Different Continents by Genotyping JC Polyomavirus in DNA From Semen Samples. J Cell Physiol. 2017;232:982–5. https://doi.org/10.1002/jcp.25686.
Comar M, Zanotta N, Croci E, Murru I, Marci R, Pancaldi C, et al. Association between the JC polyomavirus infection and male infertility. PloS One. 2012;7:e42880. https://doi.org/10.1371/journal.pone.0042880.
Bandeira AC, Campos GS, Rocha VF, de Freitas Souza BS, Soares MB, Oliveira AA, et al. Prolonged shedding of Chikungunya virus in semen and urine: A new perspective for diagnosis and implications for transmission. IDCases. 2016;6:100–3. https://doi.org/10.1016/j.idcr.2016.10.007.
Gregor KM, Michaely LM, Gutjahr B, Rissmann M, Keller M, Dornbusch S, et al. Rift Valley fever virus detection in susceptible hosts with special emphasis in insects. Sci Rep. 2021;11:9822. https://doi.org/10.1038/s41598-021-89226-z.
Haneche F, Leparc-Goffart I, Simon F, Hentzien M, Martinez-Pourcher V, Caumes E, et al. Rift Valley fever in kidney transplant recipient returning from Mali with viral RNA detected in semen up to four months from symptom onset, France, autumn 2015. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2016;21. https://doi.org/10.2807/1560-7917.ES.2016.21.18.30222.
Karamolahi S, Yazdi RS, Zangeneh M, Makiani MJ, Farhoodi B, Gilani MAS. Impact of hepatitis B virus and hepatitis C virus infection on sperm parameters of infertile men. Int J Reprod Biomed. 2019;17:551–6. https://doi.org/10.18502/ijrm.v17i8.4820.
La Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Sperm DNA damage in patients with chronic viral C hepatitis. Eur J Intern Med. 2012;23:e19–24. https://doi.org/10.1016/j.ejim.2011.08.011.
Hofny ERM, Ali MEM, Taha EA, Nafeh HM, Sayed DS, Abdel-Azeem HG, et al. Semen and hormonal parameters in men with chronic hepatitis C infection. Fertil Steril. 2011;95:2557–9. https://doi.org/10.1016/j.fertnstert.2011.05.014.
Safarinejad MR, Kolahi AA, Iravani S. Evaluation of semen variables, sperm chromosomal abnormalities and reproductive endocrine profile in patients with chronic hepatitis C. BJU Int. 2010;105:79–86. https://doi.org/10.1111/j.1464-410X.2009.08720.x.
Durazzo M, Premoli A, Di Bisceglie C, Bertagna A, Faga E, Biroli G, et al. Alterations of seminal and hormonal parameters: An extrahepatic manifestation of HCV infection? World J Gastroenterol. 2006;12:3073–6. https://doi.org/10.3748/wjg.v12.i19.3073.
López Zúñiga MÁ, Chueca N, de Salazar A, Fernández Caballero JA, Gutierrez Valencia A, Vinuesa García D, et al. Genetic diversity of HIV in seminal plasma remains higher than in blood after short-term antiretroviral therapy. Sex Transm Infect. 2020;96:337–41. https://doi.org/10.1136/sextrans-2020-054439.
Houzet L, Matusali G, Dejucq-Rainsford N. Origins of HIV-infected leukocytes and virions in semen. J Infect Dis. 2014;210(Suppl 3):S622–30. https://doi.org/10.1093/infdis/jiu328.
Anderson JA, Ping L-H, Dibben O, Jabara CB, Arney L, Kincer L, et al. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS Pathog. 2010;6:e1001053. https://doi.org/10.1371/journal.ppat.1001053.
Kariuki SM, Selhorst P, Norman J, Cohen K, Rebe K, Williamson C, et al. Detectable HIV-1 in semen in individuals with very low blood viral loads. Virol J. 2020;17:29. https://doi.org/10.1186/s12985-020-01300-6.
Gianella S, Smith DM, Vargas MV, Little SJ, Richman DD, Daar ES, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57:441–7. https://doi.org/10.1093/cid/cit252.
Gianella S, Mehta SR, Strain MC, Young JA, Vargas MV, Little SJ, et al. Impact of seminal cytomegalovirus replication on HIV-1 dynamics between blood and semen. J Med Virol. 2012;84:1703–9. https://doi.org/10.1002/jmv.23398.
Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. https://doi.org/10.1093/infdis/jir700.
Politch JA, Mayer KH, Welles SL, O’Brien WX, Xu C, Bowman FP, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS Lond Engl. 2012;26:1535–43. https://doi.org/10.1097/QAD.0b013e328353b11b.
Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. https://doi.org/10.1097/olq.0b013e318141fe9b.
La Vignera S, Vicari E, Condorelli RA, D’Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review). Int J Androl. 2011;34:e330–47. https://doi.org/10.1111/j.1365-2605.2011.01200.x.
Waters L, Gilling-Smith C, Boag F. HIV infection and subfertility. Int J STD AIDS. 2007;18:1–6. https://doi.org/10.1258/095646207779949871.
Savasi V, Parisi F, Oneta M, Laoreti A, Parrilla B, Duca P, et al. Effects of highly active antiretroviral therapy on semen parameters of a cohort of 770 HIV-1 infected men. PLoS ONE. 2019;14:e0212194. https://doi.org/10.1371/journal.pone.0212194.
Dondero F, Rossi T, D’Offizi G, Mazzilli F, Rosso R, Sarandrea N, et al. Semen analysis in HIV seropositive men and in subjects at high risk for HIV infection. Hum Reprod Oxf Engl. 1996;11:765–8. https://doi.org/10.1093/oxfordjournals.humrep.a019251.
Goulart ACX, Farnezi HCM, França JPBM, dos Santos A, Ramos MG, Penna MLF. HIV, HPV and Chlamydia trachomatis: impacts on male fertility. JBRA Assist Reprod. 2020;24:492–7. https://doi.org/10.5935/1518-0557.20200020.
Shehu-Xhilaga M, de Kretser D, Dejucq-Rainsford N, Hedger M. Standing in the way of eradication: HIV-1 infection and treatment in the male genital tract. Curr HIV Res. 2005;3:345–59. https://doi.org/10.2174/157016205774370375.
Almeida R, das N, Braz-de-Melo HA, Santos I de O, Corrêa R, Kobinger GP, Magalhaes KG. The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells. 2020;9:E1006. https://doi.org/10.3390/cells9041006.
Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D, et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N Engl J Med. 2018;378:1377–85. https://doi.org/10.1056/NEJMoa1711038.
Gaskell KM, Houlihan C, Nastouli E, Checkley AM. Persistent Zika Virus Detection in Semen in a Traveler Returning to the United Kingdom from Brazil, 2016. Emerg Infect Dis. 2017;23:137–9. https://doi.org/10.3201/eid2301.161300.
Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, et al. Virus and Antibody Dynamics in Travelers With Acute Zika Virus Infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;66:1173–80. https://doi.org/10.1093/cid/cix967.
D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–8. https://doi.org/10.1056/NEJMc1604449.
Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2016;21. https://doi.org/10.2807/1560-7917.ES.2016.21.32.30314.
Payne K, Kenny P, Scovell JM, Khodamoradi K, Ramasamy R. Twenty-First Century Viral Pandemics: A Literature Review of Sexual Transmission and Fertility Implications in Men. Sex Med Rev. 2020;8:518–30. https://doi.org/10.1016/j.sxmr.2020.06.003.
Varjasi G, Póka R. Zika virus infection in pregnancy. Orv Hetil. 2017;158:563–71. https://doi.org/10.1556/650.2017.30728.
Mlera L, Bloom ME. Differential Zika Virus Infection of Testicular Cell Lines. Viruses. 2019;11:E42. https://doi.org/10.3390/v11010042.
Robinson CL, Chong ACN, Ashbrook AW, Jeng G, Jin J, Chen H, et al. Male germ cells support long-term propagation of Zika virus. Nat Commun. 2018;9:2090. https://doi.org/10.1038/s41467-018-04444-w.
Matusali G, Houzet L, Satie A-P, Mahé D, Aubry F, Couderc T, et al. Zika virus infects human testicular tissue and germ cells. J Clin Invest. 2018;128:4697–710. https://doi.org/10.1172/JCI121735.
Halabi J, Jagger BW, Salazar V, Winkler ES, White JP, Humphrey PA, et al. Zika Virus Causes Acute and Chronic Prostatitis in Mice and Macaques. J Infect Dis. 2020;221:1506–17. https://doi.org/10.1093/infdis/jiz533.
Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika Virus in Body Fluids - Final Report. N Engl J Med. 2018;379:1234–43. https://doi.org/10.1056/NEJMoa1613108.
Yang Y, Jia C-W, Ma Y-M, Zhou L-Y, Wang S-Y. Correlation between HPV sperm infection and male infertility. Asian J Androl. 2013;15:529–32. https://doi.org/10.1038/aja.2013.36.
Capra G, Nyitray AG, Lu B, Perino A, Marci R, Schillaci R, et al. Analysis of persistence of human papillomavirus infection in men evaluated by sampling multiple genital sites. Eur Rev Med Pharmacol Sci. 2015;19:4153–63.
Laprise C, Trottier H, Monnier P, Coutlée F, Mayrand M-H. Prevalence of human papillomaviruses in semen: a systematic review and meta-analysis. Hum Reprod Oxf Engl. 2014;29:640–51. https://doi.org/10.1093/humrep/det453.
Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196:1146–52. https://doi.org/10.1086/521629.
Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–57. https://doi.org/10.1086/507432.
Rintala MAM, Pöllänen PP, Nikkanen VP, Grénman SE, Syrjänen SM. Human papillomavirus DNA is found in the vas deferens. J Infect Dis. 2002;185:1664–7. https://doi.org/10.1086/340421.
Isaguliants M, Krasnyak S, Smirnova O, Colonna V, Apolikhin O, Buonaguro FM. Genetic instability and anti-HPV immune response as drivers of infertility associated with HPV infection. Infect Agent Cancer. 2021;16:29. https://doi.org/10.1186/s13027-021-00368-1.
Perino A, Giovannelli L, Schillaci R, Ruvolo G, Fiorentino FP, Alimondi P, et al. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril. 2011;95:1845–8. https://doi.org/10.1016/j.fertnstert.2010.11.047.
Jeršovienė V, Gudlevičienė Ž, Rimienė J, Butkauskas D. Human Papillomavirus and Infertility. Med Kaunas Lith. 2019;55:E377. https://doi.org/10.3390/medicina55070377.
Capra G, Schillaci R, Bosco L, Roccheri MC, Perino A, Ragusa MA. HPV infection in semen: results from a new molecular approach. Epidemiol Infect. 2019;147:e177. https://doi.org/10.1017/S0950268819000621.
Garolla A, Pizzol D, Foresta C. The role of human papillomavirus on sperm function. Curr Opin Obstet Gynecol. 2011;23:232–7. https://doi.org/10.1097/GCO.0b013e328348a3a4.
Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PloS One. 2011;6:e15036. https://doi.org/10.1371/journal.pone.0015036.
Tangal S, Taşçı Y, Pabuçcu EG, Çağlar GS, Haliloğlu AH, Yararbaş K. DNA fragmentation index and human papilloma virus in males with previous assisted reproductive technology failures. Turk J Urol. 2019;45:12–6. https://doi.org/10.5152/tud.2018.96393.
MAR and Viral Disease n.d. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-MAR-in-patients-with-viral-disease ().
Depuydt CE, Donders GGG, Verstraete L, Vanden Broeck D, Beert JFA, Salembier G, et al. Infectious human papillomavirus virions in semen reduce clinical pregnancy rates in women undergoing intrauterine insemination. Fertil Steril. 2019;111:1135–44. https://doi.org/10.1016/j.fertnstert.2019.02.002.
Garolla A, Engl B, Pizzol D, Ghezzi M, Bertoldo A, Bottacin A, et al. Spontaneous fertility and in vitro fertilization outcome: new evidence of human papillomavirus sperm infection. Fertil Steril. 2016;105:65–72.e1. https://doi.org/10.1016/j.fertnstert.2015.09.018.
Depuydt CE, Donders G, Verstraete L, Vanden Broeck D, Beert J, Salembier G, et al. Time has come to include Human Papillomavirus (HPV) testing in sperm donor banks. Facts Views Vis ObGyn. 2018;10:201–5.
Eggert-Kruse W, Reuland M, Johannsen W, Strowitzki T, Schlehofer JR. Cytomegalovirus (CMV) infection--related to male and/or female infertility factors? Fertil Steril. 2009;91:67–82. https://doi.org/10.1016/j.fertnstert.2007.11.014.
Habibi M, Bahrami A, Morteza A, Sadighi Gilani MA, Hassanzadeh G, Ghadami M, et al. Study of cytomegalovirus infection in idiopathic infertility men referred to Shariati hospital, Tehran. Iran. Iran J Reprod Med. 2014;12:151–4.
Bai S, Li Y, Wan Y, Guo T, Jin Q, Liu R, et al. Sexually transmitted infections and semen quality from subfertile men with and without leukocytospermia. Reprod Biol Endocrinol RBE. 2021;19:92. https://doi.org/10.1186/s12958-021-00769-2.
Wald A, Matson P, Ryncarz A, Corey L. Detection of herpes simplex virus DNA in semen of men with genital HSV-2 infection. Sex Transm Dis. 1999;26:1–3. https://doi.org/10.1097/00007435-199901000-00001.
Ochsendorf FR. Sexually transmitted infections: impact on male fertility. Andrologia. 2008;40:72–5. https://doi.org/10.1111/j.1439-0272.2007.00825.x.
Abdulmedzhidova AG, Kurilo LF, Shileĭko LV, Makarova NP, Klimova RR, Kushch AA. Asymptomatic genital herpes infection and infertility in males. Urol Mosc Russ. 1999;2007:56–9.
Kotronias D, Kapranos N. Detection of herpes simplex virus DNA in human spermatozoa by in situ hybridization technique. Vivo Athens Greece. 1998;12:391–4.
Godet AN, Soignon G, Koubi H, Bonnafous P, Agut H, Poirot C, Gautheret-Dejean A. Presence of HHV-6 genome in spermatozoa in a context of couples with low fertility: what type of infection? Andrologia. 2015;47(5):531-5. https://doi.org/10.1111/and.12299. Epub 2014 May 20. https://pubmed.ncbi.nlm.nih.gov/24846813/.
Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant. 2015;50:1030–6. https://doi.org/10.1038/bmt.2015.76.
Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22:144–55. https://doi.org/10.1002/rmv.715.
Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:5563–8. https://doi.org/10.1073/pnas.0913586107.
Nacheva EP, Ward KN, Brazma D, Virgili A, Howard J, Leong HN, et al. Human herpesvirus 6 integrates within telomeric regions as evidenced by five different chromosomal sites. J Med Virol. 2008;80:1952–8. https://doi.org/10.1002/jmv.21299.
Hall CB, Caserta MT, Schnabel K, Shelley LM, Marino AS, Carnahan JA, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. 2008;122:513–20. https://doi.org/10.1542/peds.2007-2838.
Chen J-W, Cui Y, Zhang XX. Investigate the impact of hepatitis B virus infection on sperm DNA integrity. Zhonghua Shi Yan He Lin Chuang Bing Xue Za Zhi Zhonghua Shiyan He Linchuang Bingduxue Zazhi Chin J Exp Clin Virol. 2011;25:345–7.
Wang Z, Liu W, Zhang M, Wang M, Wu H, Lu M. Effect of Hepatitis B Virus Infection on Sperm Quality and Outcomes of Assisted Reproductive Techniques in Infertile Males. Front Med. 2021;8:744350. https://doi.org/10.3389/fmed.2021.744350.
Huang J-M, Huang T-H, Qiu H-Y, Fang X-W, Zhuang T-G, Liu H-X, et al. Effects of hepatitis B virus infection on human sperm chromosomes. World J Gastroenterol. 2003;9:736–40. https://doi.org/10.3748/wjg.v9.i4.736.
Tasdelen Fisgin N, Aydin BK, Sarikaya H, Tanyel E, Esen S, Sunbul M, et al. Oxidative stress and antioxidant defense in patients with chronic hepatitis B. Clin Lab. 2012;58:273–80.
Dikici I, Mehmetoglu I, Dikici N, Bitirgen M, Kurban S. Investigation of oxidative stress and some antioxidants in patients with acute and chronic viral hepatitis B and the effect of interferon-alpha treatment. Clin Biochem. 2005;38:1141–4. https://doi.org/10.1016/j.clinbiochem.2005.10.006.
Wang K, Wang B, Fan X, Lin Y, Shui W. Oxidative stress in patients with chronic hepatitis B. Zhonghua Shi Yan He Lin Chuang Bing Xue Za Zhi Zhonghua Shiyan He Linchuang Bingduxue Zazhi Chin J Exp Clin Virol. 2004;18:172–4.
Qian L, Li Q, Li H. Effect of hepatitis B virus infection on sperm quality and oxidative stress state of the semen of infertile males. Am J Reprod Immunol N Y N. 1989;2016(76):183–5. https://doi.org/10.1111/aji.12537.
Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80(Suppl 2):844–50. https://doi.org/10.1016/s0015-0282(03)00983-x.
Huang J, Zhong Y, Fang X, Xie Q, Kang X, Wu R, et al. Hepatitis B virus s protein enhances sperm apoptosis and reduces sperm fertilizing capacity in vitro. PloS One. 2013;8:e68688. https://doi.org/10.1371/journal.pone.0068688.
Kang X, Xie Q, Zhou X, Li F, Huang J, Liu D, et al. Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions. PloS One. 2012;7:e33471. https://doi.org/10.1371/journal.pone.0033471.
Su F-H, Chang S-N, Sung F-C, Su C-T, Shieh Y-H, Lin C-C, et al. Hepatitis B virus infection and the risk of male infertility: a population-based analysis. Fertil Steril. 2014;102:1677–84. https://doi.org/10.1016/j.fertnstert.2014.09.017.
Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos LH, Henrique Jacomo R, et al. Presence of SARS-CoV-2 RNA in Semen-Cohort Study in the United States COVID-19 Positive Patients. Infect Dis Rep. 2021;13:96–101. https://doi.org/10.3390/idr13010012.
Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod Oxf Engl. 2021;36:1520–9. https://doi.org/10.1093/humrep/deab026.
Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–8. https://doi.org/10.1016/j.fertnstert.2020.05.028.
Yang M, Chen S, Huang B, Zhong J-M, Su H, Chen Y-J, et al. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur Urol Focus. 2020;6:1124–9. https://doi.org/10.1016/j.euf.2020.05.009.
Achua JK, Chu KY, Ibrahim E, Khodamoradi K, Delma KS, Iakymenko OA, et al. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections on Testis. World J Mens Health. 2021;39:65–74. https://doi.org/10.5534/wjmh.200170.
Schroeder M, Tuku B, Jarczak D, Nierhaus A, Bai T, Jacobsen H, et al. The majority of male patients with COVID-19 present low testosterone levels on admission to Intensive Care in Hamburg, Germany: a retrospective cohort study 2020:https://doi.org/10.1101/2020.05.07.20073817.
Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. J Sex Med. 2021;18:256–64. https://doi.org/10.1016/j.jsxm.2020.11.007.
Burke CA, Skytte AB, Kasiri S, Howell D, Patel ZP, Trolice MP, et al. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J Assist Reprod Genet. 2021;38:785–9. https://doi.org/10.1007/s10815-021-02119-y.
Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: A cross-sectional, pilot study. Andrologia. 2021;53:e13912. https://doi.org/10.1111/and.13912.
Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology. 2021;9:99–106. https://doi.org/10.1111/andr.12939.
Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–22. https://doi.org/10.1007/s40618-020-01261-1.
Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients†. Biol Reprod. 2020;103:4–6. https://doi.org/10.1093/biolre/ioaa050.
Situ J, Wang W, Long F, Yang W, Yang C, Wei D, et al. Hepatitis E viral infection causes testicular damage in mice. Virology. 2020;541:150–9. https://doi.org/10.1016/j.virol.2019.12.009.
El-Mokhtar MA, Seddik MI, Osman AOB, Mahmoud AA, Mandour SA, Radwan E, et al. No evidence of HEV genotype 1 infections harming the male reproductive system. Virology. 2021;554:37–41. https://doi.org/10.1016/j.virol.2020.12.002.
Horvatits T, Varwig-Janssen D, Schulze Zur Wiesch J, Lübke R, Reucher S, Frerk S, et al. No link between male infertility and HEV genotype 3 infection. Gut. 2020;69:1150–1. https://doi.org/10.1136/gutjnl-2019-319027.
Hu C, Huang Y, Su J, Wang M, Zhou Q, Zhu B. Detection and analysis of variants of JC polyomavirus in urine samples from HIV-1-infected patients in China’s Zhejiang Province. J Int Med Res. 2018;46:1024–32. https://doi.org/10.1177/0300060517746297.
Franzén J, Ramqvist T, Bogdanovic G, Grün N, Mattson J, Dalianis T. Studies of human polyomaviruses, with HPyV7, BKPyV, and JCPyV present in urine of allogeneic hematopoietic stem cell transplanted patients with or without hemorrhagic cystitis. Transpl Infect Dis Off J Transplant Soc. 2016;18:240–6. https://doi.org/10.1111/tid.12500.
Urbano PRP, Oliveira RR, Romano CM, Pannuti CS, Fink MCD da S. Occurrence, genotypic characterization, and patterns of shedding of human polyomavirus JCPyV and BKPyV in urine samples of healthy individuals in São Paulo, Brazil. J Med Virol 2016;88:153–158. https://doi.org/10.1002/jmv.24318.
Zambrano A, Kalantari M, Simoneau A, Jensen JL, Villarreal LP. Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. The Prostate. 2002;53:263–76. https://doi.org/10.1002/pros.10157.
Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, et al. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66:717–22 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2.
Moretti E, Federico MG, Giannerini V, Collodel G. Sperm ultrastructure and meiotic segregation in a group of patients with chronic hepatitis B and C. Andrologia. 2008;40:286–91. https://doi.org/10.1111/j.1439-0272.2008.00855.x.
Pike JFW, Polley EL, Pritchett DY, Lal A, Wynia BA, Roudebush WE, et al. Comparative analysis of viral infection outcomes in human seminal fluid from prior viral epidemics and Sars-CoV-2 may offer trends for viral sexual transmissibility and long-term reproductive health implications. Reprod Health. 2021;18:123. https://doi.org/10.1186/s12978-021-01172-1.
Perry DL, Huzella LM, Bernbaum JG, Holbrook MR, Jahrling PB, Hagen KR, et al. Ebola Virus Localization in the Macaque Reproductive Tract during Acute Ebola Virus Disease. Am J Pathol. 2018;188:550–8. https://doi.org/10.1016/j.ajpath.2017.11.004.
PREVAIL III Study Group, Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, et al. A Longitudinal Study of Ebola Sequelae in Liberia. N Engl J Med. 2019;380:924–34. https://doi.org/10.1056/NEJMoa1805435.
Schindell BG, Webb AL, Kindrachuk J. Persistence and Sexual Transmission of Filoviruses. Viruses. 2018;10:E683. https://doi.org/10.3390/v10120683.
World Health Organization. Clinical care for survivors of Ebola virus disease: interim guidance: World Health Organization; 2016.
Purpura LJ, Choi MJ, Rollin PE. Zika virus in semen: lessons from Ebola. Lancet Infect Dis. 2016;16:1107–8. https://doi.org/10.1016/S1473-3099(16)30330-9.
Tuominen H, Rautava J, Kero K, Syrjänen S, Collado MC, Rautava S. HPV infection and bacterial microbiota in the semen from healthy men. BMC Infect Dis. 2021;21:373. https://doi.org/10.1186/s12879-021-06029-3.
de Lima Bossi R, Valadares JB, Del Puerto HL, Rocha MG, Braga LC, Sampaio MA, et al. Prevalence of human papillomavirus (HPV) in the semen of patients submitted to assisted reproductive technology treatment in a private clinic in Brazil. JBRA Assist Reprod. 2019;23:205–9. https://doi.org/10.5935/1518-0557.20190009.
Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod Oxf Engl. 2019;34:209–17. https://doi.org/10.1093/humrep/dey348.
Condijts T, Bourdeaud’huy L, Tilleman K, Lierman S, Dewinter C, Padalko E. Swim-up as a strategy for isolation of spermatozoa without viral incorporation in men with chronic hepatitis B: A pilot study. Andrologia. 2020;52:e13732. https://doi.org/10.1111/and.13732.
Abou-Setta AM. Transmission risk of hepatitis C virus via semen during assisted reproduction: how real is it? Hum Reprod Oxf Engl. 2004;19:2711–7. https://doi.org/10.1093/humrep/deh509.
Gantner P, Assoumou L, Leruez-Ville M, David L, Suzan-Monti M, Costagliola D, et al. HIV-1-RNA in seminal plasma correlates with detection of HIV-1-DNA in semen cells, but not with CMV shedding, among MSM on successful antiretroviral regimens. J Antimicrob Chemother. 2016;71:3202–5. https://doi.org/10.1093/jac/dkw271.
Lambert-Niclot S, Tubiana R, Beaudoux C, Lefebvre G, Caby F, Bonmarchand M, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002-2011 survey. AIDS Lond Engl. 2012;26:971–5. https://doi.org/10.1097/QAD.0b013e328352ae09.
Zafer M, Horvath H, Mmeje O, van der Poel S, Semprini AE, Rutherford G, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril. 2016;105:645–655.e2. https://doi.org/10.1016/j.fertnstert.2015.11.028.
Semprini AE, Macaluso M, Hollander L, Vucetich A, Duerr A, Mor G, et al. Safe conception for HIV-discordant couples: insemination with processed semen from the HIV-infected partner. Am J Obstet Gynecol. 2013;208(402):e1–9. https://doi.org/10.1016/j.ajog.2013.02.009.
Acknowledgments
The authors thank the Januário Cicco Maternity School of the Federal University of Rio Grande do Norte for their collaboration.
Funding
This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES).
Author information
Authors and Affiliations
Contributions
B. H. D. R. d. A. and D. C. F. L. conceived the study; wrote the manuscript (draft preparation); B.H.D.R.d.A. and D.C.F.L reviewed and edited the paper; D.C.F.L., J.F.A., M.d.M.G.T., and M.T.F.C.d.O. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Albuquerque, B.H.D.R., de Oliveira, M.T.F.C., Aderaldo, J.F. et al. Human seminal virome: a panel based on recent literature. Basic Clin. Androl. 32, 16 (2022). https://doi.org/10.1186/s12610-022-00165-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12610-022-00165-9