Abstract
L-Ascorbic acid, commonly known as vitamin C, has been used not only for disease prevention and in complementary and alternative medicine, but also for anti-aging purposes. However, the scientific evidence is not yet sufficient. Here, we review the physiological functions of vitamin C and its relationship with various pathological conditions, including our previous findings, and discuss the prospects of its application in healthy longevity. In summary, vitamin C levels are associated with lifespan in several animal models. Furthermore, clinical studies have shown that the blood vitamin C levels are lower in middle-aged and older adults than in younger adults. Lower blood vitamin C levels have also been observed in various pathological conditions such as chronic kidney disease and chronic obstructive pulmonary disease in the elderly. These observations suggest the implications of vitamin C in age-related pathological mechanisms owing to its physiological functions.
Similar content being viewed by others
Background
Among the members of the vitamin family, l-ascorbic acid (vitamin C) is particularly well known to the public, and functional foods and supplements are widely available. Vitamin C is a water-soluble antioxidant that was discovered as an anti-scurvy factor. Therefore, long-term vitamin C deficiency can cause scurvy. The main symptoms of scurvy include gingival hemorrhage, arthralgia, impaired wound healing, perifollicular hemorrhage, and ecchymoses [1]. Blood vitamin C levels of healthy individuals are around 50 µM, and levels below 11 µM increase the risk of scurvy [2]. Under physiological pH conditions in aqueous solution, vitamin C most commonly exists in its monoanionic form, ascorbate. Dehydroascorbic acid (DHA), an oxidized form of ascorbate, is generated via redox reactions [3]. In contrast, in vivo, DHA is reduced to ascorbate by dehydroascorbate reductase. In this article, ascorbate is described as vitamin C and L-ascorbic acid.
A reference value of 95–110 mg/day for vitamin C intake is recommended for healthy adults to maintain an adequate status [4]. There have been concerns regarding the accumulation and deposition of a vitamin C metabolite, oxalate, in the kidneys with increased intake of vitamin C [5]. However, recent examinations indicate that excess vitamin C is not associated with urinary stones or kidney injury [6]. Thus, vitamin C has few adverse effects, and high-dose therapy has been used worldwide. However, its efficacy remains unclear.
Notably, lower blood vitamin C levels were observed in various age-related pathological conditions [7, 8]. These manifestations are thought to be closely related to lower blood vitamin C levels in middle- and older-aged adults. Nevertheless, previous reports on vitamin C have not been summarized for some age-related diseases, but only for specific diseases. In this article, we review the physiological functions of vitamin C and its relationship with various pathological conditions, and discuss the implications of vitamin C in age-related diseases, including our previous findings.
Main text
Physiological functions of vitamin C
Vitamin C is a scavenger of reactive oxygen species (ROS) and an important cofactor in enzymatic reactions [9]. The enzymatic reactions catalyzed in the presence of vitamin C are categorized two main types, 2-oxoglutarate (α-ketoglutarate) dependent (or -independent) iron (Fe2+)-containing dioxygenases and copper (Cu2+)-containing monooxygenases. In Fe2+-containing dioxygenases reactions, vitamin C acts on hydroxylation and is involved in collagen polymerization, tyrosine metabolism, proteolysis of hypoxia-inducible factor (HIFα), and epigenetic regulations such as DNA, RNA, and histone demethylation [10]. In contrast, Cu2+-containing monooxygenase reactions with vitamin C catalyze the synthesis of peptide hormones and catecholamines [9]. These properties of vitamin C are thought to maintain biological functions and lead to anti-inflammatory and immune-boosting effects. Vitamin C is also believed to act as a cofactor in the Fe2+-containing dioxygenase reactions involved in carnitine synthesis [11]. However, we demonstrated that vitamin C is not essential for carnitine biosynthesis in vivo and suggested that glutathione (GSH) may compensate for vitamin C in this pathway [12].
Furthermore, vitamin C promotes iron absorption [13]. Vitamin C increases the absorption efficiency in the intestinal tract by reducing Fe3+ to Fe2+ in food.
In addition, vitamin C is known to recycle vitamin E (α-tocopherol) [14]. Vitamin E prevents lipid peroxidation by reducing lipid peroxyl radicals [15]. In contrast, vitamin C restores the antioxidant capacity of vitamin E by donating hydrogen atoms to the vitamin E radicals. Subsequently, oxidized vitamin C is regenerated by GSH in vivo [16]. Previously, we suggested that the interaction between vitamin C and vitamin E is tissue-specific and demonstrated that vitamin C spares vitamin E levels mainly in the liver in a mouse model [17].
Thus, vitamin C functions extensively in biological reactions.
Studies on vitamin C and lifespan
Many vertebrates synthesize vitamin C in vivo. However, primates, including humans, lack the ability to synthesize vitamin C due to mutations in the L-gulono-γ-lactone oxidase (Gulo) gene, the final enzyme in the pathway that biosynthesizes vitamin C from glucose [18]. Therefore, humans must consume external vitamin C to prevent scurvy. In contrast, mice and rats, which are commonly used models for pharmacological experiments, do not harbor mutations in Gulo. Therefore, Gulo-knockout (KO) mice [19], senescence marker protein-30/gluconolactonase (SMP30/GNL)-KO mice [20], osteogenic disorder Shionogi (ODS) rats [21], and guinea pigs [22] are used as rodent models deficient in vitamin C synthesis. Interestingly, studies on Gulo-KO and SMP30/GNL-KO mice have shown that feeding small amounts of vitamin C, which does not cause scurvy, and continued breeding under conditions of vitamin C shortage, shortens their lifespan [23, 24]. SMP30 was found as a protein that decreases with aging in the liver and kidneys of mice and rats [25]. Subsequently, SMP30 was identified as GNL, an enzyme involved in the vitamin C biosynthesis pathway [20]. Our previous studies have shown that vitamin C deficiency or insufficiency leads to age-related disease-like symptoms (e.g., hearing loss [26], UV-induced cataracts [27], epidermal atrophy [28], and impaired physical function [29, 30]) in SMP30/GNL-KO mice. Furthermore, chronic vitamin C deficiency in ODS rats results in senile disease-like lesions such as osteoporosis and emphysema [31].
In Caenorhabditis elegans, the efficacy of vitamin C administration in extending lifespan has been shown [32], but other studies have reported no lifespan extension by vitamin C because it acts as a pro-oxidant [33]. In Drosophila, no effect of vitamin C administration on lifespan has been observed [34].
In addition, long-term administration of vitamin C to wild-type mice, which have the ability to synthesize vitamin C, did not alter their lifespan compared to unadministered mice [35]. However, the lifespan was shortened in a mouse model of Werner's syndrome, a disease of premature aging, compared to that in wild-type mice, whereas vitamin C administration resulted in a lifespan equivalent to that of wild-type mice [35].
Vitamin C has been shown to exhibit anti-aging properties in several animal models. In contrast, vitamin C supplementation had no effect on mortality in a large human study [36]. However, various confounding factors may have influenced the results. Recently, a positive correlation between vitamin C intake and telomere length has been reported [37]. The new physiological effects of vitamin C are expected to be elucidated in the future. Furthermore, a simple sensing methodology for the rapid detection of vitamin C has been developed [38], and vitamin C levels should be easily monitored to diagnose health.
Vitamin C and pathologies
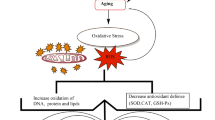
Blood vitamin C levels are lower in men than in women on average [39]. Reasons for this include the fact that men have larger bodies and a higher percentage of smokers. Notably, lower blood vitamin C levels have been observed in middle-aged and older adults than in younger adults in both men and women [40]. One reason for this is that older adults are more likely to be prone to chronic diseases. Therefore, vitamin C levels are closely associated with aging and diseases (Fig. 1).
In our laboratory, plasma vitamin C levels in patients with chronic kidney disease (CKD) were analyzed [7]. It was revealed that elderly patients with CKD had lower plasma vitamin C levels (27.1 ± 13.9 µM) than healthy elderly individuals (42.3 ± 15.5 µM). In particular, patients on hemodialysis had a higher risk of developing scurvy because the plasma vitamin C levels were 7.2 ± 3.9 µM after the hemodialysis, and the proportion of DHA increased. This may be due to the removal of vitamin C from the blood by hemodialysis and unbalanced nutritional intake due to dietary therapy. Some patients with diabetes have low blood and urine vitamin C levels due to poor vitamin C reabsorption or renal leakage caused by renal impairment [41]. In contrast, vitamin C supplementation may improve glycemic control and blood pressure in patients with type 2 diabetes [42].
Furthermore, our previous study has revealed that plasma vitamin C levels were lower in patients with chronic obstructive pulmonary disease (COPD) (31.2 ± 13.9 µM) [8]. A recent meta-analysis [43] showed that patients with COPD who received 400 mg of vitamin C daily had significantly improved respiratory function (forced expiratory volume in one second [FEV1%] and FEV1/forced vital capacity [FVC]) compared to the placebo group. In addition, a prospective cohort study in Europe [44] examined the association between blood vitamin C levels and respiratory diseases. The results showed that in a population with low (mean 28 µM [3–41 µM]) and high (mean 79 µM [66–242 µM]) blood vitamin C levels, the group with higher levels had a significantly lower risk of developing lung cancer and pneumonia. Nevertheless, in a U.S. study involving men aged 50 years and older who received either 500 mg/day of vitamin C or placebo and were followed up for an average of 8 years to assess cancer incidence [45], it was found that vitamin C intake had no effect on the incidence of lung cancer. Furthermore, an analysis showed no evidence of a causal association between circulating vitamin C levels and the risk of lung, breast, prostate, colon, and rectal cancers [46].
In a study of patients with cancer, low plasma vitamin C levels were more prevalent in patients undergoing cancer chemotherapy or immunotherapy than in pre-surgery patients [47]. In other words, cancer treatment likely affects blood vitamin C levels. Currently, no clear efficacy of high-dose intravenous vitamin C therapy has been demonstrated for cancer [48]. However, vitamin C may be effective against several types of cancer, and clinical trials are ongoing worldwide.
Blood vitamin C levels are also reduced by respiratory tract infections such as coronavirus infections and sepsis [49, 50]. These levels tend to be lower in critically ill patients [49, 50]. Vitamin C may have immunomodulatory functions, such as the suppression of pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP), and reduce oxidative stress, which are increased during infection [51]. However, the therapeutic efficacy of vitamin C has been inconsistent among clinical trials and ineffective in large-scale studies [52, 53]. The effect of vitamin C on infections may be influenced by the baseline blood vitamin C levels in the individual [54].
Significantly increased odds of coronary artery disease were also observed in individuals with deficient plasma vitamin C levels [55]. It is plausible that chronic insufficiency of vitamin C, due to both inadequate nutritional intake and increased consumption in the body, contributes to the progression of chronic diseases involving inflammatory components.
There is also a significant decrease in plasma vitamin C levels in patients with Alzheimer’s disease (AD) compared to healthy controls [56]. However, no association has been reported between vitamin C levels and cognitive dysfunction in AD [57]. Notably, higher blood vitamin C levels are associated with a reduction in apolipoprotein E (APOE) E4-related risks of cognitive decline in women [58]. The APOE gene ɛ4 is a strong genetic risk for AD. Further studies are warranted to elucidate the exact mechanistic role of vitamin C in the pathophysiology and prevention of AD.
It has also been reported that middle- and older-aged men and women with lower plasma vitamin C levels (< 50 µM) have lower estimated skeletal muscle mass than those with high levels (≥ 50 µM) [59]. Our study showed a decrease in skeletal muscle weight due to long-term vitamin C deficiency in SMP30/GNL-KO mice, and this loss of muscle weight was reversed by vitamin C administration [30]. The amount of ROS contributes to protein degradation in skeletal muscles and vitamin C may affect it [29]. In addition, a cross-sectional study of elderly women (70–84 years old) showed a positive correlation between blood vitamin C levels, muscle strength, and physical performance [60]. Similarly, a negative correlation of blood vitamin C concentration with frailty severity has been reported in elderly individuals (≥ 75 years old) [61]. Muscle decline is one of the most significant causes of frailty, and maintaining high blood vitamin C levels may play a role in its prevention.
Moreover, a meta-analysis showed that a higher vitamin C intake was associated with a lower risk of osteoporosis [62]. Vitamin C deficiency is thought to cause collagen loss, leading to a decreased bone mineral density. In contrast, recent studies using Gulo-KO mice have shown that the epigenetic functions of vitamin C are central to osteoblastogenesis and bone formation [63]. Epigenetic abnormalities are associated with the development of many diseases [64], and the epigenomic modulatory effects of vitamin C are promising [65]. Interestingly, vitamin C intake has been associated with systemic development and the immune response by epigenetic regulation [66], and may have an effect on epigenetic age, an indicator of biological age.
Although the results of these studies varied depending on the dosage and duration of the study, the diverse physiological effects of vitamin C have been linked to various diseases. As a common cause, vitamin C levels are thought to be affected mainly by oxidative stress and acute or chronic inflammatory reactions (Fig. 1). However, the cause of low blood vitamin C levels may differ depending on the type of disease. Further mechanistic studies on the in vivo effects of vitamin C are required.
Conclusions
Lower blood vitamin C levels have been observed in various pathological conditions and older adults. Therefore, maintaining a sufficient level of vitamin C may be beneficial for the prevention of age-related diseases and may be the key to longevity.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- DHA:
-
Dehydroascorbic acid
- GNL:
-
Gluconolactonase
- Gulo:
-
L-Gulono-γ-lactone oxidase
- IL-6:
-
Interleukin-6
- ODS:
-
Osteogenic disorder Shionogi
- ROS:
-
Reactive oxygen species
- SMP30:
-
Senescence marker protein-30
- TNF-α:
-
Tumor necrosis factor-alpha
References
Gandhi M, Elfeky O, Ertugrul H, Chela HK, Daglilar E (2023) Scurvy: rediscovering a forgotten disease. Diseases 11(2):78
Dresen E, Lee ZY, Hill A, Notz Q, Patel JJ, Stoppe C (2023) History of scurvy and use of vitamin C in critical illness: a narrative review. Nutr Clin Pract 38(1):46–54
Sato Y, Uchiki T, Iwama M, Kishimoto Y, Takahashi R, Ishigami A (2010) Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull 33(3):364–369
German Nutrition Society (DGE) (2015) New reference values for vitamin C intake. Ann Nutr Metab 67(1):13–20
Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M (2010) Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 5(7):e11414
Prier M, Carr AC, Baillie N (2018) No reported renal stones with intravenous vitamin C administration: a prospective case series study. Antioxidants (Basel) 7(5):68
Doshida Y, Itabashi M, Takei T, Takino Y, Sato A, Yumura W, Maruyama N, Ishigami A (2021) Reduced plasma ascorbate and increased proportion of dehydroascorbic acid levels in patients undergoing hemodialysis. Life 11(10):1023
Kodama Y, Kishimoto Y, Muramatsu Y, Tatebe J, Yamamoto Y, Hirota N, Itoigawa Y, Atsuta R, Koike K, Sato T, Aizawa K, Takahashi K, Morita T, Homma S, Seyama K, Ishigami A (2017) Antioxidant nutrients in plasma of Japanese patients with chronic obstructive pulmonary disease, asthma-COPD overlap syndrome and bronchial asthma. Clin Respir J 11(6):915–924
Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková L, Mladěnka P, Oemonom OBOT (2021) Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 13(2):615
Liu X, Khan A, Li H, Wang S, Chen X, Huang H (2022) Ascorbic acid in epigenetic reprogramming. Curr Stem Cell Res Ther 17(1):13–25
Rebouche CJ (1991) Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr 54(6):1147S-1152S
Furusawa H, Sato Y, Tanaka Y, Inai Y, Amano A, Iwama M, Kondo Y, Handa S, Murata A, Nishikimi M, Goto S, Maruyama N, Takahashi R, Ishigami A (2008) Vitamin C is not essential for carnitine biosynthesis in vivo: verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biol Pharm Bull 31(9):1673–1679
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22(1):18–35
Igarashi O, Yonekawa Y, Fujiyama-Fujihara Y (1991) Synergistic action of vitamin E and vitamin C in vivo using a new mutant of wistar-strain rats, ods, unable to synthesize vitamin C. J Nutr Sci Vitaminol 37(4):359–369
Leng X, Kinnun JJ, Marquardt D, Ghefli M, Kučerka N, Katsaras J, Atkinson J, Harroun TA, Feller SE, Wassall SR (2015) α-Tocopherol is well designed to protect polyunsaturated phospholipids: MD simulations. Biophys J 109(8):1608–1618
Linster CL, Van Schaftingen E (2007) Vitamin C biosynthesis, recycling and degradation in mammals. FEBS J 274(1):1–22
Sato A, Takino Y, Yano T, Fukui K, Ishigami A (2022) Determination of tissue-specific interaction between vitamin C and vitamin e in vivo using senescence marker protein-30 knockout mice as a vitamin c synthesis deficiency model. Br J Nutr 128(6):993–1003
Inai Y, Ohta Y, Nishikimi M (2003) The whole structure of the human nonfunctional l-gulono-gamma-lactone oxidase gene—the gene responsible for scurvy—and the evolution of repetitive sequences thereon. J Nutr Sci Vitaminol 49(5):315–319
Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM (2010) Low ascorbic acid and increased oxidative stress in gulo(−/−) mice during development. Brain Res 1349:143–152
Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, Goto S, Nishikimi M, Maruyama N, Ishigami A (2006) Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci USA 103(15):5723–5728
Ikeda S, Horio F, Kakinuma A (1998) Ascorbic acid deficiency changes hepatic gene expression of acute phase proteins in scurvy-prone ODS rats. J Nutr 128(5):832–838
Nishikimi M, Kawai T, Yagi K (1992) Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem 267(30):21967–21972
Aumailley L, Warren A, Garand C, Dubois MJ, Paquet ER, Le Couteur DG, Marette A, Cogger VC, Lebel M (2016) Vitamin C modulates the metabolic and cytokine profiles, alleviates hepatic endoplasmic reticulum stress, and increases the life span of Gulo−/−mice. Aging 8(3):458–483
Ishigami A, Kondo Y, Nanba R, Ohsawa T, Handa S, Kubo S, Akita M, Maruyama N (2004) SMP30 deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span. Biochem Biophys Res Commun 315(3):575–580
Kondo Y, Ishigami A (2016) Involvement of senescence marker protein-30 in glucose metabolism disorder and non-alcoholic fatty liver disease. Geriatr Gerontol Int 16(1):4–16
Kashio A, Amano A, Kondo Y, Sakamoto T, Iwamura H, Suzuki M, Ishigami A, Yamasoba T (2009) Effect of vitamin C depletion on age-related hearing loss in SMP30/GNL knockout mice. Biochem Biophys Res Commun 390(3):394–398
Ishikawa Y, Hashizume K, Kishimoto S, Tezuka Y, Nishigori H, Yamamoto N, Kondo Y, Maruyama N, Ishigami A, Kurosaka D (2012) Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp Eye Res 94(1):85–89
Sato Y, Arai KY, Nishiyama T, Nomura Y, Kishimoto Y, Aizawa S, Maruyama N, Ishigami A (2012) Ascorbic acid deficiency leads to epidermal atrophy and UVB-induced skin pigmentation in SMP30/GNL knockout hairless mice. J Invest Dermatol 132(8):2112–2115
Takisawa S, Funakoshi T, Yatsu T, Nagata K, Aigaki T, Machida S, Ishigami A (2019) Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci Rep 9(1):4702
Takisawa S, Takino Y, Lee J, Machida S, Ishigami A (2022) Vitamin C is essential for the maintenance of skeletal muscle functions. Biology 11(7):955
Kono K, Hayakawa M, Asai K, Kuzuya F, Harada T (1988) The relationship between illness in the aged and in inherently scorbutic rats (ODS rats) [in Japanese]. Nihon Ronen Igakkai Zasshi 25(5):508–514
Shibamura A, Ikeda T, Nishikawa Y (2009) A method for oral administration of hydrophilic substances to caenorhabditis elegans: effects of oral supplementation with antioxidants on the nematode lifespan. Mech Ageing Dev 130(9):652–655
Bonuccelli G, Brooks DR, Shepherd S, Sotgia F, Lisanti MP (2023) Antibiotics that target mitochondria extend lifespan in C. Elegans. Aging 15(21):11764–11781
Massie HR, Shumway ME, Whitney SJ, Sternick SM, Aiello VR (1991) Ascorbic acid in drosophila and changes during aging. Exp Gerontol 26(5):487–494
Massip L, Garand C, Paquet ER, Cogger VC, O’Reilly JN, Tworek L, Hatherell A, Taylor CG, Thorin E, Zahradka P, Le Couteur DG, Lebel M (2010) Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J 24(1):158–172
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297(8):842–857
Cai Y, Zhong YD, Zhang H, Lu PL, Liang YY, Hu B, Wu H (2023) Association between dietary vitamin C and telomere length: A cross-sectional study. Front Nutr 10:1025936
Manikandan R, Kim J, Ishigami A, Cho JY, Kim JH, Han JT, Lee J, Chang SC (2023) Dispersant-free supra single-walled carbon nanotubes for simultaneous and highly sensitive biomolecule sensing in ex vivo mouse tissues. Carbon 213:118275
Carr AC, Lykkesfeldt J (2023) Factors affecting the vitamin C dose-concentration relationship: implications for global vitamin c dietary recommendations. Nutrients 15(7):1657
Carr AC, Lykkesfeldt J (2023) Does aging affect vitamin C status relative to intake? Findings from NHANES 2017–2018. Nutrients 15(4):892
Ebenuwa I, Violet PC, Padayatty S, Wang Y, Wang Y, Sun H, Adhikari P, Smith S, Tu H, Niyyati M, Wilkins K, Levine M (2022) Abnormal urinary loss of vitamin C in diabetes: prevalence and clinical characteristics of a vitamin C renal leak. Am J Clin Nutr 116(1):274–284
Mason SA, Keske MA, Wadley GD (2021) Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care 44(2):618–630
Lei T, Lu T, Yu H, Su X, Zhang C, Zhu L, Yang K, Liu J (2022) Efficacy of vitamin C supplementation on chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 17:2201–2216
Myint PK, Wilson AM, Clark AB, Luben RN, Wareham NJ, Khaw KT (2019) Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European prospective investigation into cancer-Norfolk population-based cohort study. Eur J Clin Nutr 73(11):1492–1500
Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE (2009) Vitamins E and C in the prevention of prostate and total cancer in men: the physicians’ health study ii randomized controlled trial. JAMA 301(1):52–62
Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, Larsson SC, Zheng JS (2021) Circulating vitamin C concentration and risk of cancers: a mendelian randomization study. BMC Med 19(1):171
White R, Nonis M, Pearson JF, Burgess E, Morrin HR, Pullar JM, Spencer E, Vissers MCM, Robinson BA, Dachs GU (2020) Low vitamin C status in patients with cancer is associated with patient and tumor characteristics. Nutrients 12(8):2338
Ngo B, Van Riper JM, Cantley LC, Yun J (2019) Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer 19(5):271–282
Arvinte C, Singh M, Marik PE (2020) Serum levels of vitamin C and vitamin D in a cohort of critically ill covid-19 patients of a north American community hospital intensive care unit in May 2020: a pilot study. Med Drug Discov 8:100064
Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 21(1):300
Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD (2020) Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients 12(12):3760
Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, Busse LW, Coopersmith CM, DeWilde C, Ely EW, Eyzaguirre LM, Fowler AA, Gaieski DF, Gong MN, Hall A, Hinson JS, Hooper MH, Kelen GD, Khan A, Levine MA, Lewis RJ, Lindsell CJ, Marlin JS, McGlothlin A, Moore BL, Nugent KL, Nwosu S, Polito CC, Rice TW, Ricketts EP, Rudolph CC, Sanfilippo F, Viele K, Martin GS, Wright DW, Investigators VICTAS (2021) Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA 325(8):742–750
Ao G, Li J, Yuan Y, Wang Y, Nasr B, Bao M, Gao M, Qi X (2022) Intravenous vitamin C use and risk of severity and mortality in COVID-19: a systematic review and meta-analysis. Nutr Clin Pract 37(2):274–281
Abioye AI, Bromage S, Fawzi W (2021) Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: a systematic review and meta-analysis. BMJ Glob Health 6(1):e003176
Crook JM, Yoon SL, Grundmann O, Horgas A, Johnson-Mallard V (2023) Subclinical vitamin C plasma levels associated with increased risk of CAD diagnosis via inflammation: results from the NHANES 2003–2006 surveys. Nutrients 15(3):584
Hamid M, Mansoor S, Amber S, Zahid S (2022) A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Front Aging Neurosci 14:970263
Ide K, Yamada H, Kawasaki Y, Yamanaka M, Kawakami N, Katsuyama Y, Yoshida H, Kim K, Shiosaki E, Sonoda A, Umegaki K, Harada K (2016) Peripheral vitamin C levels in Alzheimer’s disease: a cross-sectional study. J Nutr Sci Vitaminol (Tokyo) 62(6):432–436
Noguchi-Shinohara M, Abe C, Yuki-Nozaki S, Dohmoto C, Mori A, Hayashi K, Shibata S, Ikeda Y, Sakai K, Iwasa K, Yokogawa M, Ishimiya M, Nakamura H, Yokoji H, Komai K, Nakamura H, Yamada M (2018) Higher blood vitamin C levels are associated with reduction of apolipoprotein E E4-related risks of cognitive decline in women: the Nakajima Study. J Alzheimers Dis 63(4):1289–1297
Lewis LN, Hayhoe RPG, Mulligan AA, Luben RN, Khaw KT, Welch AA (2020) Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J Nutr 150(10):2789–2798
Saito K, Yokoyama T, Yoshida H, Kim H, Shimada H, Yoshida Y, Iwasa H, Shimizu Y, Kondo Y, Handa S, Maruyama N, Ishigami A, Suzuki T (2012) A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J Gerontol A Biol Sci Med Sci 67(3):295–301
Sharma Y, Popescu A, Horwood C, Hakendorf P, Thompson C (2021) Prevalence of hypovitaminosis C and its relationship with frailty in older hospitalised patients: a cross-sectional study. Nutrients 13(6):2117
Malmir H, Shab-Bidar S, Djafarian K (2018) Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br J Nutr 119(8):847–858
Thaler R, Khani F, Sturmlechner I, Dehghani SS, Denbeigh JM, Zhou X, Pichurin O, Dudakovic A, Jerez SS, Zhong J, Lee JH, Natarajan R, Kalajzic I, Jiang YH, Deyle DR, Paschalis EP, Misof BM, Ordog T, van Wijnen AJ (2022) Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat Commun 13(1):5883
la Torre A, Lo Vecchio F, Greco A (2023) Epigenetic mechanisms of aging and aging-associated diseases. Cells 12(8):1163
Camarena V, Wang G (2016) The epigenetic role of vitamin C in health and disease. Cell Mol Life Sci 73(8):1645–1658
Keshawarz A, Joehanes R, Ma J, Lee GY, Costeira R, Tsai PC, Masachs OM, Bell JT, Wilson R, Thorand B, Winkelmann J, Peters A, Linseisen J, Waldenberger M, Lehtimäki T, Mishra PP, Kähönen M, Raitakari O, Helminen M, Wang CA, Melton PE, Huang RC, Pennell CE, O’Sullivan TA, Ochoa-Rosales C, Voortman T, van Meurs JBJ, Young KL, Graff M, Wang Y, Kiel DP, Smith CE, Jacques PF, Levy D (2023) Dietary and supplemental intake of vitamins C and E is associated with altered DNA methylation in an epigenome-wide association study meta-analysis. Epigenetics 18(1):2211361
Acknowledgements
We would like to thank Editage (http://www.edita ge.com) for English language editing.
Funding
This work was supported by JSPS KAKENHI Grant Number 21K17697.
Author information
Authors and Affiliations
Contributions
AS, YK, and AI were contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sato, A., Kondo, Y. & Ishigami, A. The evidence to date: implications of l-ascorbic acid in the pathophysiology of aging. J Physiol Sci 74, 29 (2024). https://doi.org/10.1186/s12576-024-00922-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12576-024-00922-7