Abstract
HCO3− secretion in distal airways is critical for airway mucosal defense. HCO3−/H+ transport across the apical membrane of airway surface epithelial cells was studied by measuring intracellular pH in luminally microperfused freshly dissected mice bronchioles. Functional studies demonstrated that CFTR, ENaC, Cl−–HCO3− exchange, Na+-H+ exchange, and Na+–HCO3− cotransport are involved in apical HCO3−/H+ transport. RT-PCR of isolated bronchioles detected fragments from Cftr, α, β, γ subunits of ENaC, Ae2, Ae3, NBCe1, NBCe2, NBCn1, NDCBE, NBCn2, Nhe1, Nhe2, Nhe4, Nhe5, Slc26a4, Slc26a6, and Slc26a9. We assume that continuous decline of intracellular pH following alkaline load demonstrates time course of HCO3− secretion into the lumen which is perfused with a HCO3−-free solution. Forskolin-stimulated HCO3− secretion was substantially inhibited by luminal application of CFTRinh-172 (5 μM), H2DIDS (200 μM), and amiloride (1 μM). In bronchioles from a cystic fibrosis mouse model, basal and acetylcholine-stimulated HCO3− secretion was substantially impaired, but forskolin transiently accelerated HCO3− secretion of which the magnitude was comparable to wild-type bronchioles. In conclusion, we have characterized apical HCO3−/H+ transport in native bronchioles. We have demonstrated that cAMP-mediated and Ca2+-mediated pathways are involved in HCO3− secretion and that apical HCO3− secretion is largely mediated by CFTR and H2DIDS-sensitive Cl−–HCO3− exchanger, most likely Slc26a9. The impairment of HCO3− secretion in bronchioles from a cystic fibrosis mouse model may be related to the pathogenesis of early lung disease in cystic fibrosis.
Similar content being viewed by others
Introduction
The airway surface liquid (ASL) is a thin layer of fluid covering the luminal surface of airway epithelium. The ASL is composed of inner periciliary liquid layer (PCL) and outer single-layer thin mucus. Proper volume/depth, viscosity, and pH of ASL are required for efficient mucociliary clearance and antimicrobial activity [11, 38, 47].
It is widely accepted that the volume/depth of PCL is determined by Cl− secretion via cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channel (CaCC) and Na+ absorption via epithelial Na+ channel (ENaC) [30, 33]. In proximal airways, Cl− secretion is mostly derived from serous cells of submucosal glands [5, 8, 17]. In distal airways, submucosal glands are absent [10, 35] and concurrent Cl− secretion and Na+ absorption was observed in surface epithelial cells [44]. Loss of CFTR function due to severe pathogenic variants in both alleles of the CFTR gene causes cystic fibrosis (CF). The initial event of CF lung disease is characterized by low PCL volume, which is thought to be achieved by defective CFTR-mediated Cl− secretion and abnormally elevated Na+ absorption via ENaC [33].
Evidence has accumulated to indicate that HCO3− transport is important in airway mucosal defense. HCO3− concentration affects physical properties of mucus [4, 39] and mucociliary transport in ex vivo pig trachea under acetylcholine (ACh) stimulation was more dependent on HCO3− secretion than Cl− secretion [13]. ASL pH in vivo newborn CF pigs was more acidic compared to wild-type and the impaired bacterial-killing activity of CF ASL was rescued by adding NaHCO3 [38]. Cellular mechanisms for HCO3− transport in airways have been studied using cultured human nasal epithelial cells [36, 37] and Calu-3 cells, a model of serous cells of submucosal glands [20, 25, 28]. However, HCO3− transport in distal airways/bronchioles is not well understood. ASL pH was more alkaline in lower airways than in upper airways in human [34]. Thus, a balance of HCO3− and H+ secretion may shift to HCO3− secretion in distal airways.
Distal airways contribute to 85–90% of the total epithelial surface area of conducting airways [10, 50]. Moreover, mucus plugging and obstruction of bronchioles are among the earliest events of CF lung disease, suggesting that regulation of epithelial ion transport in distal airways is critical for normal lung physiology [46]. However, the assessment of ion transport in distal airways/bronchioles has been limited because of the small size, complex anatomy and relative inaccessibility of structures [10]. Measurement of transmembrane potential in sheep, porcine, and human bronchioles identified Na+ and Cl− conductive pathways [2, 6, 9]. Aquaporin-mediated transepithelial water permeability was identified in guinea pig bronchioles [18]. Measurement of transepithelial potentials by a capillary-Ussing chamber revealed concurrent fluid secretion and absorption and HCO3− secretion in human bronchioles [45]. However, characteristics and cellular mechanisms of HCO3− transport in distal airways/bronchioles have not been fully investigated.
In the present study, HCO3− transport in surface epithelial cells of native bronchioles was studied by measuring intracellular pH (pHi) in luminally microperfused freshly dissected mice bronchioles. HCO3− transport in bronchioles from a CF mouse model was also studied.
Methods
Ethics approval
The study was approved by the Ethical Committee of Nagoya University on Animal Use for Experiment (approval No. M210457-003) and the Recombinant DNA Experiment Safety Committee of Nagoya University (approval No. 20-93).
Isolation of bronchioles from mice lung
A CF mouse model in which the F508del mutation was introduced in the mouse Cftr with the C57BL/6J genetic background (ΔF mouse) [53] was purchased from the Jackson Laboratory (Bar Harbor, ME). ΔF mice and their wild-type littermates were bled in Center for Research of Laboratory Animals and Medical Research Engineering, Nagoya University. Mice (8–10 weeks of age) of either sex were suffocated with CO2. The thorax was opened and the ice-cold standard HCO3−-buffered solution was gently injected into the trachea to fill the lungs. The lungs were then removed and the segments of conducting bronchioles (the third or fourth branches, 150–180 μm in inner diameter) were micro-dissected using sharpened needles in the ice-cold standard HCO3−-buffered solution.
Solutions
The standard HCO3−-buffered solution contained (mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 d-glucose, and 25 mM NaHCO3, and was equilibrated with 95% O2–5% CO2. The 25 mM HCO3−–0% CO2 solution was gassed with 100% O2 (pH: ~ 7.8) and thus was nominally free of CO2. The standard Hepes-buffered solution contained (mM): 130 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 d-glucose, and 10 Na-Hepes, and was equilibrated with 100% O2. The Cl−-free HCO3−-buffered solution contained (mM): 115 Na-gluconate, 2.5 K2HPO4, 1 CaSO4, 1 MgSO4, 10 d-glucose, and 25 mM NaHCO3, and was equilibrated with 95% O2–5% CO2. The Cl−-free Hepes-buffered solution contained (mM): 130 Na-gluconate, 2.5 K2HPO4, 1 CaSO4, 1 MgSO4, 10 d-glucose, and 10 Na-Hepes, and was equilibrated with 100% O2. The Na+-free HCO3−-buffered solution contained N-methyl-d-glucamine (NMDG) in place of NaCl, choline bicarbonate in place of NaHCO3, and 10 μM atropine to prevent the possible activation of muscarinic receptors by choline. The Na+-free HEPES-buffered solution contained NMDG-Cl in place of NaCl, and Hepes-acid in place of Na-Hepes. In the HCO3−-buffered solution containing 20 mM NH4Cl, the concentration of NaCl was reduced to maintain osmolarity. All solutions, except for the 25 mM HCO3−–0% CO2 solution, were adjusted to pH 7.4 at 37 ℃.
Microperfusion of isolated bronchioles
The lumen of the isolated bronchiole segments was microperfused by applying a method to microperfuse isolated pancreatic ducts [22]. One end of bronchiole was cannulated for luminal microperfusion (Fig. 1a and b). The concentric pipette arrangement consisted of an outer holding pipette, an inner perfusion pipette, and a silica inner capillary for exchange of solutions. The combination of inner silica capillary and waste line enables rapid exchange of luminal solutions. The lumen was perfused at 20–30 μl/min while the bath was perfused at ~ 3 ml/min and maintained at 37 ℃. The luminal perfusate leaving the other end of the bronchiole was diluted and washed away by the much greater flow of solution through the bath, which prevented the luminal perfusate from gaining access to the basal surface of the bronchiole.
Luminal microperfusion of an isolated bronchiole and measurement of intracellular pH. a, b The proximal end of an isolated bronchiole (inner diameter: ~ 150 μm) is held and cannulated for luminal microperfusion (20–30 μl/min). The representative bronchiole is bifurcated. The luminal perfusate leaving the distal end of the bronchiole is diluted and washed away by the flow (~ 3 ml/min) of solution through the bath. c Fluorescence of BCECF in surface epithelial cells lining the bronchiole. Small regions of the surface epithelium are selected (such as a rectangle) and the intracellular pH (pHi) is estimated by microfluorometry at 37 °C. d Isolated bronchioles were bilaterally perfused with the standard HCO3−-buffered solution and NH4Cl (20 mM) was applied to the lumen. Time course changes of pHi are shown as means ± SD of 5 experiments
Measurement of intracellular pH of bronchiole surface epithelium
The intracellular pH (pHi) in the epithelial cells was estimated by microfluorometry using the pH-sensitive fluoroprobe 2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF). After cannulating a bronchiole for luminal microperfusion, the epithelial cells were loaded with BCECF by perfusing the lumen with a solution containing the acetoxymethyl ester BCECF-AM (5 μM) for 10 min. Small regions of the bronchiole surface epithelium (Fig. 1c) were illuminated alternately at 430 and 480 nm and fluorescence was measured at 530 nm (F430 and F480). Values of pHi were calculated from the F480/F430 ratio after correction for the endogenous tissue fluorescence measured prior to loading with BCECF. Calibration data were obtained by the high K+-nigericin technique [36, 48].
Reverse transcriptase-polymerase chain reaction
Messenger RNA expression of several ion transporters and channels was examined in isolated bronchioles and tracheal mucosa by polymerase chain reaction (PCR) (Table 1). Primers were derived from published sequences with GenBank accession numbers. The PCR protocol was: 96 ℃, 25 s; 60 ℃, 30 s; 72 ℃, 40 s; 35 cycles. Templates for positive controls were complementary DNAs (cDNAs) prepared from lung, kidney, heart, colonic mucosa, brain and stomach mucosa. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers (452 bp) were used for the positive controls.
Materials
BCECF-AM was obtained from Invitrogen (Carlsbad, USA); 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (H2DIDS) was from Molecular Probes (Eugene, USA); forskolin, amiloride, CFTRinh-172 and other standard laboratory chemicals were from Sigma (St. Louis, USA).
Statistics
Data are presented as the means ± SD unless otherwise indicated. Tests for statistically significant differences were made with Student’s t-test.
Results
Isolated bronchioles from CF mice (ΔF/ΔF mice) were used in experiments shown in Fig. 8. Isolated bronchioles from wild-type mice were used in the other experiments (Figs. 1, 2, 3, 4, 5, 6, 7).
Basal pHi in bronchiole epithelial cells and the response to luminal NH4 +
When isolated bronchioles were bilaterally (bath and lumen) perfused with the standard HCO3−-buffered solution containing 25 mM HCO3− and 5% CO2 (pH 7.4), basal pHi was 6.94 ± 0.03 (n = 64, mean ± SD). When isolated bronchioles were bilaterally perfused with the standard Hepes-buffered (HCO3−–CO2-free) solution (pH 7.4), basal pHi was 6.77 ± 0.03 (n = 40). Basal pHi in the presence of HCO3−–CO2 was significantly (p < 0.01) higher compared to that in the absence of HCO3−–CO2. When NH4Cl (20 mM) was applied to the lumen in the presence of HCO3−–CO2, pHi showed typical time-course changes by NH4+ pulse [40]. Addition of NH4Cl caused quick alkalinization (NH3 influx) followed by slower decline and removal of NH4Cl caused quick acidification (NH3 efflux) followed by slower recovery to the baseline (Fig. 1d). This suggests that H+/HCO3− transport is active in this preparation.
Effects of luminal HCO3 −–CO2 removal on pHi in microperfused bronchioles
When isolated bronchioles were first bilaterally perfused with the standard HCO3−-buffered solution and the luminal perfusate was switched to the standard Hepes-buffered (HCO3−–CO2-free) solution (Fig. 2a), pHi quickly increased from 6.94 ± 0.02 to 7.05 ± 0.02 (n = 8) and then gradually decreased towards a value (6.86 ± 0.03) lower than the baseline in 10 min. To distinguish between the separate effects of removal of CO2 and HCO3− from the lumen, a solution was prepared which first contained 25 mM HCO3− but which was equilibrated with 100% O2 (pH: ~ 7.8) and thus was nominally free of CO2. When the luminal perfusate was switched to the 25 mM HCO3−–0% CO2 solution, pHi quickly increased to 7.21 ± 0.06 (n = 8) and the alkalinization was sustained (Fig. 2b). Thus, the transient alkalinization and the subsequent recovery (acidification) by removal of luminal HCO3−–CO2 was most likely due to CO2 diffusion of out of the cell followed by HCO3− efflux (Fig. 2a). Most of the HCO3− efflux was probably via the apical membrane due to the steep HCO3− gradient between the cell and the lumen (HCO3− concentration was close to zero). H+ influx was not likely involved in the subsequent acidification because Na+–H+ exchanger would work for H+ extrusion in this condition.
Effects of luminal HCO3−–CO2 removal on pHi in bronchiole epithelial cells. a The experimental protocol used for the measurement of HCO3− efflux across the apical membrane of microperfused bronchioles. Isolated bronchioles were first bilaterally perfused with the standard HCO3−-buffered solution and the luminal perfusate was switched to the standard Hepes-buffered HCO3−–CO2-free solution. It was assumed that the transient alkalinization was due to CO2 diffusion out of the cell and the subsequent recovery was due to HCO3− efflux mostly across the apical membrane. Means ± SD of 8 experiments. b Isolated bronchioles were first bilaterally perfused with the standard HCO3−-buffered solution and the luminal perfusate was switched to the solution which first contained 25 mM HCO3− but which was equilibrated with 100% O2 (pH: ~ 7.8) and thus was nominally free of CO2. Means ± SD of 8 experiments
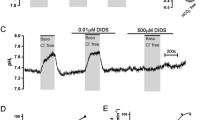
Effects of luminal application of forskolin, CFTRinh-172, H2DIDS, and amiloride on apical HCO3 − efflux in bronchiole epithelial cells
The mechanisms for HCO3− efflux across the apical membrane were examined using the protocol of Fig. 2a. After HCO3−–CO2 was removed from the luminal perfusate, forskolin (5 μM), the activator of adenylate cyclase, was applied to the lumen as indicated (Fig. 3a). Stimulation with forskolin transiently accelerated the pHi decline by 97% (p < 0.01) (n = 8, Fig. 3a and f) of control (without forskolin stimulation: blue line in Fig. 3a). The late phase of pHi decline (at midpoint pHi of 6.95) was also accelerated by 47% (p < 0.05) (Fig. 3a and g) of control. The data suggest that elevation of intracellular cAMP activates HCO3− secretion in a biphasic manner: initial large response followed by sustained activation, in mice bronchiole epithelial cells.
Effects of luminal application of forskolin, CFTRinh-172, H2DIDS, and amiloride on apical HCO3− efflux. a–e Isolated bronchioles were first bilaterally perfused with the standard HCO3−-buffered solution and the luminal perfusate was switched to the standard Hepes-buffered HCO3−–CO2-free solution. After HCO3−–CO2 was removed from the luminal perfusate, forskolin (5 μM) was applied to the lumen. Time course changes of pHi in the absence (a) or presence of CFTRinh-172 (5 μM) in the lumen (b), H2DIDS (200 μM) in the lumen (c), both CFTRinh-172 (5 μM) and H2DIDS (200 μM) in the lumen (d), or amiloride (1 μM) in the lumen (e) are shown as means ± SD of 8 experiments, respectively. The blue line in a indicates mean change of pHi without forskolin stimulation as a reference. f Early-phase pHi decline (ΔpH for 1 min) just after forskolin stimulation. #p < 0.01 compared with control (without forskolin stimulation). *p < 0.01 compared with forskolin alone. &p < 0.05. g Late-phase pHi decline (ΔpH/min at midpoint pHi of 6.95) under forskolin stimulation (red dashed lines in a-e). #p < 0.05 compared with control (without forskolin stimulation). *p < 0.01, **p < 0.05 compared with forskolin alone. &p < 0.01
CFTRinh-172 (5 μM) and H2DIDS (200 μM) in the lumen inhibited the forskolin-stimulated transient pHi decline by 69% (n = 8, p < 0.01) (Fig. 3b and f) and 65% (n = 8, p < 0.01) (Fig. 3c and f), respectively. Luminal CFTRinh-172 and H2DIDS also slowed down the late phase of pHi decline (at midpoint pHi of 6.95) by 54% (p < 0.01) (Fig. 3b and g) and 33% (p < 0.05) (Fig. 3c and g), respectively. The data suggest that both CFTR and H2DIDS-sensitive HCO3− transporter and/or HCO3−-permeable anion channel partly mediate cAMP-stimulated HCO3− secretion. The forskolin-stimulated transient pHi decline in the presence of both CFTRinh-172 and H2DIDS (Fig. 3d and f) was significantly (p < 0.05) smaller compared to that in the presence of CFTRinh-172 or H2DIDS alone (Fig. 3b, c, and f). The late-phase of pHi decline in the presence of both CFTRinh-172 and H2DIDS (Fig. 3d and g) was significantly (p < 0.05) slower compared to that in the presence of H2DIDS alone (Fig. 3c and g).
Luminal application of CFTRinh-172 and H2DIDS by themselves induced a transient small dip of pHi (Fig. 3b and c). The transient pHi dip largely disappeared when CFTRinh-172 and H2DIDS were simultaneously applied to the lumen (Fig. 3d). This suggests that CFTR and H2DIDS-sensitive HCO3− transporter/channel compensate each other for apical HCO3− efflux. We speculate on the mechanisms as follows. CFTR inhibition would hyperpolarize the cell, which would induce transient HCO3− efflux via a HCO3−-permeable anion channel or an electrogenic HCO3− transporter (such as 1Cl−–2HCO3− exchanger). If H2DIDS-sensitive HCO3- transport is electrogenic, luminal H2DIDS would hyperpolarize the cell, which would induce transient HCO3− efflux via CFTR.
To examine the role of ENaC in HCO3− secretion, a relatively low concentration of amiloride (1 μM) [32] was applied to the lumen. Amiloride (1 μM) in the lumen inhibited the forskolin-stimulated transient pHi decline by 63% (n = 8, p < 0.01) (Fig. 3e and f), but did not significantly affect the late phase of pHi decline (Fig. 3e and g). The data suggest that ENaC is involved in the regulation of HCO3− secretion. The transient dip of pHi by luminal amiloride (Fig. 3e) likely indicates apical HCO3− efflux which is accelerated by membrane hyperpolarization.
When isolated bronchioles were bilaterally perfused with the standard HCO3−-buffered solution, application of CFTRinh-172 (5 μM) to the lumen caused a transient increase of pHi by 0.022 ± 0.003 unit (n = 8, data not shown). The pHi increase was not observed in the absence of HCO3−–CO2 and enhanced by 77% (p < 0.05) by stimulation with forskolin (5 μM) (data not shown). The data suggest that CFTR is involved in HCO3− secretion in a physiological condition.
Effects of luminal Cl− removal on pHi in bronchiole epithelial cells
To examine the activity of Cl−–HCO3− exchange in the apical membrane, effects of luminal Cl− removal on pHi were examined. When isolated bronchioles were bilaterally perfused with the standard Hepes-buffered HCO3−–CO2-free solution, removal of luminal Cl− (by replacement with gluconate) caused a slight decline of pHi (Fig. 4a and e). In contrast, when isolated bronchioles were bilaterally perfused with the standard HCO3−-buffered solution, luminal Cl− removal caused a reversible increase of pHi by 0.14 ± 0.03 unit (n = 8) over ~ 4 min period (Fig. 4b and e), most likely due to influx of luminal HCO3− in exchange for intracellular Cl−. When the activity of apical Cl−–HCO3− exchange is shown as the initial rate of pHi increase upon luminal Cl− removal, the activity is not affected by forskolin (5 μM) in the lumen (Fig. 4c and e) and largely (p < 0.01) inhibited by H2DIDS (200 μM) in the lumen (Fig. 4d and e). The data suggest that H2DIDS-sensitive Cl−–HCO3− exchanger is localized in the apical membrane.
Effects of luminal Cl− removal on pHi in bronchiole epithelial cells. a–d Isolated bronchioles were first bilaterally perfused with the standard Hepes-buffered HCO3−–CO2-free solution (a) or the standard HCO3−-buffered solution (b–d). Luminal Cl− was removed by replacement with gluconate in the absence (a, b) or presence of luminal forskolin (5 μM) (c) or luminal H2DIDS (200 μM) (d). Time course changes of pHi are shown as means ± SD of 8 experiments, respectively. e The activity of apical Cl−–HCO3− exchange is shown as the initial rate of pHi increase upon luminal Cl− removal (red dashed lines in a–d). *p < 0.01
Na+-dependent H+ extrusion across the apical membrane of bronchiole epithelial cells
To examine whether Na+–H+ exchanger (NHE) and Na+–HCO3− cotransporter (NBC) are localized in the apical membrane, luminal Na+-dependent H+ extrusion was examined. In the absence of HCO3−–CO2, removal of luminal Na+ (by replacement with NMDG) caused a continuous decline of pHi and restoration of Na+ to the lumen caused a recovery (Fig. 5a). When the activity of luminal Na+-dependent H+ extrusion is shown as the initial pHi increase (ΔpH for 1 min) upon restoration of luminal Na+, the activity was completely (p < 0.01) inhibited by amiloride (100 μM) in the lumen (Fig. 5b and f). The data suggest that NHE is localized in the apical membrane.
Na+-dependent H+ extrusion across the apical membrane of bronchiole epithelial cells. a–e Isolated bronchioles were first bilaterally perfused with the standard Hepes-buffered HCO3−–CO2-free solution (a, b) or the standard HCO3−-buffered solution (c–e). Luminal Na+ was removed by replacement with NMDG and restored to the lumen in the absence (a, c) or presence of luminal amiloride (100 μM) (b, d) or combination of luminal amiloride (100 μM) and H2DIDS (200 μM) (e). Time course changes of pHi are shown as means ± SD of 8 experiments. f The activity of luminal Na+-dependent H+ extrusion is shown as the initial increase/decrease of pHi (ΔpH for 1 min) upon restoration of luminal Na+. *p < 0.01, **p < 0.05
The activity of luminal Na+-dependent H+ extrusion in the presence of HCO3−–CO2 (Fig. 5c and f) was significantly (p < 0.05) greater compared to that in the absence of HCO3−–CO2 (Fig. 5a and f), partially inhibited by amiloride (100 μM) in the lumen (Fig. 5d and f), and completely inhibited by a combination of amiloride (100 μM) and H2DIDS (200 μM) in the lumen (Fig. 5e and f). The data suggest that NBC is localized in the apical membrane.
Effects of luminal amiloride on pHi in bronchiole epithelial cells
While lower concentrations of amiloride inhibit ENaC with IC50 of 1 μM [32], higher concentrations of amiloride (0.5–1 mM) also inhibit apical NHE in human bronchial epithelium [49]. Figure 6 shows the effects of various concentrations of luminal amiloride (1, 10, and 100 μM) on basal pHi (Fig. 6). To examine the relative contribution of ENaC and apical NHE in H+/HCO3− transport in a physiological condition, we examined concentration-dependent effects of luminal amiloride rather than a more specific inhibitor of NHE such as ethylisopropyl amiloride (EIPA).
Effects of luminal amiloride on pHi in bronchiole epithelial cells. a–f Isolated bronchioles were first bilaterally perfused with the standard Hepes-buffered HCO3−–CO2-free solution (a, c, e) or the standard HCO3−-buffered solution (b, d, f). Amiloride was applied to the lumen as indicated at concentrations of 1 μM (a, b), 10 μM (c, d) or 100 μM (e, f). Time course changes of pHi are shown as means ± SD of 8 experiments. g Increase or decrease of pHi (ΔpH for 5 min) by luminal application of amiloride at various concentrations
In the absence of HCO3−–CO2, luminal application of amiloride caused concentration-dependent decline of pHi (Fig. 6a, c, e, g).
In the presence of HCO3−–CO2, luminal application of 1 μM amiloride caused an increase in pHi by 0.03 ± 0.01 (n = 8, Fig. 6b and g). Luminal 100 μM amiloride caused a transient increase followed by a continuous decline in pHi by 0.06 ± 0.01 (n = 8) in 5 min (Fig. 6f and g). Luminal 10 μM amiloride (Fig. 5d and g) caused an intermediate pattern of pHi changes of those by 1 μM and 100 μM amiloride.
Thus, the effects of lower concentration of apical amiloride on basal pHi were dependent on the presence of HCO3−–CO2, which suggests that ENaC is involved in the regulation of HCO3− transport. The data also indicate that apical NHE is involved in the regulation of basal pHi.
Messenger RNA expression of ion transporters and channels in bronchiole epithelial cells
Expression of Cftr, ENaC subunits, and Slc4, Slc9, and Slc26 families of transporters in isolated bronchioles and tracheal mucosa was examined by RT-PCR (Fig. 7). Amplified fragments from Cftr, α, β, γ subunits of ENaC, Slc4a2 (Ae2), Slc4a3 (Ae3), Slc4a4 (NBCe1), Slc4a5 (NBCe2), Slc4a7 (NBCn1), Slc4a8 (NDCBE), Slc4a10 (NBCn2), Slc9a1 (Nhe1), Slc9a2 (Nhe2), Slc9a4 (Nhe4), Slc9a5 (Nhe5), Slc26a4 (Pendrin), Slc26a6 (Pat1), and Slc26a9 were detected in isolated bronchioles and tracheal mucosa. Fragments from Slc9a3 (Nhe3) and Slc26a3 (Dra) were not detected in isolated bronchioles and tracheal mucosa.
Messenger RNA expression of ion transporters and channels in bronchiole epithelial cells. Messenger RNA was extracted from tracheal mucosa (T), isolated bronchioles (B), colon (C), kidney (K), stomach (S), heart (H), lung (L), and brain (Br) and reverse transcribed. PCR was performed using each cDNA as template and with gene-specific primers (Table 1). GAPDH was used as a reference. ‒: PCR was performed in the absence of RT enzyme. M: 100-bp DNA ladder
Basal pHi and apical HCO3 − efflux in bronchiole epithelial cells from CF mice
Basal pHi in the presence of HCO3−–CO2 in isolated bronchioles from ΔF/ΔF mice (6.97 ± 0.02, n = 6) was slightly but significantly (p < 0.05) higher compared to bronchioles from wild-type mice (6.94 ± 0.02, n = 8, blue line) (Fig. 8a and d). Initial increase of pHi (ΔpH) by removal of luminal HCO3−–CO2 was also significantly (p < 0.01) greater in ΔF/ΔF bronchioles compared to wild-type bronchioles (Fig. 8a and e). The data suggest that basal HCO3− secretion is impaired in CF bronchioles. The rate of pHi decline at midpoint pHi of 6.95 was significantly (p < 0.05) slower in CF bronchioles compared to wild-type bronchioles (Fig. 8a and g).
Apical HCO3− efflux in bronchiole epithelial cells from CF mice. a–c Isolated bronchioles from ΔF/ΔF mice were first bilaterally perfused with the standard HCO3−-buffered solution and the luminal perfusate was switched to the standard Hepes-buffered HCO3−–CO2-free solution. After HCO3−–CO2 was removed from the luminal perfusate, forskolin (5 μM, b) or ACh (10 μM, c) was applied to the lumen. Means ± SD of 5–6 experiments, respectively. Blue lines indicate mean changes of pHi in wild-type bronchioles as references. d Basal pHi in the presence of HCO3−–CO2 in wild-type (n = 8) and ΔF/ΔF (n = 6) bronchioles. #p < 0.05. e Transient increase of pHi (ΔpH) by removal of luminal HCO3−–CO2 in wild-type and ΔF/ΔF bronchioles. #p < 0.01. f Early-phase pHi decline (ΔpH for 1 min) just after stimulation with forskolin or acetylcholine. #p < 0.01 compared to wild-type. *p < 0.01, **p < 0.05 compared with control (without stimulation). &p < 0.01. g Late-phase pHi decline (ΔpH/min at midpoint pHi of 6.95) under stimulation with forskolin or ACh (red dashed lines in a–c). *p < 0.05 compared with control (without stimulation). #p < 0.05 compared to wild-type
Stimulation with luminal forskolin (5 μM) transiently accelerated pHi decline (apical HCO3− efflux) in ΔF/ΔF bronchioles (Fig. 8b and f) and the acceleration was comparable to wild-type bronchioles (blue line). Forskolin failed to accelerate the late phase of pHi decline (at midpoint pHi of 6.95) in ΔF/ΔF bronchioles (Fig. 8b and g). The data suggest that cAMP stimulation transiently activated HCO3− secretion in CF bronchioles probably via activation of a HCO3−-permeable anion channel or a HCO3− transporter, but failed to induce sustained increase of HCO3− secretion.
Luminal application of ACh induced a transient increase of transepithelial ion current in mice and pig tracheal epithelium [16, 21]. Application of ACh (10 μM) to the lumen transiently accelerated pHi decline (apical HCO3− efflux) in wild-type bronchioles (blue line in Fig. 8c) and the acceleration was greater than forskolin (Fig. 8f, p < 0.01). The ACh-induced transient acceleration of pHi decline was reduced by 45% (p < 0.01) in ΔF/ΔF bronchioles (Fig. 8c and f). Luminal ACh did not affect the late phase of pHi decline in both wild-type and ΔF/ΔF bronchioles (Fig. 8c and g). The data indicate that ACh stimulation transiently activated HCO3− secretion in wild-type bronchioles and that the ACh-induced enhancement of HCO3− secretion was substantially reduced in CF bronchioles. This suggests that CFTR partly mediates ACh-induced HCO3− secretion in addition to CaCC in mice bronchiole epithelial cells.
Discussion
In the present study, HCO3− transport in surface epithelial cells of native bronchioles was studied by measuring pHi in luminally microperfused freshly dissected mice bronchioles. HCO3− transport in bronchioles from CF mice was also studied. Although some connective tissue was attached to the outside of bronchioles (Fig. 1), surface epithelial cells were successfully loaded with BCECF from the lumen and pHi was measured as long as 30 min. The present study focused on HCO3−/H+ transport across the apical membrane, since rapid exchange of luminal solutions was achieved in our preparation [22].
Intracellular pH in surface epithelial cells of mice bronchioles
Human and rodent bronchioles are lined with columnar to cuboidal epithelium which is composed of ciliated and nonciliated (Clara) cells [35]. In the present study, basal pHi of surface epithelial cells in isolated mice bronchioles was ~ 6.94 in bilateral (bath and lumen) presence of 25 mM HCO3− and 5% CO2. The value is similar to the basal pHi of cultured human nasal epithelial cells (~ 6.94) in the same experimental condition [37]. The relatively low basal pHi likely resulted from higher pCO2 in the lumen compared to the physiological in vivo situation where the luminal side of the epithelial layer is exposed to air.
Ion transporters and channels localized in the apical membrane of bronchiole epithelial cells
In the present study, functional studies suggested that CFTR and H2DIDS-sensitive HCO3− transporter and/or HCO3−-permeable anion channel mediate cAMP-stimulated HCO3− secretion and ENaC, H2DIDS-sensitive Cl−–HCO3− exchangers, NHE, and NBC are involved in HCO3−/H+ transport across the apical membrane of surface epithelial cells of mice bronchioles (Fig. 9). This is supported by mRNA expression of Cftr, ENaC subunits, and Slc4, Slc9, and Slc26 families of transporters (Fig. 7).
A hypothetical model for H+/HCO3− transport across the apical membrane of airway surface epithelial cells in mice bronchiole. HCO3− secretion across the apical membrane is largely mediated by CFTR and Slc26a9 Cl−–HCO3− exchanger. CaCC is also involved in HCO3− secretion. An unknown HCO3−-permeable anion channel or HCO3− transporter is upregulated in CF bronchioles. ENaC is involved in the regulation of HCO3− transport but the mechanisms are not clear. NHE2 and NBC contribute to the regulation of intracellular and ASL pH
The activity of H2DIDS-sensitive Cl−–HCO3− exchanger was detected in the apical membrane (Fig. 4) and probably mediated part of cAMP-stimulated HCO3− secretion (Fig. 3c). The candidate molecules are Slc4a2 (Ae2), Slc4a3 (Ae3), Slc26a4 (Pendrin), Slc26a6, and Slc26a9 of which mRNA expression was detected in isolated bronchioles (Fig. 7). In human bronchial epithelia, SLC26A4 (Pendrin) colocalized with CFTR in the apical membrane of ciliated surface cells and mediated most of HCO3− secretion when pretreated with IL-4 [24]. SLC26A9 is prominently expressed in brain and on apical membrane of airway epithelial cells and gastric mucosa [3, 31]. A missense variant of SLC26A9 found in a patient of diffuse bronchiectasis failed to activate CFTR in a heterologous expression system [7]. While Slc26a4 (Pendrin) is H2DIDS-insensitive, Slc26a9 is sensitive to H2DIDS. Thus, Slc26a9 is likely the major apical Cl−–HCO3− exchanger in mice bronchioles.
Apical H+ secretion via H+/K+ ATPase and vacuolar H+-ATPase was reported in airways [52]. Our present study identified the activities of NHE and NBC in the apical membrane of mice bronchioles (Figs. 5, 6) which may contribute to the regulation of intracellular and ASL pH.
NHE activity was detected in the apical membrane and mediated H+ secretion in tracheal epithelial cells from sheep [1]. The candidate molecules of apical NHE in mice bronchioles are Slc9a1 (Nhe1), Slc9a2 (Nhe2), Slc9a4 (Nhe4), and Slc9a5 (Nhe5) of which mRNA expression was detected (Fig. 7). NHE2 is known to be expressed in the lung, predominantly localized to the apical membrane of epithelial cells [19], and relatively sensitive to amiloride [51]. Thus, NHE2 is likely the major apical Na+–H+ exchanger in mice bronchioles.
While SlC4A4 (NBCe1) and SLC4A5 (NBCe2) were identified in the basolateral membrane of Calu-3 cells [27], NBC isoforms have not been identified in the apical membrane of airway epithelium. Messenger RNA of all NBC isoforms: Slc4a4 (NBCe1), Slc4a5 (NBCe2), Slc4a7 (NBCn1), Slc4a8 (NDCBE), and Slc4a10 (NBCn2) was detected in isolated mice bronchioles (Fig. 7). Our present study cannot identify the membrane localization of the NBC isoforms.
Mechanisms and regulation of HCO3 − secretion in bronchiole epithelial cells
Surface airway epithelial cells as well as serous cells of the submucosal glands secrete Cl− and HCO3− in response to agents increasing intracellular cAMP (VIP, noradrenaline, etc.) and/or Ca2+ (ACh, histamine, etc.) [41]. It is generally accepted that cAMP-mediated secretion involves CFTR and Ca2+-mediated secretion involves CaCC encoded by TMEM16A/ANO1 [15]. Cyclic AMP- and Ca2+-mediated agonists independently and additively increased HCO3− secretion in human bronchioles [45]. However, it has been noted that muscarinic responses of fluid secretion are reduced in submucosal glands from patients with cystic fibrosis [42] and a recent study demonstrated a crosstalk of CFTR and TMEM16A in CFBE cells [29].
We assume that continuous decline of pHi following alkaline load (Figs. 2, 3, 8) demonstrates time course of HCO3− secretion into the lumen which is perfused with the HCO3−-free solution. Forskolin biphasically stimulated HCO3− secretion: transiently accelerated HCO3− secretion just after application and increased the rate of steady-state HCO3− secretion (Fig. 3a). ACh transiently accelerated HCO3− secretion, but did not increase the steady-state HCO3− secretion (Fig. 8c). The data indicate that both cAMP-mediated and Ca2+-mediated pathways are involved in HCO3− secretion in mice bronchiole epithelial cells.
Luminal CFTRinh-172 and H2DIDS substantially inhibited both transient and steady-state phases of forskolin-stimulated HCO3− secretion (Fig. 3). CFTR was localized not only in serous cells of submucosal glands [17, 23], but also in the apical membrane of surface epithelium of proximal to distal airways in human [26]. GlyH101-sensitive HCO3− transport was detected in human bronchioles [45]. Our present data suggest that both CFTR and H2DIDS-sensitive HCO3− transporter (likely SLC26A9 Cl−–HCO3− exchanger shown in Fig. 4) and/or HCO3−-permeable anion channel are involved in apical HCO3− secretion (Fig. 9).
A relatively low concentration of amiloride in the lumen inhibited transient phase of forskolin-stimulated HCO3− secretion (Fig. 3e). The data suggest that ENaC is involved in the regulation of HCO3− transport, which is consistent with amiloride (1 μM)-induced pHi increase in the presence of HCO3−–CO2 (Fig. 6). The cellular mechanisms for the involvement of ENaC in HCO3− secretion are not clear.
HCO3 − secretion in CF bronchiole epithelial cells
ASL pH was more acidic in trachea of CF pigs under basal and methacholine-stimulated conditions [38]. Lower pH of ASL was also observed in nasal epithelium of CF patients [34, 52], while the other study did not find differences in ASL pH of bronchus between CF patients and control [43]. Combination of forskolin and 3-isobutyl-1-methylxanthine alkalinized ASL of cultured bronchial epithelium of normal subjects but acidified CF ASL [12].
In the present study, HCO3− secretion was studied in bronchioles isolated from a CF mouse model in which the F508del mutation (most frequent pathogenic variant of CFTR) was introduced (ΔF mouse) (Fig. 8). Although CF mice do not display severe lung disease as observed in humans, an impaired ability to stretch/expand the peripheral lung compartment and increased distances between gas exchange surfaces which are early pulmonary phenotype of human CF were found in young (8–16 weeks old) ΔF/ΔF mice [14]. Our present study demonstrated higher level of basal pHi in the presence of HCO3−–CO2 and larger increase of pHi by removal of luminal HCO3−–CO2 in CF bronchioles (Fig. 8), which indicate that basal HCO3− secretion is reduced in CF distal airways.
The effects of forskolin and ACh on HCO3− secretion in CF bronchioles (Fig. 8) were unexpected. While forskolin stimulation transiently accelerated HCO3− secretion in CF bronchioles (comparable to wild-type bronchioles, Fig. 8b), ACh-induced acceleration of HCO3− secretion was substantially reduced in CF bronchioles (Fig. 8c). The data are consistent with the presence of a crosstalk of cAMP- and Ca2+-mediated pathways of HCO3− secretion. The data also suggest that a cAMP-activated HCO3−-permeable anion channel or HCO3− transporter was upregulated in CF bronchioles.
The present study has some limitations. (1) The intracellular buffering capacity is not measured and the rate of H+/HCO3− flux is not inferred from changes in pHi. (2) Information of membrane potential is not available and the electrochemical potential gradient for HCO3− across the apical membrane is not accurately predicted. (3) RT-PCR of isolated bronchioles does not identify the cell types (ciliated or nonciliated) and the membrane (apical or basolateral) in which transporters/channels are located.
In summary, we have characterized HCO3−/H+ transport across the apical membrane of surface epithelial cells of native mice bronchioles. We have demonstrated that cAMP-mediated and Ca2+-mediated pathways are involved in HCO3− secretion and that apical HCO3− secretion is largely mediated by CFTR and Cl−–HCO3− exchange. The impairment of HCO3− secretion in CF bronchioles may be related to the pathogenesis of early lung disease in CF.
Availability of data and materials
All data generated or analyzed during this study are included in the manuscript.
Code availability
Not applicable.
References
Acevedo M, Steele LW (1993) Na+-H+ exchanger in isolated epithelial tracheal cells from sheep. Involvement in tracheal proton secretion. Exp Physiol 78:383–394. https://doi.org/10.1113/expphysiol.1993.sp003692
Al-Bazzaz FJ, Tarka C, Farah M (1991) Microperfusion of sheep bronchioles. Am J Physiol 260:594–602. https://doi.org/10.1152/ajplung.1991.260.6.L594
Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34:494–515. https://doi.org/10.1016/j.mam.2012.07.009
Ambort D, Johansson ME, Gustafsson JK, Ermund A, Hansson GC (2012) Perspectives on mucus properties and formation–lessons from the biochemical world. Cold Spring Harb Perspect Med 2:a014159. https://doi.org/10.1101/cshperspect.a014159
Aritake H, Tamada T, Murakami K, Gamo S, Nara M, Kazama I, Ichinose M, Sugiura H (2021) Effects of indacaterol on the LPS-evoked changes in fluid secretion rate and pH in swine tracheal membrane. Pflugers Arch 473:883–896. https://doi.org/10.1007/s00424-021-02560-z
Ballard ST, Schepens SM, Falcone JC, Meininger GA, Taylor AE (1992) Regional bioelectric properties of porcine airway epithelium. J Appl Physiol 73:2021–2027. https://doi.org/10.1152/jappl.1992.73.5.2021
Bakouh N, Bienvenu T, Thomas A, Ehrenfeld J, Liote H, Roussel D, Duquesnoy P, Farman N, Viel M, Cherif-Zahar B, Amselem S, Taam RA, Edelman A, Planelles G, Sermet-Gaudelus I (2013) Characterization of SLC26A9 in patients with CF-like lung disease. Hum Mutat 34:1404–1414. https://doi.org/10.1002/humu.22382
Ballard ST, Spadafora D (2007) Fluid secretion by submucosal glands of the tracheobronchial airways. Respir Physiol Neurobiol 159:271–277. https://doi.org/10.1016/j.resp.2007.06.017
Blouquit S, Morel H, Hinnrasky J, Naline E, Puchelle E, Chinet T (2002) Characterization of ion and fluid transport in human bronchioles. Am J Respir Cell Mol Biol 27:503–510. https://doi.org/10.1165/rcmb.4869
Blouquit-Laye S, Chinet T (2007) Ion and liquid transport across the bronchiolar epithelium. Respir Physiol Neurobiol 159:278–282. https://doi.org/10.1016/j.resp.2007.03.007
Coakley RD, Boucher RC (2001) Regulation and functional significance of airway surface liquid pH. JOP 2:294–300
Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O’Neal WK, Boucher RC (2003) Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 100:16083–16088. https://doi.org/10.1073/pnas.2634339100
Cooper JL, Quinton PM, Ballard ST (2013) Mucociliary transport in porcine trachea: differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol 304:184–190. https://doi.org/10.1152/ajplung.00143.2012
Darrah RJ, Mitchell AL, Campanaro CK, Barbato ES, Litman P, Sattar A, Hodges CA, Drumm ML, Jacono FJ (2016) Early pulmonary disease manifestations in cystic fibrosis mice. J Cyst Fibros 15:736–744. https://doi.org/10.1016/j.jcf.2016.05.002
Danahay H, Gosling M (2020) TMEM16A: an alternative approach to restoring airway anion secretion in cystic fibrosis? Int J Mol Sci 21:2386. https://doi.org/10.3390/ijms21072386
Dittrich NP, Kummer W, Clauss WG, Fronius M (2015) Luminal acetylcholine does not affect the activity of the CFTR in tracheal epithelia of pigs. Int Immunopharmacol 29:166–172. https://doi.org/10.1016/j.intimp.2015.08.010
Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM (1992) Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2:240–248. https://doi.org/10.1038/ng1192-240
Folkesson HG, Matthay MA, Frigeri A, Verkman AS (1996) Transepithelial water permeability in microperfused distal airways. Evidence for channel-mediated water transport. J Clin Invest 97:664–671. https://doi.org/10.1172/JCI118463
Fuster DG, Alexander RT (2014) Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch 466:61–76. https://doi.org/10.1007/s00424-013-1408-8
Garnett JP, Hickman E, Tunkamnerdthai O, Cuthbert AW, Gray MA (2013) Protein phosphatase 1 coordinates CFTR-dependent airway epithelial HCO3- secretion by reciprocal regulation of apical and basolateral membrane Cl–HCO3- exchangers. Br J Pharmacol 168:1946–1960. https://doi.org/10.1111/bph.12085
Hollenhorst MI, Lips KS, Wolff M, Wess J, Gerbig S, Takats Z, Kummer W, Fronius M (2012) Luminal cholinergic signalling in airway lining fluid: a novel mechanism for activating chloride secretion via Ca2+-dependent Cl- and K+ channels. Br J Pharmacol 166:1388–1402. https://doi.org/10.1111/j.1476-5381.2012.01883.x
Ishiguro H, Steward MC, Yamamoto A (2011) Microperfusion and micropuncture analysis of ductal secretion. Pancreapedia Exocrine Pancreas Knowl Base. https://doi.org/10.3998/panc.2011.16
Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, Dalemans W (1993) Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J 6:169–176
Kim D, Huang J, Billet A, Abu-Arish A, Goepp J, Matthes E, Tewfik MA, Frenkiel S, Hanrahan JW (2019) Pendrin mediates bicarbonate secretion and enhances cystic fibrosis transmembrane conductance regulator function in airway surface epithelia. Am J Respir Cell Mol Biol 60:705–716. https://doi.org/10.1165/rcmb.2018-0158OC
Kim D, Kim J, Burghardt B, Best L, Steward MC (2014) Role of anion exchangers in Cl− and HCO3− secretion by the human airway epithelial cell line Calu-3. Am J Physiol Cell Physiol 307:208–219. https://doi.org/10.1152/ajpcell.00083.2014
Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC (2005) Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16:2154–2167. https://doi.org/10.1091/mbc.e04-11-1010
Kreindler JL, Peters KW, Frizzell RA, Bridges RJ (2006) Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta 1762:704–710. https://doi.org/10.1016/j.bbadis.2006.06.005
Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ (2004) Acid and base secretion in the Calu-3 model of human serous cells. Am J Physiol Lung Cell Mol Physiol 287:1274–1283. https://doi.org/10.1152/ajplung.00036.2004
Lérias J, Pinto M, Benedetto R, Schreiber R, Amaral M, Aureli M, Kunzelmann K (2018) Compartmentalized crosstalk of CFTR and TMEM16A (ANO1) through EPAC1 and ADCY1. Cell Signal 44:10–19. https://doi.org/10.1016/j.cellsig.2018.01.008
Lee RJ, Foskett JK (2010) Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am J Physiol Lung Cell Mol Physiol 298:L210-231. https://doi.org/10.1152/ajplung.00342.2009
Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J (2002) Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem 277:14246–14254. https://doi.org/10.1074/jbc.M111802200
Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K (1998) The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest 102:15–21. https://doi.org/10.1172/JCI2729
Mall M, Boucher RC (2006) Pathogenesis of pulmonary disease in cystic fibrosis. In: Bush A, Alton EWFW, Davies JC, Griesenbach U, Jaffe A (eds) Cystic fibrosis in the 21st century. Karger, Basel, pp 116–121 https://doi.org/10.1159/000088489
McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EW (2003) Airway surface pH in subjects with cystic fibrosis. Eur Respir J 21:37–42. https://doi.org/10.1183/09031936.03.00027603
Meyerholz DK, Suarez CJ, Dintzis SM, Frevert CW (2018) Respiratory system. In: Treuting PM, Dintzis SM, Montine KS Eds. Comparative anatomy and histology, a mouse, rat, and human atlas. 2nd edn. Academic Press, Cambridge, pp 147–162. https://doi.org/10.1016/B978-0-12-802900-8.00009-9
Paradiso AM (1997) ATP-activated basolateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am J Physiol 273:L148–L158. https://doi.org/10.1152/ajplung.1997.273.1.L148
Paradiso AM, Coakley RD, Boucher RC (2003) Polarized distribution of HCO3- transport in human normal and cystic fibrosis nasal epithelia. J Physiol 548:203–218. https://doi.org/10.1113/jphysiol.2002.034447
Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487:109–113. https://doi.org/10.1038/nature11130
Quinton PM (2008) Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372:415–417. https://doi.org/10.1016/S0140-6736(08)61162-9
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61:296–434. https://doi.org/10.1152/physrev.1981.61.2.296
Saint-Criq V, Gray MA (2017) Role of CFTR in epithelial physiology. Cell Mol Life Sci 74:93–115. https://doi.org/10.1007/s00018-016-2391-y
Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS (2005) Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J 19:431–433. https://doi.org/10.1096/fj.04-2879fje
Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, Montgomery ST, Nguyen T, Kreda SM, Kicic A, Noble PB, Button B, Stick SM (2017) Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun 8:1409. https://doi.org/10.1038/s41467-017-00532-5
Shamsuddin AK, Quinton PM (2012) Surface fluid absorption and secretion in small airways. J Physiol 590:3561–3574. https://doi.org/10.1113/jphysiol.2012.230714
Shamsuddin AKM, Quinton PM (2019) Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human bronchioles. Am J Physiol Lung Cell Mol Physiol 316:953–960. https://doi.org/10.1152/ajplung.00545.2018
Sheppard MN (1995) The pathology of cystic fibrosis. In: Hodson ME, Geddes DM (eds) Cystic fibrosis. Chapman & Hall Medical, London, pp 131–149
Smith JJ, Travis SM, Greenberg EP, Welsh MJ (1996) Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 8:229–236. https://doi.org/10.1016/s0092-8674(00)81099-5
Thomas JA, Buchsbaum RN, Zimniak A, Racker E (1979) Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18:2210–2218. https://doi.org/10.1021/bi00578a012
Urbach V, Hélix N, Renaudon B, Harvey BJ (2002) Cellular mechanisms for apical ATP effects on intracellular pH in human bronchial epithelium. J Physiol 543:13–21. https://doi.org/10.1113/jphysiol.2001.015180
Weibel ER (1963) Morphometry of the Human Lung. Springer, Berlin. https://doi.org/10.1007/978-3-642-87553-3
Xu H, Ghishan FK, Kiela PR (2018) SLC9 gene family: function, expression, and regulation. Compr Physiol 8:555–583. https://doi.org/10.1002/cphy.c170027
Zajac M, Dreano E, Edwards A, Planelles G, Sermet-Gaudelus I (2021) Airway surface liquid pH regulation in airway epithelium: current understandings and gaps in knowledge. Int J Mol Sci 22:3384. https://doi.org/10.3390/ijms22073384
Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr, Capecchi MR, Welsh MJ, Thomas KR (1995) A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest 96:2051–2064. https://doi.org/10.1172/JCI118253
Funding
Libin Liu received Japanese Government (MEXT) Scholarship and China Scholarship Council (CSC) Scholarship. This work was supported by grants from the Japan Society for the Promotion of Science and the Japanese study group for pediatric rare and intractable hepato-biliary-pancreatic diseases provided by the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LL, AY, MY, IT, NN, MN, YK, TF, MH, EN, TT, and HI. The first draft of the manuscript was written by LL and HI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee on Animal Use for Experiment (approval No. M210457-003) and the Recombinant DNA Experiment Safety Committee (approval No. 20-93) of Nagoya University.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, L., Yamamoto, A., Yamaguchi, M. et al. Bicarbonate transport of airway surface epithelia in luminally perfused mice bronchioles. J Physiol Sci 72, 4 (2022). https://doi.org/10.1186/s12576-022-00828-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12576-022-00828-2