Abstract
Purpose
To describe the spectrum of bacterial and fungal pathogens in cases of endophthalmitis requiring evisceration and report their antimicrobial susceptibilities.
Methods
Retrospective, consecutive, and descriptive case series of endophthalmitis that underwent evisceration from January 2004 to December 2017. Vitreous samples from all patients had been investigated for bacteria and fungus using institutional protocol. Bacterial isolates were identified using analytical profile index (API) system until 2010 and Vitek-2 compact system (bioMérieux, France), thereafter. The susceptibility of bacterial isolates to a variety of antibiotics was determined by the Kirby-Bauer disk-diffusion method.
Results
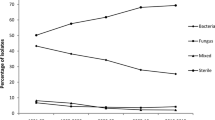
Of 791 cases reviewed, culture positivity was reported in 388 cases (48.92%). Commonest clinical setting of endophthalmitis necessitating evisceration was post-microbial keratitis (58%), followed by post-trauma and post-cataract surgery (14–15%). The commonest isolate was Streptococcus pneumoniae, seen in 68 samples overall (17.52%). One hundred and eighty-three isolates (47.16%) were gram-positive, 86 (22.16%) were gram-negative, and fungi constituted 137 (35.3%) isolates. Streptococcus pneumoniae was the commonest gram-positive bacterial isolate seen in 68/183 samples (37.15%). Among gram-negative organisms, the commonest was Pseudomonas aeruginosa seen in 47/86 (54.65%). Aspergillus spp. formed the commonest fungal isolate, 58/137 (42.33%). The susceptibility of the gram-positive bacteria was highest with vancomycin, 136/147 (92.51%) and for gram-negative bacteria was seen best with imipenem 24/29 (82.75%). Susceptibility to ceftazidime was 31/61 (50.81%) in 31/61.
Conclusion
Endophthalmitis due to Pneumococci, Aspergillus, and Pseudomonas can be very fulminant and progress to require evisceration in spite of prompt and appropriate treatment.
Similar content being viewed by others
Summary statement
Endophthalmitis is the most severe form of intraocular infection. Due to multiple factors, sometimes the infections progress in spite of timely and appropriate management and necessitates evisceration. The current paper discusses the clinical setting, microbiologic profile, and antibiotic susceptibility pattern of cases of endophthalmitis that required evisceration.
Introduction
Endophthalmitis is an ocular condition characterized by inflammation of the inner coats of the eye followed by exudation in the vitreous cavity [1]. It necessitates prompt and early management with intraocular antibiotics often combined with a pars plana vitrectomy. Many a times, in spite of prompt management, the condition may progress and either cause a painful blind eye or convert into a panophthalmitis with the infection spreading to the sclera and the Tenon’s capsule. In such situations, the eye often needs to undergo evisceration [2, 3]. Lu, et al. in their paper on risk factors for endophthalmitis requiring evisceration or enucleation described an evisceration rate of 14.3% [2]. Tsai, et al. in their paper on the same subject reported a higher evisceration/enucleation rate of 23.2% [3].
The probable reasons for the progression in spite of prompt and appropriate management could include relatively virulent organisms with possible high antibiotic resistance pattern. Current existing literature does not have adequate description about the spectrum of causative organisms and their antimicrobial susceptibility patterns in cases of endophthalmitis that eventually require evisceration. In the current communication, we report the above in such cases treated at our center over the past decade.
Materials and methods
This is a retrospective, non-comparative, descriptive, consecutive case series of patients treated at L.V. Prasad Eye Institute, Hyderabad, India, both in-house and referred from January 2004 to December 2017. The microbiology records of all cases of endophthalmitis that underwent evisceration were reviewed. The study was approved by the Institutional Review Board, and it adhered to the Tenets of the Declaration of Helsinki. Vitreous samples from all patients had been investigated for bacteria and fungus using institutional protocol [4]. Bacterial isolates were identified using analytical profile index (API) system until 2010 and Vitek-2 compact system (bioMérieux, France), thereafter. The susceptibility of bacterial isolates to a variety of antibiotics was determined by the Kirby-Bauer disk-diffusion method. Fungal species were identified based on their colony and microscopic characteristics. Susceptibility test for fungal isolates were not performed.
Eviscerated samples were transported to the microbiology laboratory immediately in a sterile bottle. The sample was examined by direct microscopy (Calcofluor-white, Gram, Giemsa stains) and culture for aerobic and anaerobic organisms. Special stains such as modified Ziehl-Neelsen using 1% H2SO4 and Gomori methenamine Silver stain were done for microscopy when indicated. For culture, the sample was inoculated on 5% sheep blood agar, 5% sheep blood chocolate agar, brain heart infusion broth, thioglycollate broth, and Sabouraud dextrose agar (SDA). All media were incubated at 37 °C for 1 week except SDA, which was incubated at 27 °C for 2 weeks for the isolation of fungi. Growth on two or more media or confluent growth on at least one solid medium at the site of inoculation or growth on one medium with consistent direct microscopy result was defined as a significant positive culture and was included in the study.
Results
A total of 6158 cases of endophthalmitis were seen at our center along the time period from January 2004 to December 2017. Of these, 791 cases of endophthalmitis underwent evisceration (12.84%). Of these cultures, positivity was reported in 388 cases (48.92%). Table 1 shows the various clinical etiologies that led to eventual evisceration. The commonest clinical setting of endophthalmitis necessitating evisceration was post-microbial keratitis which accounted for more than half (58%) of all the cases. This was followed by endophthalmitis post-trauma and post-cataract surgery (14–15%). Of the total 388 culture positive cases, the commonest isolate reported was Streptococcus pneumoniae, seen in 68 samples overall (17.52%). One hundred and eighty-three isolates (47.16%) were gram-positive organisms, 86 (22.16%) were gram-negative organisms, and fungi constituted 137 (35.3%) isolates. Among gram-positive organisms, Streptococcus pneumoniae was the commonest gram-positive bacterial isolate and the commonest gram-positive coccus seen in 68/183 samples (37.15%). The commonest gram-positive bacillus was Bacillus species 6/183 samples (6.55%). Among gram-negative organisms, the commonest isolate was Pseudomonas aeruginosa seen in 47/86 (54.65%). Aspergillus spp. formed the commonest isolate among fungi, 58/137 (42.33%). The detailed isolate list is described in Table 2.
The susceptibility of the gram-positive bacteria was highest with vancomycin, 136/147 samples tested (92.51%) followed by cefazolin118/149 samples tested (79.19%). That for gram-negative bacteria was seen best with imipenem24/29 samples tested (82.75%) followed by gatifloxacin 137/196 samples tested (69.89%), moxifloxacin101/158 samples tested (63.92%), and ofloxacin 118/199 samples tested (59.29%). Susceptibility to ceftazidime was found to be 50.81% in 31/61 samples tested. The detailed susceptibility list is described in Table 3.
Discussion
The current study showed that the commonest isolate in cases of endophthalmitis undergoing evisceration was Streptococcus pneumoniae followed by Aspergillus and Pseudomonas aeruginosa respectively. Only one previous study on evisceration following endophthalmitis has discussed the causative organisms [3]. In that study, the authors described 20 eyes that underwent evisceration following endophthalmitis. The commonest reported organism in that subset was Pseudomonas aeruginosa. Aspergillus accounted for one eye whereas Streptococcus pneumoniae was not reported. Studies on endophthalmitis secondary to Streptococcus pneumoniae suggest a poor visual outcome with a high rate of poor anatomic outcome. Miller et al., in their study on pneumococcal endophthalmitis, reported 3/27 (11.11%) eyes needing evisceration [5].The low evisceration rate in their study could be attributed to the fact that the antimicrobial susceptibilities to the commonly used intravitreal antibiotics like vancomycin, gatifloxacin, cefazolin, and ciprofloxacin, in their study was 100%. Conversely, in the current study, the pneumococcal endophthalmitis subgroup (68 eyes) showed a varied sensitivity pattern to the common antibiotics used. In contrast to Miller et al., in another study by Soriano et al. [6], the evisceration rates in 36 cases of Streptococcus pneumoniae endophthalmitis was 47.22%. This indirectly is in agreement with our study observation that Streptococcus pneumoniae is an important cause of evisceration following endophthalmitis. This relatively poor outcome has been hypothesized due to a very high degree of inflammatory response evoked by Streptococcus pneumoniae by its exotoxins and enzymes [7,8,9,10]. A commonly known virulence factor is a polysaccharide capsule that releases pneumococci from the host by preventing phagocytosis. Another potent virulence factor is pneumolysin which inhibits host responses such as antibody synthesis and lymphocyte proliferation. Inflammation is caused by the virulence factor of cell wall components, which are thought to be the main cause of symptoms. Aspergillus is a common organism causing endophthalmitis, more so in the Indian sub-continent as compared to the western world, where molds like Candida form the major etiology of fungal endophthalmitis [11,12,13]. In cases of fungal endophthalmitis especially with filamentous fungi, evisceration or enucleation rates as high as 25% have been reported [12, 13]. Occurrence in immuno-competent individuals common in the Indian sub-continent and a low index of suspicion initially often leads to a delay in the diagnosis of fungal endophthalmitis. This delay especially in case of Aspergillus like fungi can cause widespread vascular spread and intraocular tissue necrosis causing loss of anatomic integrity and necessitating evisceration [14].
EIfrig et al [15] in their study on Pseudomonas endophthalmitis reported an evisceration/enucleation rate of 64%. Similarly, high evisceration rates post Pseudomonas endophthalmitis were also reported by other workers [16, 17].This is attributable to the widespread and rapid tissue necrosis caused by Pseudomonas toxins. These toxins are known to disrupt cellular membranes and epithelial barriers and cause cytotoxicity [18, 19].
The current study specifically looked at the microbiologic profile and antibiotic susceptibility pattern of bacteria associated with endophthalmitis that underwent evisceration. The study also shows that the commonest predisposing clinical setting that culminates into need for evisceration is endophthalmitis following keratitis and perforated corneal ulcer. Other than the microbiologic profile, various other clinical factors like duration of the infection, etiology of endophthalmitis, type of treatment, associated trauma, and comorbid systemic factors may have a role in the final outcome, but the current study was not designed to look at those factors. In conclusion, the current communication suggests that though traditionally speaking gram-positive bacterial endophthalmitis has a relatively better treatment outcome as compared to gram-negative endophthalmitis, in cases progressing the evisceration, the incidence of Streptococcus pneumonia, Pseudomonas, and Aspergillus was very high.
References
Mamalis N (2002) Endophthalmitis. J Cataract Refrac Surg 28:729–730
Lu X, Ng DS, Zheng K, Peng K, Jin C, Xia H et al (2016) Risk factors for endophthalmitis requiring evisceration or enucleation. Sci Rep 15:6
Tsai Y, Tseng S (2001) Risk factors in endophthalmitis leading to evisceration or enucleation. Ophthalmic Surg Lasers 32:208–212
Kunimoto DY, Das TP, Sharma S, Jalali S, Majji AB, Gopinathan U et al (1999) Microbiologic spectrum and susceptibility of isolates: part 1. Postoperative endophthalmitis. Am J Ophthalmol 128:240–242
Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Corey RP, Miller D (2004) Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol 138:231–236
Soriano F, Perez-Trallero E, Pallares R, Meseguer MA, Fleites A, Gene A et al (2006) Streptococcus pneumoniae endophthalmitis: a study of 36 cases with special reference to antibiotic resistance and treatment options. Clin Microbiol Infect 12:519–526
Chan SM, Hodge WG, Leonard BC (1998) Postoperative Streptococcus pneumoniae endophthalmitis complicated by meningitis. Arch Ophthalmol 116:951–953
Shrader SK, Band JD, Lauter CB, Murphy P (1990) The clinical spectrum of endophthalmitis: incidence, predisposing factors, and features influencing outcome. J Infect Dis 162:115–120
Rai P, He F, Kwang J, Engelward BP, Chow VTK (2016) Pneumococcalpneumolysin induces DNA damage and cell cycle arrest. Sci Rep 30:22972
Paton JC, Rowan-Kelly B, Ferrante A (1984) Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun 43:1085–1087
Han DP, Wisniewski SR, Wilson LA et al (1996) Spectrum and susceptibilities of micorbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol 122:1–17
Wykoff CC, Flynn HW Jr, Miller D, Scott IU, Alfonso EC (2008) Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology 115:1501–1507
Leung EH, Kuriyan AE, Flynn WW Jr, Relhan N, Huang LC, Miller D (2016) Am J Ophthalmol 172:45–50
Rao NA, Hidayat A (2000) Trans Am OphthalmolSoc 98:183–194
Eifrig C, Scott IU, Flynn HW Jr, Miller D (2003) Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology 110:1714–1717
Sridhar J, Kuriyan AE, Flynn HW Jr, Miller D (2015) Endophthalmitis caused by Pseudomonas aeruginosa: clinical features, antibiotic susceptibilities and treatment outcomes. Retina 35:1101–1106
Falavarjani KG, Alemzadeh SA, Habibi A, Hadavandkhani A, Askari S, Pourhabibi A (2017) Pseudomonas aeruginosa endophthalmitis: clinical outcomes and antibiotic susceptibilities. OculImmunolInflamm 25:377–381
Soong G, Parker D, Magargee M, Prince A (2008) The type II toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J Bacteriol 190:2814–2821
Barbieri JT (2000) Pseudomonas aeruginosaExoenxyme S, a bifunctional type-III secreted cytotoxin. Int J Med Microbiol 290:381–387
Acknowledgements
Not applicable.
Funding
Funding was received from the Hyderabad Eye Research foundation to analyze the microbiology samples.
Availability of data and materials
Please contact the corresponding author for data requests.
Disclosures
None of the authors have any disclosures to make.
Author information
Authors and Affiliations
Contributions
VPD carried out the manuscript writing, proofreading, and drafted the final copy. JJ carried out microbiologic assessment. AP and RRP participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee approval was taken from the LV Prasad Eye Institute Hyderabad Ethics committee and Institutional Review Board for this research work. No animals were used in this research.
Consent for publication
Not applicable as no identity revealing photographs or data of any patient were used in the manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dave, T.V., Dave, V.P., Sharma, S. et al. Infectious endophthalmitis leading to evisceration: spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J Ophthal Inflamm Infect 9, 9 (2019). https://doi.org/10.1186/s12348-019-0174-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12348-019-0174-y