Abstract
Background
Air pollution is associated with adverse health effects, especially on the respiratory and cardiovascular systems, but according to recent research, even in cognitive health, metabolic, and immune systems. The objective was to analyse the effect of long-term exposure to air pollution on selected immune system parameters, 8-isoprostane a parameter of oxidative stress, and alpha-1-antitrypsin a protease inhibitor.
Methods
The number of 381 probands aged 35–65 from two differently polluted regions was included. Lifetime exposures to PM10, PM2.5, NO2, B(a)P, and benzene for each proband were calculated based on historical pollutant concentrations observed. The selected blood parameters were analysed in relation to independent variables (air pollutants, socioeconomic factors, etc.) using multiple regression. Possible covariates were determined. In its end, the study was conceived as a case–control study, and the odds ratio was quantified, expressing the strength of the association of the monitored parameters with the region.
Results
The average lifetime exposures to air pollution were significantly different between the two regions. Significant effects of the region were observed on IgM, IL-6, 8-isoprostane, and alpha-1-antitrypsin levels. The strongest positive association was observed between 8-isoprostane levels and benzene, PM2.5, PM10 and B(a)P. Odds ratio was 3.21 (95%CI 1.61–6.38). A significant negative association between all pollutants and IgM levels was observed even with covariate adjustment. Odds ratio was 1.80 (95%CI 1.15–2.82). A significant negative association between the alpha-1-antitrypsin levels and PM10, PM2.5, and benzene was found, independent of smoking as a covariate factor. Odds ratio was 1.77 (95%CI 1.09–2.87). In the case of IL-6, a significant effect of especially sleep as a covariate was observed. After covariates adjustment, a significant positive association between the IL-6 levels and PM10 and benzene was only observed. The odds ratio was 1.95 (95%CI 1.28–2.97).
Conclusions
The study confirmed that long-term exposure to air pollutants is associated with reduced levels of the protease inhibitor alpha-1-antitrypsin and decreased immune system performance by IgM. Furthermore, long-term exposure to air pollutants was associated with increased oxidative stress in humans, measured by 8-isoprostane levels. Residents who live in an industrial, environmentally polluted region showed elevated levels of IL-6.
Similar content being viewed by others
Introduction
Adverse health effect of air pollution
Air pollution represents the most significant environmental risk to human health. Long-term exposure to air pollutants is associated with an increased risk of premature death, especially due to respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and cardiovascular diseases [1,2,3,4,5,6,7,8]. Furthermore, recent epidemiological studies have also linked such long-term exposures with negative effects on the metabolic system, cognitive health (i.e. cognitive decline and the development of dementia), and the immune system [9,10,11,12], especially exposure to fine particles.
The mechanisms of adverse health effects related to air pollution are complex. They are mainly linked to direct or indirect reactive oxygen and nitrogen species (ROS and RNS, respectively) production. Oxidative stress can activate the inflammatory pathways that lead to immune cell activation and cytokine production [13, 14], and also the epigenetic mechanisms (change in histone tail modifications, microRNA expression, and DNA methylation) that contribute to the development and maintenance of inflammation [15].
Immune system and air pollution
Thus, in part, immune disturbances might mediate the effects of air pollutants on human health [16]. The respiratory system, the main site of exposure to air pollution, represents the main interface between inhalable air pollutants and the immune system [17]. Thus, epithelial cells and professional immune cells of airways can be stimulated by air pollution and triggered cellular signalling pathways. As a result, multicellular immune responses can be perturbated, leading to the onset of disease [17]. Studies also suggest that air pollution can influence skin integrity and, in this way, can have a significant adverse impact on the immune system [18]. Air pollutants can bind to the stratum corneum, may penetrate the epidermal barrier, and can enter the systemic circulation [18]. At the same time, inflammation associated with exposure to air pollution can cause skin ageing, allergic contact dermatitis, atopic dermatitis, psoriasis, acne, and skin cancer primarily [18].
Interleukins and alpha-1-antitrypsin
Several studies have reported associations between long-term (or lifetime) exposure to air pollution and chronic changes in immune markers [19,20,21]. The documented effects of air pollution primarily particulate matter (PM), nitrogen dioxide (NO2), and ozone, include increased presence of inflammatory and immune cells and inflammatory mediators, such as neutrophils, mast cells and interleukins [22]. For interleukins, it is primarily interleukin 6 (IL-6) [23,24,25] and interleukin 8 (IL-8) [25, 26], which have been shown to be significantly correlated with air pollution. Alpha-1-antitrypsin (A1AT) is an acute-phase protein within the immune system [27]. A1AT plays a crucial role in protecting tissues from enzymes of inflammatory cells. There is also evidence of a positive association between levels of A1AT and air pollution, especially PM10 and PM2.5 [28].
Imunoglobulins
A positive relationship has also been found between the concentration of immunoglobulins (Ig) and PMs, especially significant for IgG and PM2.5 and less significant for PMs and IgM, IgA, or IgE [29]. But, for example, Zhao et al. [30] have investigated immunoglobulin levels in traffic in Shanghai police officers and found that exposure to PM2.5 was associated with a decrease in IgA and increases in IgM, IgG, and IgE. According to Wang et al. and their systematic review and meta-analysis [31], ambient air pollutants increase the risk of IgE-mediated allergic diseases, including eczema, atopic dermatitis, and allergic rhinitis.
Marker of oxidative stress
Among the biological markers related to oxidative stress in vivo, 8-isoprostane (IsoP) is known as a stable and specific product of lipid peroxidation [32]. IsoP is formed endogenously and can be measured in urine, plasma, exhaled breath condensates, amniotic fluid, saliva, and tissues [33]. The IsoP concentration increases in response to ROS production. Along with decreased antioxidant defence, can induce tissue damage. Therefore, it contribute to pathophysiological changes such as those seen in asthma [34, 35]. A growing number of studies link IsoP concentrations with outdoor air pollutants, especially PM2.5 [36,37,38,39,40], but these studies are mainly focused on short-term exposure.
Long-term exposure to air pollution
Therefore, it is important to focus research on the study of the associations between air pollution and immune system parameters. Furthermore, recent studies consistently show that adverse health effects triggered by air can also be observed at very low concentrations (lower than current limit values) [41,42,43,44,45]. As a response, the WHO published an update in 2021 of the WHO Global Air Quality Guidelines that adjusted the levels of key air pollutants in the downward air quality guidelines for long-term (lifetime) exposure [46]. The highest concentrations of certain air pollutants within the European Union occur in some localities in the Czech Republic (CR) [47, 48]. On the other hand, there are localities that can be characterised as relatively environmentally clean, i.e. with air pollution below governmentally set limit values. The objective of this study is to analyse the effect of long-term exposure to air pollution on selected parameters of the immune system, 8-Isoprostane and alpha-1 antitrypsin in differently polluted localities in the CR.
Most related research examines short-term exposure. Fewer studies focus on long-term exposure, and many of them were conducted relatively long ago or examined individual air pollutants. Therefore, there is less evidence of the combined effects of air pollution in general. The present study assesses the effect of long-term exposure, using an originally developed method of historical time series analysis of air pollutant concentrations, which allows quantifying individual lifetime exposure of air pollutants of each proband. In additional, the selection of two differently polluted regions allows the investigation to be carried out as an analytical epidemiological study. Thus, it is possible to quantify the strength of the association of combined long-term exposure to air pollution (represented by environmentally distinct regions) and aberrations of selected parameters. Analysing dependence at this level may contribute to understanding the causal relationship between long-term exposure to air pollution and changes in the parameters of the immune system and related parameters, which can induce an adverse health effect.
Methods
Study settings
The subcohort of the research project Healthy Aging in Industrial Environment (HAIE) carried out in 2018–2023 (in acknowledgement) was used. The HAIE study addresses the assessment of the effects of air pollution and lifestyle on health and ageing in environmentally different regions of the CR. The presented study analyses the parameters of the immune system and related parameters in relation to different independent variables (parameters of air pollution, socioeconomic factors, etc.) using multiple regression analysis. The present study is conceived as a case–control study and quantifies the strength of the statistical association (odds ratio) between selected parameters and environmentally distinct regions. Individuals with aberrated immune system parameters can be understood as a group of cases and vice versa. Two environmentally distinct regions represent different risk factors of long-term exposure to air pollution.
Location and study group
The number of 381 probands aged 35–65 years from two differently polluted regions of CR was included in this study (see Fig. 1). Two hundred and one probands (98 males, 103 females) were from the Moravian-Silesian region (MSR), which lies in the northeast part of CR. Probands were mainly recruited from the Ostrava (the capital of this region) and Karvina (one out of six districts in the MSR) districts. These two districts of the MSR have long been among the most air-polluted areas in the CR and Central and Western Europe [48]. This region has a long history in heavy industry (primary metallurgy, steel, and coke production) and the chemical industry [49, 50]. Currently, the main sources of air pollution are industry, energy production, local heating, road traffic, and the emission of dust and gas pollutants from local municipal energy sources [49, 50]. Therefore, this locality is considered a high-polluted region (HPR) for the purpose of this study.
The number of 180 probands (88 males, 92 females) were from the South Bohemia region (SBR), which lies in the southwest part of CR. In contrast, this region, which consists of seven districts, is one of the least environmentally polluted areas of CR. The probands were mainly recruited from the district České Budějovice (the capital of this region). Historically, this region has been characterised by fish farming and forestry and has extensive agricultural areas. Although the industry with a focus on manufacturing activities developed in the last century, the concentrations of air pollutants are below the limit values [51]. Therefore, this region is considered a low-polluted region (LPR).
The selection of probands was random, with an equal representation of men and women from a cohort of approximately 3700 people representing the population living in both areas. Part of the exclusion criteria for the random selection of probands in both regions was good health, without the presence of serious diseases. To be included in the study, a person had to have signed an informed consent form, have lived in the area for at least half of their life and have lived in the area for at least five years as a child (up to 15 years) and for at least the last ten years of their life. The person must not have planned to move out of the area in the next 12 months.
Input data
Long-term exposure to air pollution
Our originally developed method for historical time series analysis of air pollutant concentrations at the level of districts was used. This method was developed for the purposes of the HAIE project (mentioned above) to estimate lifetime exposures. The methodology of historical time series analysis was published by Michalik et al. [50]. The methodology of lifetime exposure calculation using this historical time series analysis was also published by Machaczka et al. [52]. The main procedures are summarised in the following text.
Historical time series analysis of air pollutant concentrations
Time series data from PM10, PM2.5, NO2, benzene, and benzo(a)pyrene from valid national databases were used for model calculations. The analysis was divided into four main steps according to the availability of the data:
-
1.
Time period 1997–2019 (available air quality data)
-
The average annual concentrations were interpreted from the available tabular overviews of data from air pollution monitoring stations and modelled area five-year average concentrations in a regular network of squares (1 × 1 km). By combining these data based on mutual relations of individual air pollution characteristics, the average annual concentrations in the entire regular network of squares were interpreted. The missing data were replaced by a linear trend determined by the closest known values. Correction was made to the annual average concentrations of territorial units for residential zones.
-
-
2.
Time period 1980–1997 (available emissions balance data)
-
Subsequently, a less accurate estimate of these concentrations was made for the years 1980, 1985, 1990, 1995 based on emission balance data for individual evaluated districts. Then, an approximation was done for all years from 1997 to 1980 using linear trend based on the closest known values.
-
-
3.
Time period 1980–1960 (different availability of data)
-
For districts where air quality monitoring station data have been available, air pollutant concentrations were estimated based on the steps mentioned above. Between 1972 and 1960, the extrapolation was based on regression and correlation analysis of air quality monitoring data, emission balance, and dust downfall.
-
For other districts, where exact data from air quality monitoring stations or emission balance data were unavailable, a constant trend was used to back-extrapolate into a deeper history.
-
-
4.
Time period before year 1960 (no exact data on air quality or emissions balance)
-
Based on documented data on the increasing volume of production, the extrapolation was carried out using a linear trend. For districts, where such data were unavailable, a constant trend was used.
-
Estimated historical time series of air pollutant concentrations were further used to quantify lifetime exposures.
Calculating lifetime exposure to air pollutants
Lifetime exposure to air pollutants was expressed by the lifetime average exposure concentration (LCxp) of the individual air pollutant x for each proband p. The calculation is based on the average concentration (ICxdy), which uses historical time series of air pollutant concentrations estimated according to the procedure mentioned above. ICxdy was calculated for the air pollutant x in district d where the proband lived, corresponding to the years y of life spent in the given region. Formula used:
where:
LCxp—mean lifetime exposure of pollutant x for each proband p expressed as lifetime average exposure concentration in μg/m3 or ng/m3 for B(a)P.
ICxdy—the average concentration of the air pollutant x in district d of the HPR or LPR where the proband lived corresponding to year y-n, where y is the year corresponding to proband age and n is the sequence 0, 1, 2, ...,y.
The lifetime exposure calculation was performed for each proband separately and consisted of averaging the annual pollutant concentrations for each year from the proband’s birth to 2019 from the district in which the proband lived in each year. For example, if a 45-year-old proband lived 20 years in District A and then 25 years in District B, the concentrations of a specific pollutant were averaged from District A from 1974–1993 and from District B from 1994–2019.
Blood samples
The probands gave 9 ml of clotted venous blood, which was placed in BD Vacutainer CAT tubes. Serum was separated from blood by centrifugation. IgA, IgG, IgM, and A1AT were determined in serum using a BN II automatic nephelometric analyzer (Siemens). The immunoglobulin E and interleukin-6 parameters were determined by Immulite (Siemens), and interleukin-8 was determined by Enzyme-linked Immunosorbent Assay (ELISA).
Plasma blood sample purification and plasma IsoP analysis were performed using an 8-isoprostane ELISA kit from Cayman Chemical Company (Ann Arbor, MI, USA) according to the manufacturer’s instructions. Each sample (125 μL) was hydrolysed and further purified using 8-isoprostane Affinity Sorbent (Cayman, Ann Arbor, MI, USA). After evaporation of the elution solution, the purified samples were dissolved in ELISA buffer (330 μL) and analysed in technical duplicates using a 50 μL sample/well. The absorbance was measured using SpectraMax®iD3 (Molecular Devices, San Jose, CA, USA) at 405 nm, and the plasma IsoP concentration was expressed as pg IsoP/mL plasma.
The lower detection limit for the assays was 0.07 g/l for IgA, 0.07 g/l for IgG (determination limit), 0.043 g/l for IgM, 2.00 IU/ml for IgE, 2.00 pg/ml for IL-6, 9.36 pg/ml for IL-8, 0.047 g/l for A1AT, 0.8 pg/ml of plasma for IsoP. For further calculations, data adjusted according to the detection limit were used, that is, if a value below the detection limit was measured, it was replaced by half the value of the given detection limit.
Socioeconomic and lifestyle factors (covariates)
All probands completed the standardised socioeconomic questionnaire (SES questionnaire) [53], which included data on gender, age, education, diet, alcohol consumption, smoking, physical activity, BMI, waist circumference, and sleep that were used for analysis. The SES questionnaire was created by selecting and modifying the questions of the standardised questionnaires used in major studies, such as, for example, EHIS (European Health Interview Survey) [54], ELSA (English Longitudinal Study of Ageing) [55], HELEN (Health, Life Style and Environment) [56], and LTEQ Physical Activity [57]. The rating scales and evaluation methods used for each of the socioeconomic and lifestyle factors are shown in Table 1.
Data processed
The data evaluation in this study uses 3 different methods:
a. First, there were comparative tests of raw data using a nonparametric test (Mann–Whitney U test) for comparison results for probands living in different regions HPR and LPR.
b. Second, we used the multiple linear regression model to identify possible influences (independent variables/covariates—not only different regions) on the investigated parameters (dependent variables/covariates)[59]. The following steps of exploratory analysis before multiple linear regression were performed. They are:
-
Smirnov–Grubbs test for outliers.
-
Shapiro–Wilk normality test and single sample Kolmogorov–Smirnov normality test of residuals.
-
In case of non-normal distribution of residuals—Box–Cox transformation,
-
Test multicollinearity by the eigenvalues of correlation matrix, condition number, and variance inflation factors,
-
Anova table of regression, i.e. F-test and p-value for significance of the regression.
-
Multiple correlation statistics (incl. multiple correlation coefficient).
-
All significant partial regression coefficients were verified by simple linear regression (for one covariate), which also had to show significance.
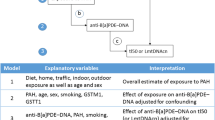
Independent covariates are lifestyle parameters and the lifetime exposure to air pollution and dependent covariates are the immune system status (immunoglobulins and cytokines), the protease inhibitor (A1AT) and the oxidative stress biomarker (IsoP). If the simultaneous effect of several covariates on the observed parameter was detected, the multiple regression model was adjusted and the effect of the covariates was subtracted. The results of the original model (crude model) and adjusted models (covariate models) are presented together. For example, the study by Azzouz et al. used a similar method of analysis [28] to investigate the associations between air pollution and established biomarkers of inflammation and cardiovascular disease.
c. The standard logistic regression method was used to calculate odds ratios (OR) with the calculation of 95% confidence intervals and statistical significance using the Chi2-test. The minimum of the difference between sensitivity and specificity and the minimum of p(Chi2) value were used to find the optimal cut-point [60].
Statistica 14 (TIBCO Software Inc, Palo Alto, USA), KyPlot 6.0. (KyensLab Inc, Japan) and Excel (Microsoft) were used for statistical analysis.
Results
The descriptive statistics of the estimated lifetime exposures to air pollutants (as independent variables) and their comparison by region and sex are shown in Table 2. The average lifetime exposure to PM10, PM2.5, NO2, benzene and B(a)P was 51.28 μg/m3, 39.28 μg/m3, 23.01 μg/m3, 2.46 μg/m3 and 4.87 ng/m3, respectively, in a high-polluted area (HPR), against 24.45 μg/m3, 19.18 μg/m3, 13.88 μg/m3, 0.82 μg/m3, 0.45 ng/m3 in a low-polluted area (LPR). So, the lifetime exposures to air pollution were almost twice as large in HPR than in LPR and thus significantly different in the general population. Interestingly, there were also differences between men and women from different regions.
Immunoglobulins—IgA, IgE, IgG, IgM, interleukins—IL-6, IL-8, protease inhibitor—A1AT, and parameter of oxidative stress—IsoP were determined from blood samples. The descriptive statistics of the results of the blood tests (as independent variables) and their comparison according to regions and sex are shown in Table 3. A significant difference between regions was found for A1AT, IgM, IL-6, and IsoP.
Information on socioeconomic and lifestyle factors was also collected from the studied population as possible covariates (Table 4). All monitored factors such as gender, age, education, diet, alcohol consumption, smoking, physical activity, BMI, waist circumference, and sleep were not statistically different between HPR and LPR, only physical activity was slightly different in women between HPR and LPR.
First, the effect of possible covariates on the selected parameters was tested by multiregression analysis (Table 5). In the case of immunoglobulins and possible covariates, there was observed an association of sex with IgA, and IgM levels; age was associated with IgG levels; alcohol consumption and BMI was associated with IgA, and IgE levels; smoking was associated with IgG levels; and diet was associated with IgA levels. In interleukins, only in IL-6 has the effect of the evaluated covariates been observed. There was found an association of BMI, and especially sleep. In addition to the parameters of the immune system, the A1AT protease inhibitor was also monitored and it was found that smoking affects its level. Also, the oxidative stress IsoP parameter was analysed, but no effect of the evaluated covariates was observed.
The statistically significant effect of the region (HPR, LPR) was found only in cases of A1AT, IgM, IL-6, and IsoP (Table 5). Only these parameters were subjected to more detailed analysis due to the main objective of the study (to evaluate the effect of air pollution on selected parameters in environmentally distinct regions). Table 6 then shows the results of the effect of long-term exposures to different air pollutants on A1AT, IgM, IL-6 and IsoP using different covariate models with different adjusted covariates identified in the previous step (Table 5) as statistically significant.
In the case of A1AT, a significant effect of lifetime exposure to PM10, PM2.5, and benzene was found on the A1AT levels independent of the influence of smoking, that is, for both crude model (M0) and the model with the adjusted effect of smoking (M1). On the other side BMI, and especially sleep played a significant role in the level of IL-6. If the effect of BMI and sleep was adjusted, significant effect on the level of IL-6 was found only for PM10 and benzene long-term exposures (covariate model M3). Even if the covariates were adjusted (covariate model M2), a statistically significant effect of long-term exposures to all air pollutants lifetime exposures on the IgM level was found. Similarly, long-term exposures to PM10, PM2.5, B(a)P, and benzene had a significant effect on the level of IsoP, and there was no significant effect of covariates.
The study design allows quantification of the strength of the statistical association by odds ratio (OR) between different long-term exposures to air pollution (represented by environmentally distinct regions HPR and LPR) and A1AT, IgM, IL-6, and IsoP. OR was calculated for each covariate model. The cut-point with the highest and statistically significant value of OR was searched for every evaluated parameter; the results are shown in Fig. 2.
An increased risk of aberrated levels of all parameters evaluated was found for HPR (that is, higher long-term exposures to air pollution). The highest statistical association strength (OR 3.21) was found in the case of IsoP when the risk of aberrated IsoP level (cut-point ≥ 35.00 pg/ml) was more than three times higher in HPR than in LPR. For the other parameters evaluated, the risk of aberration was approximately 2 times higher (OR 1.77–2.06) in HPR than in LPR.
Discussion
The difference between populations from environmentally distinct regions allowed us to study the link between long-term risk exposures and adverse health effects. The populations of the present study are characterised by significantly different long-term (lifetime) exposures to air pollution (Table 2). The findings of the present study indicate an association between lifetime exposures to air pollution and aberrated levels of some selected parameters of the immune system, the parameter of oxidative stress, and the protease inhibitor.
The effect of covariates on the levels of selected parameters
First, the effect of possible covariates was tested (Table 5). For example, according to a study by Gonzalez-Quintela [61], sex, age, alcohol consumption, smoking, and obesity should be considered when interpreting serum levels of IgA, IgG, and IgM. Sex differences in female immunoglobulin levels are attributed to hormonal effects on B lymphocytes [62, 63]. IgM levels have been reported to be higher in females than in males [61, 64]. In addition, in the present study, higher levels of IgM were found in females. On the contrary, higher levels of IgA were found in males, which corresponds to the results of other studies [61, 65, 66]. Also, immunoglobulin levels tend to increase with age [61, 67]. An increase in IgA and IgG levels may reflect the accumulation of chronic inflammatory conditions with ageing [61]. This corresponds to the results of the present study, in which age was associated with IgG. In studies, a parallel association has been observed between IgA and IgG levels and IL-6 levels, which is a marker of inflammation and a co-factor for immunoglobulin synthesis [61]. Regarding alcohol consumption, studies show a positive association with IgA levels [61, 68], also found in the present study too and also at IgE. For example, studies show that the increase in IgA levels in heavy drinkers is selective and does not affect IgM or IgG [61]. In addition, IgG and IgM levels tend to be lower in low-to-moderate alcohol consumers than in abstainers [61, 68]. IgA levels can also be affected by diet, as also the results of the present study show. The microbiome of the gastrointestinal tract is associated with B cell maturation, activation, and IgA antibody responses [69]. The results of studies demonstrate the interaction between dietary components, the gut microbiome, and autoantibody production [69]. Studies also show a negative association between smoking and IgG levels [61, 70], which was also found in the present study. Various mechanisms are involved in the effects of smoking on serum immunoglobulin concentrations. They may include direct effects on B cells and indirect effects on T cells and antigen-presenting cells, which could affect the switching of the immunoglobulin class and/or differential survival of naive B cells or memory B cells [70]. In vitro studies demonstrate that nicotine inhibits immunoglobulin production similarly to cigarette smoke extract. This could explain why smokers have significantly lower serum IgG, most of which is derived from antigen-stimulated B cells [70].
In interleukins, only in IL-6 has the effect of the evaluated covariates been observed. There was found to be an association between BMI and sleep with IL-6 levels. The strongest effect was observed in sleep. Studies show that alterations in sleep duration and quality increase IL-6 levels [71]. In general, acute and chronic reductions in sleep duration and quality have been shown to induce an increase in pro-inflammatory cytokine levels, and IL-6 appears to be one of the most important. Therefore, it is also referred to as the “sleep factor” that enhances sleep drive according to the circadian rhythm [71,72,73]. The level of IL-6 was found to be positively associated with BMI, which other studies also support by describing higher levels of IL-6 in obese patients [74, 75]. No effect of the evaluated covariates on the level of IL-8 has been observed. Other studies show that, for example, smoking [76] and BMI [77] can affect IL-8 levels.
In addition to immune system parameters, related parameters, such as the protease inhibitor A1AT, were also monitored. Regarding possible covariates, smoking can influence A1AT levels, but the relationship is more complex. Research has shown that smoking can cause increased oxidative stress and reduced levels of serum A1AT in cigarette smokers [78]. However, it is also important that increased levels of serum A1AT can occur in response to inflammation [79]. For example, studies [80,81,82] have found that serum levels of A1AT in active smokers were significantly higher than in non-smokers. Chronic smoking leads to elevation of A1AT, especially in the endothelium of the lung periphery. These changes are only modestly reflected in A1AT in the sputum, while plasma A1AT significantly reflects systemic manifestations related to smoking [80,81,82]. In the present study, a positive association of smoking was found with the A1AT level.
Also, the oxidative stress IsoP parameter was analysed, but no effect of the evaluated covariates was observed. For example, studies mention the influence of smoking when chronic healthy smokers have higher levels of IsoP in plasma and also in urine, indicating the prooxidative effect of smoking. [83, 84].
The effect of long-term air pollution exposure on the levels of selected parameters
Only at levels of IsoP, IgM, IL-6 and A1AT significant effects of the region were observed, that is, the effect of different lifetime exposures to air pollution (Table 6). Therefore, these parameters were subjected to a more detailed follow-up analysis. The effect of particular air pollutants was observed on these parameters. Without considering the influence of possible covariates (crude model M0), lifetime exposures to PM10, PM2.5, and benzene were negatively associated with levels of A1AT and IgM and positively associated with levels of IsoP and IL-6. Lifetime exposures to NO2 were negatively associated only with the levels of IgM. Lifetime exposure to B(a)P was negatively associated with the IgM levels, and positively associated with levels of IL-6 and IsoP. Different covariate models with different adjusted factors identified in the previous step as statistically significant were used to analyse the effect of only air pollution on the parameters evaluated (Table 5).
In the case of A1AT, with the adjusted effect of smoking (covariate model M1), still long-term exposure to PM10, PM2.5 and benzene was negatively associated with the level of A1AT, as in the crude model (M0). Most studies focus on A1AT deficiency and the effect of air pollution. A1AT deficiency is a genetic disorder that predisposes to the development of chronic obstructive pulmonary disease [85]. Long-term exposure to air pollution is associated with decreased lung function in patients with A1AT deficiency [86]. However, there are only a few studies that deal with the effect of long-term exposure to air pollution on the level of A1AT in healthy individuals. For example, according to previously published results [28] in the Malmö Diet and Cancer subcohort, evidence of a positive association has been found between increased levels of A1AT and moderate long-term exposure to air pollution, especially to PM10 and PM2.5. These findings support inflammation as a mechanism behind the association between air pollution and cardiovascular disease. Another study suggested that continued exposure to high levels of pollution could lead to reduced levels of A1AT in plasma, potentially compounding impaired lung function in children living in highly polluted areas [87]. This discrepancy deserves further investigation. However, studies show that lower levels of A1AT are associated with a risk of spontaneous cervical artery dissections [88], type II diabetes mellitus [89], type 1 human immunodeficiency virus (HIV) infection [90], and spontaneous abortions [91].
If the effect of BMI and sleep was adjusted (covariate model M3), a significant effect on the level of IL-6 was found for PM10 and benzene long-term exposures only. Studies found evidence of a positive association, especially between long-term PM2.5 and serum IL-6 [21, 25]. For example, according to the results of the Tripathy et al. study [92], exposure to PM2.5 was associated with increased IL-6 production by stimulated immune cells, but exposure to pollutants was not related to the circulating level of IL-6.
The effect of long-term exposures to PM10, PM2.5, B(a)P, NO2 and benzene on IgM level was found to be statistically significant, even if sex was adjusted (covariate model M2). A positive association has been observed between long-term exposure to those air pollutants and the IgM levels. Studies found a positive association between PMs and immunoglobulin concentration with different significant associations for IgM [29, 30]. For example, Hadnagy et al. [93] conducted a study in adults and Zhao et al. [30] conducted a study on traffic police officers. They found that IgM was significantly increased in relation to PM, specifically IgA in adults and IgA, IgG, and IgE in traffic police officers. On the contrary, Leonardi [29] conducted a study in children and observed a significant positive association between PM and especially IgG. In general, these results suggest that long-term exposure to PM can cause inflammation of the airways and activation of the cellular and humoral immune system [29]. A systematic review [94] has linked benzene to an immunosuppressive effect on the adaptive immune system and can activate the innate immune system to cause inflammation. In particular, benzene significantly reduces the number of white blood cells and natural killer cells and increases pro-inflammatory biomarkers at low levels of exposure. However, the specific impact of benzene on IgM is not directly described.
The covariates had no significant effect on the level of IsoP, a marker of oxidative stress, as mentioned above. Similarly, long-term exposures to PM10, PM2.5, B(a)P and benzene significantly affected the IsoP level, but with a positive association. Studies focus on short-term exposure to air pollution and its effect on IsoP. For example, Hashemzadeh’s study [38] in children showed that short-term exposure to traffic-related air pollution could increase the 8-isoprostane of exhaled breath condensate. In a study by Rossner et al. [36], a positive relationship was observed between lipid peroxidation (levels of IsoP in plasma) and air pollution during three seasons (winter 2009, summer 2009, and winter 2010), especially for exposure to B(a)P, PM2.5, and benzene.
Strength of the association (odds ratio) of the monitored parameters with the region where the probands live
Although the associations are not strong, significant associations with air pollution were found for the protease inhibitor A1AT (OR = 1.77, the effect of smoking was subtracted), for IgM (OR = 1.80, the effect of sex was subtracted), and for the oxidative stress biomarker IsoP (OR = 3.21, the effect of other covariates was not observed). For cytokine IL-6, after subtracting the effect of BMI and sleep, significant association with PM10 and benzene long-term exposures was observed, OR = 1.95. For all findings, it can be stated that they are also relevant to earlier studies, which are quite rarely conducted [29, 37, 88, 92].
Limitations
Like any study, this one also has certain limitations. First of all, it is a relatively low number of probands—about 381 in total. Ideally, there should be several times more, which unfortunately was not feasible in practice. For this reason, the statistical significance of the results is “on the edge” in some cases. Another partial limitation may be the fact that some selection criteria related to exposure, namely all places of residence listed in the proband’s questionnaire (from his birth to the present, i.e. several decades ago), could not be verified. On the other hand, we consider the strong side of the study to be strict selection criteria for equal selection in all age categories for both sexes and in both studied regions of a total cohort of approximately 4000 volunteers who were also selected according to the same criteria.
Conclusion
This research uses original methods to estimate individual lifetime exposures to polluted air and is designed as an analytical study in its conclusion. The results may contribute to understanding of the causal relationship between long-term exposure to air pollution and adverse health effects induced by aberration of the immune system and related parameters. Hypotheses about the reduction of protease inhibitor A1AT levels and immune system performance were confirmed, mainly due to the decrease in IgM immunoglobulin level, in association with long-term exposures to air pollutants. This work also showed an association of exposure to air pollutants with increased oxidative stress in humans, measured as IsoP levels, and increased levels of the cytokine IL-6 in residents who live most of their lives in an industrial, environmentally polluted region.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A1AT:
-
Alpha-1-antitrypsin
- AGE:
-
Age
- ALC:
-
Alcohol consumption
- Avg:
-
Arithmetic mean
- B(a)P:
-
Benzo(a)pyrene
- CR:
-
Czech Republic
- DIET:
-
Diet
- EDU:
-
Education
- HPR:
-
High-polluted region
- Ig:
-
Immunoglobulin
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IsoP:
-
8-Isoprostane
- LC:
-
Average lifetime exposure of air pollutant
- LQ:
-
Lower quartile
- LPR:
-
Low-polluted region
- M0:
-
Crude model
- M1-3:
-
Covariate model
- Max:
-
Maximum
- Med:
-
Median
- Min:
-
Minimum
- MSR:
-
Moravian-Silesian region
- N:
-
Number of variables
- NS:
-
Not significant
- OR:
-
Odds ration
- PA:
-
Physical activity
- Par:
-
Parameters
- PM:
-
Particulate matter
- ROS/RON:
-
Reactive oxygen and nitrogen species
- S:
-
Statistical significance
- SBR:
-
South Bohemia region
- SLEEP:
-
Sleep
- SMK:
-
Smoking
- UQ:
-
Upper quartile
- WAIST:
-
Waist circumference
References
World Health Organization (2016) Ambient air pollution: a global assessment of exposure and burden of disease WHO library cataloguing-in-publication data: WHO. ISBN 978–92–4–151135–3.
Doiron D, de Hoogh K, Probst-Hensch N et al (2019) Air pollution, lung function and COPD: results from the population-based UK biobank study. Eur Respir J. https://doi.org/10.1183/13993003.02140-2018
Beelen R, Raaschou-Nielsen O, Stafoggia M et al (2014) Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. The Lancet 383:785–795. https://doi.org/10.1016/S0140-6736(13)62158-3
Di Q, Wang Y, Zanobetti A et al (2017) Air pollution and mortality in the medicare population. New Engl J Med 376:2513–2522. https://doi.org/10.1056/NEJMoa1702747
Pope CA, Lefler JS, Ezzati M et al (2019) Mortality risk and fine particulate air pollution in a large representative cohort of U.S. adults. Environ Health Persp. https://doi.org/10.1289/EHP4438
Yazdi MD, Wang Y, Di Q et al (2021) Long-term effect of exposure to lower concentrations of air pollution on mortality among US medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health 5:e689–e697. https://doi.org/10.1016/S2542-5196(21)00204-7
Brook RD, Rajagopalan S, Pope CA et al (2010) Particulate matter air pollution and cardiovascular disease. Circulation 121:2331–2378. https://doi.org/10.1161/CIR.0b013e3181dbece1
Liu S, Jørgensen JT, Ljungman P et al (2021) Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. https://doi.org/10.1183/13993003.03099-2020
Ohlwein S, Kappeler R, Kutlar Joss M et al (2019) Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Publ Health 64:547–559. https://doi.org/10.1007/s00038-019-01202-7
Chen H, Kwong JC, Copes R et al (2017) Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 108:271–277. https://doi.org/10.1016/j.envint.2017.08.020
Peters R, Ee N, Peters J et al (2019) Air pollution and dementia: a systematic review. J Alzheimers Dis 70:S145–S163. https://doi.org/10.3233/JAD-180631
Schraufnagel DE, Balmes JR, Cowl CT et al (2019) Air pollution and noncommunicable diseases. Chest 155:417–426. https://doi.org/10.1016/j.chest.2018.10.041
Li F, An Z, Li H et al (2019) Involvement of oxidative stress and the epidermal growth factor receptor in diesel exhaust particle-induced expression of inflammatory mediators in human mononuclear cells. Mediat Inflamm 2019:1–8. https://doi.org/10.1155/2019/3437104
Kohchi C, Inagawa H, Nishizawa T, Soma G (2009) ROS and innate immunity. Anticancer Res 29(3):817–821
Shukla A, Bunkar N, Kumar R et al (2019) Air pollution associated epigenetic modifications: transgenerational inheritance and underlying molecular mechanisms. Sci Total Environ 656:760–777. https://doi.org/10.1016/j.scitotenv.2018.11.381
Prunicki M, Cauwenberghs N, Ataam JA et al (2020) Immune biomarkers link air pollution exposure to blood pressure in adolescents. Environ health. https://doi.org/10.1186/s12940-020-00662-2
Glencross DA, Ho T-R, Camiña N et al (2020) Air pollution and its effects on the immune system. Free Radical Bio Med 151:56–68. https://doi.org/10.1016/j.freeradbiomed.2020.01.179
Serafini MM, Maddalon A, Iulini M, Galbiati V (2022) Air pollution: possible interaction between the immune and nervous system? Int J Env Res Pub He. https://doi.org/10.3390/ijerph192316037
Mostafavi N, Vlaanderen J, Chadeau-Hyam M et al (2015) Inflammatory markers in relation to long-term air pollution. Environ Int 81:1–7. https://doi.org/10.1016/j.envint.2015.04.003
Pope CA, Bhatnagar A, McCracken JP et al (2016) Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119:1204–1214. https://doi.org/10.1161/CIRCRESAHA.116.309279
Hajat A, Allison M, Diez-Roux AV et al (2015) Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation. Epidemiology 26:310–320. https://doi.org/10.1097/EDE.0000000000000267
Vawda S, Mansour R, Takeda A et al (2014) Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a huge systematic review. Am J Epidemiol 179:432–442. https://doi.org/10.1093/aje/kwt269
Chen R, Li H, Cai J et al (2018) Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Persp. https://doi.org/10.1289/EHP1447
Zhang X, Staimer N, Gillen DL et al (2016) Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ Res 150:306–319. https://doi.org/10.1016/j.envres.2016.06.019
Alfaro-Moreno E, Torres V, Miranda J et al (2009) Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environ Res 109:528–535. https://doi.org/10.1016/j.envres.2009.02.010
Becker S, Dailey L, Soukup JM et al (2005) TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharm 203:45–52. https://doi.org/10.1016/j.taap.2004.07.007
Epstein FH, Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 340:448–454. https://doi.org/10.1056/NEJM199902113400607
Azzouz M, Xu Y, Barregard L et al (2022) Air pollution and biomarkers of cardiovascular disease and inflammation in the Malmö diet and cancer cohort. Environ Health. https://doi.org/10.1186/s12940-022-00851-1
Leonardi GS, Houthuijs D, Steerenberg PA et al (2001) Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of central Europe. Inhal Toxicol 12:1–14. https://doi.org/10.1080/08958370050164833
Zhao J, Gao Z, Tian Z et al (2013) The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med 70:426–431. https://doi.org/10.1136/oemed-2012-100864
Wang H, Li X-B, Chu X-J et al (2022) Ambient air pollutants increase the risk of immunoglobulin e-mediated allergic diseases: a systematic review and meta-analysis. Environ Sci Pollut R 29:49534–49552. https://doi.org/10.1007/s11356-022-20447-z
Roberts LJ, Morrow JD (2000) Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Bio Med 28:505–513
Milne GL, Dai Q, Roberts LJ (2015) The isoprostanes—25 years later. BBA Mol Cell Biol L 1851:433–445. https://doi.org/10.1016/j.bbalip.2014.10.007
Kelly FJ (2003) Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60:612–616. https://doi.org/10.1136/oem.60.8.612
Wood LG, Gibson PG, Garg ML (2003) Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J 21:177–186. https://doi.org/10.1183/09031936.03.00017003a
Rossner P, Rossnerova A, Spatova M et al (2013) Analysis of biomarkers in a Czech population exposed to heavy air pollution. Part II: chromosomal aberrations and oxidative stress. Mutagenesis 28:97–106. https://doi.org/10.1093/mutage/ges058
Havet A, Zerimech F, Sanchez M et al (2018) Outdoor air pollution, exhaled 8-isoprostane and current asthma in adults: the EGEA study. Eur Respir J. https://doi.org/10.1183/13993003.02036-2017
Hashemzadeh B, Idani E, Goudarzi G et al (2019) Effects of PM2.5 and NO2 on the 8-isoprostane and lung function indices of FVC and FEV1 in students of Ahvaz city. Iran Saudi J Biol Sci 26:473–480. https://doi.org/10.1016/j.sjbs.2016.11.008
Li W, Wilker EH, Dorans KS et al (2016) Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham heart study. J Am Heart Assoc. https://doi.org/10.1161/JAHA.115.002742
De S, Khan Q, Kushwah GDS et al (2021) Oxidative biomarkers of exhaled breath condensate in adults exposed to traffic-related air pollution: a case-control study. J Breath Res. https://doi.org/10.1088/1752-7163/ac09fa
Brauer M, Brook JR, Christidis T et al (2019) Mortality-air pollution associations in low exposure environments (MAPLE): phase 1. Res Rep Health Eff Inst 2019(203):1–87
Brunekreef B, Strak M, Chen J et al (2021) Mortality and morbidity effects of long-term exposure to low-level PM2.5, BC, NO2, and O3 an analysis of European cohorts in the ELAPSE project. Res Rep Health Eff Inst 2021(208):1–127
Dominici F, Schwartz J, Di Q et al (2019) Assessing adverse health effects of long-term exposure to low levels of ambient air pollution: phase 1. Res Rep Health Eff Inst 2019(200):1–51
Chen J, Hoek G (2020) Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int. https://doi.org/10.1016/j.envint.2020.105974
Stafoggia M, Oftedal B, Chen J et al (2022) Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health 6:e9–e18. https://doi.org/10.1016/S2542-5196(21)00277-1
World Health Organization (2021). WHO global air quality guidelines. Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. WHO: Geneva. Licence: CC BY-NC-SA 3.0 IGO
Sicard P, Agathokleous E, De Marco A et al (2021) Urban population exposure to air pollution in Europe over the last decades. Environ Sci Eur. https://doi.org/10.1186/s12302-020-00450-2
European Environment Agency (2023) Europe’s air quality status 2023. Available via: https://www.eea.europa.eu/publications/europes-air-quality-status-2023. Accesed 8 Apr 2024.
Jiřík V, Machaczka O, Miturová H et al (2016) Air pollution and potential health risk in Ostrava region—a review. Cent Eur J Publ Heal 24:S4–S17. https://doi.org/10.21101/cejph.a4533
Michalik J, Machaczka O, Jirik V et al (2022) Air pollutants over industrial and non-industrial areas: historical concentration estimates. Atmosphere. https://doi.org/10.3390/atmos13030455
Czech Statistical Office (2021) Regional administration of the CZSO in české budějovice: characteristics of the region. https://www.czso.cz/csu/xc/charakteristika_kraje. 1 May 2023.
Machaczka O, Jiřík V, Janulková T et al (2023) Comparisons of lifetime exposures between differently polluted areas and years of life lost due to all-cause mortality attributable to air pollution. Environ Sci Eur. https://doi.org/10.1186/s12302-023-00778-5
Šlachtová H, Skýbová D, Dalecká A et al (2019) Healthy aging in the industrial environment - launch of the research project. Hygiena 64:31–34. https://doi.org/10.21101/hygiena.a1649
Czech Statistical Office (2020). European health interview survey (EHIS). https://www.czso.cz/csu/vykazy/european-health-interview-survey-ehis.1 May 2023
Steptoe A, Breeze E, Banks J, Nazroo J (2014) Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 42:1640–1648. https://doi.org/10.1093/ije/dys168
Žejglicová K, Kratěnová J, Lustigová M, Malý M (2016) Trends in health indicators in the urban middle-aged population in the Czech republic in 1998–2010. Public Health 137:81–87
Godin G (2011) The godin-shephard leisure-time physical activity questionnaire. Health Fit J Can 4(1):18–22
Watson NF, Badr MS, Belenky G et al (2015) Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep 38(6):843–844. https://doi.org/10.5665/sleep.4716
Merrill RM (2008) Environmental epidemiology principles and methods. Jones & Bartlett Learning
Unal I (2017) Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Method M 2017:1–14. https://doi.org/10.1155/2017/3762651
Gonzalez-Quintela A, Alende R, Gude F et al (2008) Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 151:42–50. https://doi.org/10.1111/j.1365-2249.2007.03545.x
Kanda N, Tamaki K (1999) Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immun 103:282–288. https://doi.org/10.1016/S0091-6749(99)70503-8
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11:411–423. https://doi.org/10.1093/humupd/dmi008
Giltay EJ, Fonk JCM, von Blomberg BME et al (2000) In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocr Metab 85:1648–1657. https://doi.org/10.1210/jcem.85.4.6562
Ferastraoaru D, Rosenstreich D (2018) IgE deficiency and prior diagnosis of malignancy. Ann Allerg Asthma Im 121:613–618. https://doi.org/10.1016/j.anai.2018.07.036
de Paula Couto TAP, Falsarella N, de Cassia Brandao de CM, de Mattos LC (2014) Total IgE plasma levels vary according to gender and age in Brazilian patients with allergic rhinitis. Clinics 69:740–744
Ichihara K, Itoh Y, Min W-K et al (2004) Diagnostic and epidemiological implications of regional differences in serum concentrations of proteins observed in six Asian cities. Clin Chem Lab Med. https://doi.org/10.1515/CCLM.2004.133
McMillan SA, Douglas JP, Archbold GP et al (1997) Effect of low to moderate levels of smoking and alcohol consumption on serum immunoglobulin concentrations. J Clin Pathol 50:819–822. https://doi.org/10.1136/jcp.50.10.819
Petta I, Fraussen J, Somers V, Kleinewietfeld M (2018) Interrelation of diet, gut microbiome, and autoantibody production. Front Immunol. https://doi.org/10.3389/fimmu.2018.00439
Tarbiah N, Todd I, Tighe PJ, Fairclough LC (2019) Cigarette smoking differentially affects immunoglobulin class levels in serum and saliva: an investigation and review. Basic Clin Pharmacol 125:474–483. https://doi.org/10.1111/bcpt.13278
Rohleder N, Aringer M, Boentert M (2012) Role of interleukin-6 in stress, sleep, and fatigue. Ann Ny Acad Sci 1261:88–96. https://doi.org/10.1111/j.1749-6632.2012.06634.x
Patel SR, Zhu X, Storfer-Isser A et al (2009) Sleep duration and biomarkers of inflammation. Sleep 32:200–204. https://doi.org/10.1093/sleep/32.2.200
Vgontzas AN, Papanicolaou DA, Bixler EO et al (1997) Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocr Metab 82:1313–1316. https://doi.org/10.1210/jcem.82.5.3950
Stenlof K, Wernstedt I, Fjallman T, Wallenius V, Wal-lenius K, Jansson JO (2003) Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J Clin Endocr Metab 88(9):4379–4383
El-Mikkawy DME, EL-Sadek MA, EL-Badawy MA, Samaha D (2020) Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt Rheumatol Rehabil. https://doi.org/10.1186/s43166-020-00003-8
Oltmanns U, Chung KF, Walters M et al (2005) Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Resp Res. https://doi.org/10.1186/1465-9921-6-74
Straczkowski M, Dzienis-Straczkowska S, Stêpieñ A et al (2002) Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-α system. J Clin Endocr Metab 87:4602–4606. https://doi.org/10.1210/jc.2002-020135
Sayyed AK, Despande KH, Suryakar AN et al (2008) Oxidative stress and serum α1—antitrypsin in smokers. Indian J Clin Bioche 23:375–377
Takei N, Suzuki M, Makita H et al (2019) Serum alpha-1 antitrypsin levels and the clinical course of chronic obstructive pulmonary disease/p. Int J Chronic Obstr 14:2885–2893
Ashley MJ (1987) Smoking, alpha 1-antitrypsin and decreased fertility in women. Med Hypotheses 22:277–285. https://doi.org/10.1016/0306-9877(87)90192-7
Petridou E, Chapuis-Cellier C, Roukas K, Lan SJ, Tzonou A, Trichopoulos D (1993) Tobacco smoking and other factors in relation to serum alpha-1-antitrypsin. Hum Biol 65(3):425–432
Linja-aho A, Mazur W, Toljamo T et al (2013) Distribution and levels of alpha-1-antitrypsin in the lung and plasma in smokers and chronic obstructive pulmonary disease. APMIS 121:11–21. https://doi.org/10.1111/j.1600-0463.2012.02936.x
Reilly M, Delanty N, Lawson JA, FitzGerald GA (1996) Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 94:19–25. https://doi.org/10.1161/01.CIR.94.1.19
Morrow JD, Frei B, Longmire AW et al (1995) Increase in circulating products of lipid Peroxidation (F 2 -isoprostanes) in smokers—smoking as a cause of oxidative damage. New Engl J Med 332:1198–1203. https://doi.org/10.1056/NEJM199505043321804
Needham M (2004) 1-antitrypsin deficiency * 3: clinical manifestations and natural history. Thorax 59:441–445. https://doi.org/10.1136/thx.2003.006510
Wood AM, Harrison RM, Semple S et al (2010) Outdoor air pollution is associated with rapid decline of lung function in-1-antitrypsin deficiency. Occup Environ Med 67:556–561. https://doi.org/10.1136/oem.2009.047589
Dziegielewska KM, Guminska M, Matthews N et al (1993) Reduced levels of alpha-1-antitrypsin in children exposed to high levels of air pollution. Biol Neonate 63(5):336–339. https://doi.org/10.1159/000243950
Vila N, Millán M, Ferrer X et al (2003) Levels of α 1 -antitrypsin in plasma and risk of spontaneous cervical artery dissections. Stroke. https://doi.org/10.1161/01.STR.0000085085.20390.A3
Sandström CS, Ohlsson B, Melander O et al (2008) An association between type 2 diabetes and α 1 -antitrypsin deficiency. Diabet Med 25:1370–1373. https://doi.org/10.1111/j.1464-5491.2008.02584.x
Bryan CL, Beard KS, Pott GB et al (2010) HIV infection is associated with reduced serum alpha-1-antitrypsin concentrations. Clin Invest Med 33:384–389. https://doi.org/10.25011/cim.v33i6.14589
Madar T, Shahaf G, Sheiner E et al (2013) Low levels of circulating alpha-1 antitrypsin are associated with spontaneous abortions. J Matern-Fetal Neonatal Med 26:1782–1787. https://doi.org/10.3109/14767058.2013.801955
Tripathy S, Marsland AL, Kinnee EJ et al (2021) Long-term ambient air pollution exposures and circulating and stimulated inflammatory mediators in a cohort of midlife adults. Environ Health Persp. https://doi.org/10.1289/EHP7089
Hadnagy W (1996) Immunological alterations in sera of persons living in areas with different air pollution. Toxicol Lett 88:147–153. https://doi.org/10.1016/0378-4274(96)03730-7
Guo H, Ahn S, Zhang L (2021) Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup Environ Med 78:377–384. https://doi.org/10.1136/oemed-2020-106517
Funding
This research was supported by the European Regional Development Fund under Grant “Healthy Aging in Industrial Environment-HAIE” (CZ.02.1.01/0.0/0.0/16_019/0000798).
Author information
Authors and Affiliations
Contributions
VJ and OM: conceptualisation of the work. OM, PR, and DK: original draft preparation. VJ, AA and OM: methodology. VJ: validation. OM, VJ, PR, and DK: data analysis and interpretation. OM: visualisation. VJ, GS, LO, EK, AA, JT and PR: review and editing. JT, PR: supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the OU Research Ethics Committee (no. 2/2018). All participants signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiřík, V., Machaczka, O., Riedlová, P. et al. The effect of air pollution on selected immune system parameters, 8-isoprostane, and alpha-1-antitrypsin of people living in environmentally distinct regions. Environ Sci Eur 36, 125 (2024). https://doi.org/10.1186/s12302-024-00948-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00948-z