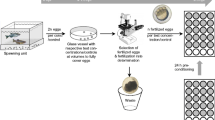
Abstract
Background
The fish embryo acute toxicity (FET) test with the zebrafish (Danio rerio) was developed to assess the acute fish toxicity of chemicals or environmental samples as a replacement for the Acute Fish Test (AFT) with juvenile fish. However, the FET is not yet established in the regulatory context. One reason is the (postulated) difference between the biotransformation capacities of embryos and juvenile fish.
The present study was designed to develop a procedure for external metabolization of test substances prior to testing in the FET. The workflow allows simultaneous exposure of the embryos to the maternal substances and their potential metabolites throughout the entire exposure period. After a 2 h incubation of the samples at 37 °C with non-toxic concentrations of a rat liver S9 homogenate or an animal-free (ewoS9R) metabolization system, freshly fertilized zebrafish embryos are added and incubated up to 120 h post-fertilization at 26 °C. Five biotransformable model substances (allyl alcohol, benzo[a]pyrene (B[a]P), chlorpyrifos (CP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and bisphenol A (BPA)) were evaluated for embryotoxicity with and without external metabolization.
Results
Only for allyl alcohol, external metabolization with both rat S9 and ewoS9R resulted in significantly higher embryotoxicity than under non-premetabolized conditions and, thus, in a better correlation of FET and AFT data. For B[a]P, CP, TDCPP and BPA, there was no relevant difference between data derived from the FET (with and without pre-metabolization) and literature AFT data; even though the FET results with and without pre-metabolization differed significantly for BPA (with rat S9 and ewoS9R) and TDCPP (rat S9 only).
Conclusions
External pre-metabolization appears a promising add-on to the FET protocol to improve the correlation with AFT data of certain biotransformable substances and might help to strengthen the FET as an alternative to the AFT and finally to reduce or replace sentient animals used for acute fish toxicity data in the regulatory context.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The acute fish test (AFT), according to OECD TG 203 [1], is commonly used in the regulatory context to screen for acute toxic effects in aquatic environments [2, 3]. Aquatic toxicity assessment is, therefore, deeply anchored in national and EU legislation. Acute fish data generated with juvenile fish are not only required for the registration of industrial chemicals exceeding production of 10 tons per year [4, 5], for plant protection products [6], veterinary medicines [7], biocides [8] and feed additives [9], but also for environmental monitoring, e.g., in the context of the European water framework directive [10]. As a consequence, the frequent application of OECD TG 203 [1] leads to a very high number of vertebrate experiments, which is not compatible with the 3Rs principle of replacing, reducing and refining animal experiments [11] and the increasing trend towards improvement of animal welfare in animal experimentation [12].
Therefore, the alternative new approach method (NAM) “fish embryo acute toxicity (FET) test” with zebrafish (Danio rerio) embryos according to OECD TG 236 [13] was developed as a substitute to the AFT [14,15,16,17,18,19]. Given that, in legal terms, embryos are not regarded as a protected life stage until they are self-feeding [20, 21], which for zebrafish happens from 120 h post-fertilization (hpf) [12], the FET is classified at least as a refinement, if not as a replacement to the AFT in the sense of Russel and Burch [11]. In various studies, a very good correlation between LC50 values from the FET and AFT has been reported [14, 18, 22,23,24].
However, the FET is still not accepted as a sole alternative to the AFT in most regulatory applications [25], except in waste water testing in Germany according to ISO 15088 [26]. One frequent argument is the assumption that embryos do not possess full metabolization capacities compared to juvenile fish relevant in AFT testing [27, 28]. Limited metabolization capacities of the embryo, however, could lead to an under- or overestimation of toxicity due to biotransformation processes catalyzed by various metabolization enzymes. In particular, one group of phase I metabolism enzymes mainly present in the liver, the cytochrome P450 monooxygenases (CYPs), are most important for bioactivation and detoxification processes as they are capable of catalyzing both oxidation and reduction reactions with broad substrate specificity, whereby the different CYP subfamilies have varying capacities for bioactivation and detoxification [29, 30].
There are various approaches across different levels of biological organization available to study and compare metabolic capacities of zebrafish at different developmental stages. However, due to the differences of applied methods and the still limited number of studies on embryonic and juvenile fish [27, 31], the comparison of metabolic capacities between zebrafish embryos and juveniles remains problematic. One method for the evaluation of metabolism differences is the consideration of the expression, biosynthesis and activity of metabolic enzymes. In a recent review, Loerracher and Braunbeck [31] pointed out that—whenever looked at in detail—all CYP activities could be demonstrated in the zebrafish embryo so far. However, in general, CYP activities [32,33,34,35,36,37] are comparatively low in Danio rerio up to 72/96 hpf compared to later larval developmental stage or in juvenile fish. More specifically, other studies by Verbueken et al. [37] and Otte et al. [38] demonstrated albeit low, but in some cases even constitutive CYP1A activity from the very beginning of zebrafish embryonic development. Another method to differentiate metabolism capacities is to study effects of individual pro-toxicants. In case of allyl alcohol, a drastic discrepancy between AFT and FET data is evident and could clearly be attributed to metabolic enzyme deficiency in zebrafish embryos [39].
To compensate for potential differences in metabolic capacities of zebrafish embryos and juveniles, previous studies included mammalian metabolic systems (rat liver microsomes) into the FET which incubated together with the fish eggs for a limited time of 1–4 h at 32 °C [40,41,42]. Due to the embryotoxicity of the microsomes [43], embryos could only be exposed for a limited time span before transfer to fresh medium for the further test duration, which ended at 48–144 hpf. In contrast, the present study was designed to clearly separate pre-metabolization at optimal mammalian temperature (37 °C) from exposure of the zebrafish embryos for a full exposure duration up to 120 hpf at 26 °C. Thus, from the very beginning of the assay, not only the maternal substances, but also potential metabolites can be tested for embryotoxicity. In addition, incubation of the embryos at temperatures above 26 °C can therefore be avoided, which might represent an additional stressor (besides exposure to the sample and metabolization enzymes).
In contrast to the studies mentioned above, the present study uses S9 homogenates instead of microsomal fractions, since the omission of one centrifugation step during production results in the persistence of cytosolic enzymes in addition to microsomal enzymes [44]. For comparison, two different S9 homogenates are used: A conventional rat liver homogenate chemically induced with phenobarbital/β-Naphthoflavone by ICCR (Roßdorf, Germany) is applied (rat S9), established in several in vitro assays (for example in mutagenicity and genotoxicity testing according to OECD TG 471 [45] and OECD TG 487 [46]). However, conventional rat liver homogenates (microsomes or S9) suffer from various shortcomings: (1) given that they originate from animal experiments, there is a conflict with the idea to establish the FET as an alternative to animal testing in the context of 3R and (2) the composition and activity (and toxicity) of rat liver homogenates may differ considerably even between batches from the same supplier [47]. To overcome the problem of animal experimentation for the production of S9 products, we also test an animal-free, biotechnologically produced S9 (ewoS9R, EWOMIS, Bruchköbel, Germany) produced under-defined conditions from suspensions of rat-derived liver cells cultured in chemically defined medium without animal-derived supplements such as fetal bovine serum. Comparable to the induction of rats for the production of conventional S9 products, rat liver cells are treated in vitro with chemical inducers to stimulate phase I metabolism with special focus on CYP1A activity. The subcellular fraction ewoS9R fraction is obtained by cell harvesting, washing, homogenization and using the supernatant after centrifugation at 9000g.
As model pro-toxicants, allyl alcohol, benzo[a]pyrene (B[a]P), chlorpyrifos (CP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and bisphenol A (BPA) are selected to evaluate the effects of rat S9 and ewoS9R, since all of these substances are metabolized via liver enzymes. The industrial chemical allyl alcohol is bioactivated via the cytosolic alcohol dehydrogenase 8a to the more toxic metabolite acrolein [39, 48, 49]. B[a]P, a polycyclic aromatic hydrocarbon, can be oxidized via microsomal CYP1A1/1A2/1B1 enzymes to various metabolites with genotoxic or endocrine disrupting properties [50,51,52,53]. The insecticide CP can be either bioactivated to the neurotoxic chlorpyrifos-oxon or detoxified to 3,5,6-trichloro-2-pyridinol via CYP2B6, 3A4, 2C19 and 1A2 activity [54,55,56,57]. TDCPP, an industrial chemical, is bioactivated by CYP2E1, 2D6, 1A2 and 2C19 present in liver S9 homogenates [58,59,60] to metabolites with elevated mutagenic activity after appropriate bioactivation [61]. The industrial chemical BPA is converted via CYPs 1A2, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, and 3A5 [62,63,64] to various metabolites with differential biological activity: Yoshihara et al. [65] and Jaeg et al. [66] showed that BPA is bioactivated by rat and mouse liver S9 to estrogenic or cytotoxic metabolites, whereas other studies reported lower estrogenic activity after metabolization [67] and lower acute toxicity of BPA metabolites in daphnids [68], if compared with the unmetabolized BPA.
To evaluate the external pre-metabolization, the present study will (a) identify optimal exposure concentrations not affecting embryonic development of both metabolization systems (rat S9 and ewoS9R) including the critical energy carrier β-dihydronicotinamide–adenine dinucleotide phosphate (NADPH), necessary for CYP activity [69] and (b) assess the metabolism capacity of both S9 homogenates exemplary for CYP1A1/1A2/1B1 activity via the fluorescence-based EROD assay [70,71,72,73]. Furthermore, embryotoxic effects of five biotransformable model substances with and without pre-metabolization will be evaluated for toxicity changes and whether external pre-metabolization in the FET increases the comparability of FET to AFT literature data, which could contribute to the acceptance of FET data and, thus, the refinement or even replacement of whole animal testing in regulatory aquatic (eco)toxicology.
Methods
Materials and stock solutions
Details on the origin of chemicals and materials used can be found in the Additional file 1: Tables S1–2, respectively. Stock solutions of benzo[a]pyrene (B[a]P, 0.25–10 mM), bisphenol A (BPA, 5–40 mM), chlorpyrifos (CP, 1–100 mM) and tris(1,3-dichloro-2-propyl)phosphate (TDCPP, 0.1–33 mM) were prepared in dimethyl sulfoxide (DMSO) and stored in brown-glass vials at 4 °C. Allyl alcohol was used as a liquid without solvent [54, 57]. The β-dihydronicotinamide–adenine dinucleotide phosphate (NADPH) stock solution (20 mM) was prepared freshly for each test day in artificial water (294 mg/L CaCl2, 123 mg/L MgSO4, 63 mg/L NaHCO3, 5.5 mg/L KCl, according to ISO 15088 [26]). Artificial water was prepared 1 day before each test day and aerated for at least 1 h; the pH was adjusted to 7.8 ± 0.2.
The rat S9 (protein concentration 33 mg/ml) was obtained from male rats (Wistar, ICCR, Roßdorf, Germany), which were induced by triplicate oral administration of 80 mg/kg body weight phenobarbital and β-naphthoflavone on 3 consecutive days. The rat S9 was stored at -80 °C and thawed maximum twice. The lyophilized ewoS9R was produced from a permanent rat liver cell line cultivated in an animal-reagent-free culture medium, and the cells were induced with CYP1A activators. One vial of lyophilized ewoS9R (stored at room temperature) was dissolved in 469 µl artificial water on the test day to adjust the resulting protein concentration to 4 mg/ml.
Zebrafish maintenance and egg production
Adult zebrafish (wild-type Danio rerio, age of 1–2 years) from the facilities of the Department of Evolutionary Ecology and Environmental Toxicology (Goethe University Frankfurt) were kept in 170 L aquaria with a flow-through system using remineralized osmosis water (26 ± 1 °C, water exchange rate of 30% per week, remineralization with NaHCO3 and sea salt (Aquaforest, Brzesko, Poland). The tank water was purified by UV-light and a biological filter. The zebrafish were fed with dry food (zebrafish SDS, Claus GmbH, Limburgerhof, Germany) twice a day and Artemia sp. (Sanders® Great Salt Lake; Ogden, Utah, USA) once a day. A constant day/night rhythm was maintained at 14/10 h. Plant imitates (weathered inert green filter material) and spawning trays prepared with 1 mm spawning grids were used for egg collection. Mass spawning with 150–200 fish was applied for egg production. Courtship and oviposition began about half an hour after the onset of light, and fertilized eggs were collected at latest 1 h after the onset of spawning.
Fish embryo acute toxicity (FET) test without metabolization
The fish embryo acute toxicity test was performed in a static setup according to OECD TG 236 [13] and Schiwy et al. [74] with the minor modifications mentioned below. Effects and lethality (for endpoints, see Additional file 1: Table S3) were recorded at 48 and shortly before 120 hpf. The test was terminated shortly before 120 hpf, and fish were euthanized by immersion in benzocaine (40 g/L in 96% ethanol).
Test substances were first examined in a standard FET to generate reference data. Therefore, the following assay concentration ranges were used: allyl alcohol 0.5–5,000 µM, B[a]P 0.25–10 µM, BPA 5–40 µM, CP 1–100 µM and TDCPP 0.1–33 µM. Test solutions were prepared by diluting stock solutions with artificial water in glass crystallizing dishes. For B[a]P, BPA, CP and TDCPP, a final DMSO concentration of 0.1% (v/v) was used. From the crystallization dishes, 10 fertilized eggs per test solution were transferred to glass-coated 96-well plates (due to hydrophobicity of the test substances) filled with 200 µl of test solution and sealed with a gas-permeable foil. Due to its liquid, hydrophilic and volatile properties, no solvent was necessary for allyl alcohol; and polystyrene well plates were double-sealed with polyester cover foils to minimize evaporation. Each test was complemented with artificial water as a negative control, 0.1% DMSO as a solvent control and 4 mg/L 3,4-dichloroaniline as a positive control. The embryos were incubated at 26 ± 1 °C until 120 hpf with a light/dark cycle of 14/10 h.
Fish embryo acute toxicity (FET) test with external pre-metabolization
Range-finding experiments were carried out to identify optimal concentrations of rat liver S9, ewoS9R and NADPH for external metabolization, for a detailed description, see Additional file 1: Method M1. For the metabolization with S9 homogenates, exposure solutions were prepared in crystallization dishes (B[a]P, BPA, CP and TDCPP) or in 6- or 24-well plates (allyl alcohol) with the same concentration ranges as mentioned above. In addition, 0.5 mM NADPH in combination with 0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R were added to the test solutions. The S9 homogenates were kept on ice when not in use. Dishes and well plates were sealed with parafilm® or polyester cover foils, respectively, and incubated for 2 h on a shaking incubator at 37 °C (optimal temperature for mammalian enzymes). Runs with 0.1% DMSO were added as a process control and two S9 homogenate process controls (0.1% DMSO + 0.5 mM NADPH + 0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R) were included in the metabolization step. After metabolization, the solutions were acclimated to 26 °C before transferring the fertilized eggs for the FET exposure solutions. The tests with pre-metabolization were carried out as described for the tests without pre-metabolization. All experiments were performed in 3–4 independent biological replicates. All data presented originated from experiments, which met the validity criteria set by OECD 236 [13]: with < 10% mortality in negative, solvent and process controls as well as > 30% mortality in the 3,4-dichloroaniline positive control at 120 hpf and ≥ 80% hatching rate in the negative and solvent control at 120 hpf.
For data analysis, concentration–response curves were generated using a non-linear regression model (least squares fit, variable slope, four parameters, constrains of bottom = 0% and top = 100%) in the software GraphPad Prism 9 (GraphPad Software, Boston, USA). The statistical comparison of concentration–response curves was carried out using an extra-sum-of-squares F test with a p value of 0.025 for two pairs of analysis (or 0.0083 in the case of BPA with four pairs of analysis). The statistical significance of different curve parameters (EC50 and hillslope) was calculated to compare the treatments including the metabolization via rat S9 or ewoS9R with the experiments without metabolization. If 50% effect could not be generated in the concentration–effect curves, only hill slopes were compared statistically between the treatments.
Impact of S9-proteins on bioavailability of chemicals
To evaluate whether the addition of S9 homogenates had an impact on the bioavailability of the test compounds, a case study with BPA was conducted. The FET methodology was conducted as described above without the energy source NADPH, but with the S9 homogenates. If similar embryotoxicities between approaches with and without NADPH were observed, this indicated binding of the model substance to the S9 proteins, but no detoxification via the metabolization enzymes.
Biochemical determination of ethoxyresorufin-O-deethylase (EROD) activities
To compare the CYP1A1/1A2/1B1 activities of the S9 homogenates, an EROD assay was conducted according to Donato and Gómez-Lechón [71]. In brief, 10 µM ethoxyresorufin, 100 µM dicumarol, 0.5 mM NADPH and 0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R were mixed in artificial water in a final volume of 200 µl in a 96-well plate. Assays were conducted under light-reduced settings to account for the light sensitivity of ethoxyresorufin. The S9 homogenates were stored on ice and were added immediately before the start of the measurement. In addition, a negative control (artificial water, 10 µM ethoxyresorufin, 100 µM dicumarol, 0.5 mM NADPH) without S9 homogenates was included for each test. The fluorescence at excitation/emission wavelengths of Em: 588 nm/Ex: 635 nm was measured kinetically for 4.5 h with a Spark multimode plate reader (Tecan, Crailsheim, Germany) pre-heated to 37 °C. The test was carried out in 3 biological replicates and 3 technical repetitions. Mean (calculated from 3 biological replicates) in relative fluorescent units (RFUs) measured with S9 homogenates minus the RFUs of the negative control were plotted against time.
Results
The range-finding experiments for the pre-metabolization step (Fig. 1 and Additional file 1: Fig. S1) revealed that 3.5 h incubation at 37 °C with a combination of 0.5 mM NADPH together with 0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R did not produce any symptoms of lethal and sublethal toxicity in the FET (≤ 10% effects at 120 hpf).
Identification of optimal pre-metabolization conditions: Effects [%] in the FET with Danio rerio at 120 h post-fertilization (hpf) after incubation at 37 °C with different NADPH-concentrations and different rat S9 (A) oder ewoS9R (B) concentrations for 3.5 h before addition of zebrafish eggs. Dotted lines indicate 10% effect (considered the non-effect threshold according to OECD TG 236). Data given as mean values ± standard deviation from independent biological replicates (n = 3–4). 0 = no effects detected, x = experiment not conducted
In these settings, both S9 homogenates clearly showed an EROD activity (Fig. 2 and Additional file 1: Fig. S2). The amount of resorufin formed increases with the addition of both S9 homogenates over the 4.5-h period. Both S9 homogenates show a steeper slope within the first 2 h followed by a gradual levelling off. The increase in EROD activity relative to the negative control was higher with rat S9 (relative fold-increase over controls: 39.1) than with ewoS9R (fold-increase: 3.8).
EROD activity respresented as relative fluorescence units (RFUs) at measuring wavelenghts of 588/635 nm in presence of 0.5 mM NADPH, 100 µM dicoumarol and 0.01 mg/ml rat S9 ( −) or 0.05 mg/ml ewoS9R ( −), respectively. Mean and error bars show relative RFU values corrected for RFUs of the negative control (n = 3) with standard deviation
In FET experiments amended with external pre-metabolization, the effects after 48 hpf (Additional file 1: Fig. S5) and 120 hpf (Fig. 3) for the five model substances were recorded with and without metabolization. The calculated EC50 values are shown in Additional file 1: Table S4. At the end of the FET tests (120 hpf), resulting embryotoxicity was significantly different after metabolization for three of five model substances (allyl alcohol, TDCPP, BPA).
Biotransformation potential of the two metabolization systems within the FET using Danio rerio embryos: The effects at 120 h post-fertilization (hpf) of allyl alcohol (A), TDCPP (B), bisphenol A (C), benzo[a]pyrene (D) and chlorpyrifos (E) are shown with or without the addition of 0.01 mg/ml rat S9 and 0.05 mg/ml ewoS9R under addition of 0.5 mM NADPH. Controls contained S9 homogenates and NADPH at the same concentrations w/o chemicals. Data are given as means ± standard deviation (n = 3–4). Model for nonlinear regression (GraphPad Prism 9): [agonist] vs. response, variable slope (four parameters), bottom = 0%, top = 100%. *Significant differences of the best-fit values (hillslope, EC50) with S9 homogenate compared to no addition of an external metabolization system
For allyl alcohol in combination with rat S9 and ewoS9R, a significantly higher embryotoxicity was observed compared to allyl alcohol without metabolization at both 48 and 120 hpf, with metabolic activation being stronger with rat S9 than with ewoS9R (Fig. 3 and Additional file 1: Fig. S5). The most frequent effects at 120 hpf without metabolization were coagulation and cardio-vascular effects; in contrast, with metabolization coagulation was most frequent (Additional file 1: Fig. S4).
For TDCPP, the two external metabolization system differed regarding their effects at both time points. At 120 hpf, the addition of rat S9 significantly increased TDCPP toxicity, whereas no significant difference was found for ewoS9R compared to effects of the unmetabolized substance (Fig. 3). However, at 48 hpf (Additional file 1: Fig. S5), the difference was significant with both S9 homogenates, and TDCPP proved more toxic with ewoS9R than with rat S9. The profile of frequent observed effects (see SI, Additional file 1: Fig. S4) was similar comparing the approaches with and without metabolization with coagulation being the most observed effect. However, the percentage of cardio-vascular effects increased with the addition of S9 homogenates.
The implementation of S9 homogenates into the FET with BPA resulted in both a decrease or increase of toxicity depending on time of development. At 120 hpf, addition of both S9-homogenates resulted in significantly lower toxicity of BPA (Fig. 3), with no hatching and cardio-vascular effects being the most frequently observed embryotoxic effects (with and without metabolization, Additional file 1: Fig. S4). In contrast, at 48 hpf (Additional file 1: Fig. S5), a reaction opposite to 120 hpf were seen, with the BPA toxicity being significantly higher only when adding ewoS9R compared to no addition metabolization.
To exclude lower toxicity due to binding to S9 homogenate proteins, S9 homogenates without addition of the energy carrier NADPH were also tested with BPA (Additional file 1: Fig. S3). Without NADPH, the proteins of S9 did not significantly alter the embryotoxicity, as the concentration–response curves were almost identical to the concentration–response relationship of chemical exposure without any S9 addition.
For B[a]P, no significant differences were found between treatments without or with addition of S9 homogenates at both 48 and 120 hpf (Fig. 3 and Additional file 1: Fig. S5). Due to precipitation at concentrations > 10 µM, a full concentration–response relationship could not be established for B[a]P. As the overall number of effects with B[a]P was very low, no frequent effects were reported in Additional file 1: Fig. S4.
Likewise, for CP, no complete concentration–response relationship could be generated due to precipitation at > 100 µM, and no significant differences could be recorded for treatment with and without S9 homogenates at 48 and 120 hpf (Fig. 3 and Additional file 1: Fig. S5). However, a slight increase in toxicity was evident when ewoS9R (48 and 120 hpf) or rat S9 (120 hpf) were added. Frequent effects without pre-metabolization at 120 hpf were cardio-vascular effects, coagulation, no hatching as well as pericardial and yolk sac edemata (Additional file 1: Fig. S4). However, following addition of S9 homogenates, coagulation was the most frequently observed effect.
Discussion
Pre-metabolization for the fish embryo toxicity test (FET)
Inclusion of an external metabolization step prior to initiation of the actual FET to allow continuous exposure of the embryos/larvae to the metabolites bears several challenges:
Temperature
First, the protocol for this study was designed to allow optimal temperatures for each system: 37 °C for incubation with the mammalian S9 homogenates and 26 °C for exposure of zebrafish embryos. For mammalian enzymes, 37 °C is usually the optimal temperature, and a lower temperature results in lower enzyme activity [75]. For zebrafish, however, higher temperatures may cause an acceleration of development [76], which compromises the comparability with FETs performed at 26 °C according to OED TG 236 [13] and may lead to conflicts with animal welfare regulations. Even more important, increased temperature over the entire test period might cause heat stress for the embryos: Although Kimmel et al. [76] did not detect developmental abnormalities at incubation temperatures up to 33 °C in zebrafish, a study by Long et al. [77] demonstrated altered gene expression using an increased temperature of 34 °C. Thus, elevated temperatures in the test create a third stressor in addition to the test compound and the addition of S9 homogenates. By separating a 2 h pre-exposure at 37 °C (without the addition of eggs) from the FET itself (26 °C), such additional stress could be avoided.
Toxicity of S9 homogenates
Both the S9 homogenates and the energy source NADPH showed embryotoxic effects in the FET. Given that effects on zebrafish embryos by liver homogenates have also been documented in literature [43], only short exposure periods of embryos to high concentrations of microsomes (0.7 mg/ml) were performed in previous studies using external metabolism [40,41,42,43]. In range-finding experiments, pre-metabolization with 1 mg/ml S9 homogenates at 37 °C followed by removal of S9 homogenates using (1) a dialysis membrane or (2) heat denaturation and centrifugation to expose the embryos to the metabolites for 120 hpf (details not shown) did not effectively eliminate the embryotoxicity of the S9 homogenates. Consequently, experiments were carried out to optimize protein contents during pre-exposure metabolization: Since the combination of the S9 homogenates (EC10 values: ~ 0.08 mg/ml) and the cofactor NADPH (EC10: 0.47 mM) proved even more toxic than the isolated components (Fig. 1 and Additional file 1: Fig. S1), final concentrations of 0.01 mg/ml (rat S9) or 0.05 mg/ml (ewoS9R) together with 0.5 mM NADPH were in the final experiments.
Species-origin of S9 homogenates
Mammalian metabolization systems have repeatedly been used for the inclusion into the FET [40,41,42]. However, a difference between the metabolization capacities of mammals and fish must be considered: Given that freshly isolated rat hepatocytes have been described to be usually metabolically more competent than fish liver cells [78], rat homogenates were preferred. Hence, the mammalian metabolization within the FET could be described as a conservative and protective approach, but using fish liver homogenates (e.g., according to OECD TG 319B [79]) should also be tested in future studies to strengthen the informative value for ecotoxicology risk assessment (for reference, see [80,81,82]). Given that the FET was designed as an alternative method to conventional animal testing [83] in the context of the 3Rs [11], the primary goal of the present study was the supplementation with a biotechnological product (such as ewoS9R), a product that is derived completely in vitro, and not from animal experiments. To the best of our knowledge, biotechnological approaches using animal-free cultures with fish cells have not been developed so far.
Comparison of FET (with and without metabolism) to AFT data
The comparison of experimental and literature-based FET (without and with pre-metabolization) and AFT data for model substances allyl alcohol, TDCPP, BPA, B[a]P and CP are summarized in Table 1.
If compared to 120 hpf FET EC50 value without pre-metabolization, allyl alcohol proved to be 9–17-fold more toxic in FETs after pre-metabolization with either S9 homogenate. This significant difference can be explained by the presence or absence of alcohol dehydrogenases, which bioactivate allyl alcohol to the more toxic metabolite acrolein [39, 48, 49]. In Danio rerio embryos, alcohol dehydrogenases are present at low levels [39], but are contained in rat S9 homogenates, thus demonstrating the ability to bioactivate allyl alcohol [84, 85]. Elevated concentrations of alcohol dehydrogenases were likely present in the rat S9 homogenate, since despite the lower protein content (0.01 mg/ml), the toxicity of allyl alcohol with rat S9 was higher than after pre-metabolization than with ewoS9R (0.05 mg/ml). This difference might be explained by the origin of the two S9 products: The biotechnologically produced ewoS9R was derived from a rat liver cell line, which had been induced for high CYP1A activity, but has never been optimized for alcohol dehydrogenase contents. In contrast, the rat S9 comprises various cell types induced with a combination of β-naphthoflavone and phenobarbital, which most likely stimulated a broader spectrum of metabolic enzymes including alcohol dehydrogenases.
The strong impact of bioactivation on allyl alcohol and hence the improvement of FET for protective risk assessment becomes clear when comparing FET with AFT data. If the standard FET without metabolization [13] was used as the basis for the risk assessment, the FET LC50 would be 220–1715 times higher than the corresponding AFT LC50. Based on the chemical safety criteria according to ECHA guidance [86], an assessment/safety factor of 1000 would be applied to acute LC50/EC50 fish toxicity data to determine the predicted no-effect concentration (PNEC). Thus, based on FET data without an additional metabolism, the environmental risk for allyl alcohol would clearly be underestimated, if compared to a risk assessment on the basis of the LC50 derived from the AFT (4.8 µM according to Klüver et al. [39]), which would be below or close to the calculated literature (8.2 µM; [39]) or experimental (1.1 µM) PNEC derived from FET data. With a 18–35-fold difference from AFT data, LC50 data from FETs with pre-metabolization provide a much better data base for calculating PNEC values (86.5 nM and 166.6 nM) than conventional FET data.
For TDCPP, a significant bioactivation was found in the FET at 120 hpf after pre-metabolization with rat S9, but not with ewoS9R. TDCPP is metabolized via CYPs 2E1, 2D6, 1A2 and 2C19 [58]. Given that during ewoS9R optimization, the focus was exclusively on CYP1A induction, the formation of the embryotoxic metabolites was likely lower using ewoS9R compared to rat S9, which most likely covers a broader range of enzyme activities. Despite the small differences in the calculated EC50 values, curves differ regarding the hillslope, resulting in larger differences for the EC10 values. Zebrafish embryos with pre-metabolization were slightly more sensitive to TDCPP (LC50 4.8/5.2 µM) than embryos without pre-metabolization (LC50 5.9 µM), which in turn were slightly more sensitive than juvenile zebrafish (LC50 10.6 µM; [87]). Interestingly, after 48 h exposure in the FET, the addition of ewoS9R resulted in a significantly higher than the addition of pre-metabolization with the rat S9; likewise, the hill slopes were different, if compared to the results without metabolization. The stronger effects at 48 hpf might be due to transient increase of toxic metabolites at the beginning of the FET followed by time-dependent degradation during subsequent development [32, 33, 37].
In the case of BPA, significant detoxification was detected following addition of both S9 homogenates. As with TDCPP, the addition of both S9 homogenates led to similar EC50 values compared to the reference without metabolization, but the hillslope of the curve with rat S9 deviated strongly from the reference without metabolization, which led to stronger differences in the EC50 values. A direct comparison to AFT data was not possible, since 50% mortality could not be reached with the concentration range used in this study. However, considering the increase of EC50 values after detoxification, an approximation to zebrafish AFT LC50 can be assumed. In contrast to 120 hpf, significant bioactivation after 48 hpf exposure in the FET was observed after pre-metabolization with ewoS9R (reduced/no pigmentation, edemata, and reduced/no blood flow). Bioactivation in the FET at 48 hpf, but detoxification at 120 hpf might reflect ambiguous data in the literature, which cover both bioactivation [65, 66] and detoxification [67, 68]. As for TDCPP, the transient formation of reactive metabolites followed by detoxification in subsequent stages of development might have triggered such a biphasic reaction of zebrafish metabolites to BPA in the FET. In fact, the presumably rapid development of metabolic capacities in zebrafish embryos from 72/96 hpf should also be considered for BPA metabolization [32, 34,35,36,37, 88]. Furthermore, hatching inhibition as a pronounced effect during later development also reported by Scopel et al. [89] can, of course, only be observed at 120 hpf. Chemical analysis of BPA and potential metabolites might help to clarify the time course of (transient) formation of metabolites via S9 homogenates or the embryos themselves.
To exclude reduced bioavailability due to binding of the test substance to the proteins of the S9 homogenates as a reason for reduced toxicity as previously demonstrated for other substance classes [90], additional experiments were carried out without the energy carrier NADPH, which, however revealed that BPA was detoxified by the S9 homogenates, since the results without the energy carrier did not differ statistically from the reference without S9.
Due to precipitation at concentrations > 10 µM, no concentration-dependent embryotoxicity could be established for B[a]P, which is in line with literature AFT data on B[a]P, which also showed no toxicity. Only Weigt et al. [91] reported a very low LC50 of 5.1 µM B[a]P in the FET. The difference could be related to the usage of 0.5% DMSO as a solvent, which may have improved the chorionic permeability of the compound [88]. B[a]P is bioactivated by the presence of CYP1A1/1A2/1B1, which was detected by the EROD assay in rat S9 and ewoS9R and was also already proven to be bioactivated in in vitro assays by rat S9 and ewoS9R [53].
As for B[a]P, exposure to CP did not result in differences between FETs with and without pre-metabolization, and there was only a minor trend towards bioactivation. This observation, however, is not in line with literature, which report LC50 values of 2.0 µM (AFT, [92]) and 3.7 µM (FET, [93]). In fact, in our experiments, precipitation occurred at concentrations > 100 µM, most likely due to the hydrophobic character of CP (logP of 4.98 [94]). Low bioavailability due to binding of CP to the vessels should have been avoided with the use of glass-coated well plates. In the FET study by Zhang et al. [93], a semi-static test design was applied, and Jeon et al. [92] conducted the AFT with glassware and acetone as solvent. Possibly the slightly different test conditions led to different outcomes.
Conclusions
To summarize, the implementation of an external pre-metabolization step into the FET significantly increased the comparability with the AFT data for allyl alcohol (cf. Table 2). For the other test compounds, this clear conclusion could not be drawn, and observed minor increases/decreases of toxicity as well as putative approximations to AFT data due to the inclusion of pre-metabolization in FET cannot to be rated as biologically relevant. The similarity of AFT and FET data (with or without pre-metabolization) for the biotransformable substances TDCPP, BPA, B[a]P and CP might well be due to comparable biotransformation of the substances by embryonic and juvenile zebrafish, or to a neglectable metabolization rate under the FET/AFT test conditions. Regarding the biological relevance of minor toxicity changes with metabolization, it should be mentioned that not only FET, but—and usually to a greater extent—also AFT data are subject to intra- and inter-laboratory variability [3]. Conventional risk assessment is usually based on AFT data from different species (i.e., not only Danio rerio); thus, inter-species variability must also be considered when comparing and interpreting differences between FET and AFT data [3]. Nevertheless, FET data obtained within one laboratory conducted with and without the implementation of S9 homogenates can indicate if metabolism significantly alters toxicity and, therefore, whether metabolism might be relevant in the context of the tested sample.
Since a very good correlation between FET and AFT data has repeatedly been documented [14, 18, 22], FETs with pre-metabolization could be applied in the future specifically for selected cases only: (1) the methodology might be applied if existing data or QSAR data indicate relevant biotransformation of the substance. (2) Complex environmental samples could also be tested in FET with and without the addition of S9 homogenates, since for such samples the impact of biotransformation on fish toxicity is extremely difficult to predict.
To further validate the FET with metabolism, other substances with and without metabolism should be tested for which FET and AFT data differ strongly (e.g., albendazole [40]). In addition, other endpoints such as early life stage behavior according to Irons et al. [99] could be investigated with a procedure including pre-metabolization to investigate metabolization in the context of neurotoxic substances. For example, behavioral changes could be investigated with the substance CP as it is metabolized to the neurotoxic metabolite CP-oxone [100].
In addition, to further increase the ecotoxicological relevance of biotransformation processes, (biotechnological) fish S9 should also be examined in the future [79]. In addition, cofactors required for phase II metabolism should be added to the assay in future studies, which could also be relevant for some substances [101]. Here, additional phase II cofactors would also have to be tested for their embryotoxicity, which might affect the concentration of the S9 mixes or NADPH that can be applied. Furthermore, the inclusion of S9 homogenates into the FET could alter the uptake of the substances into the fish embryo cells and the cell excretion via transporters, as the substances properties after metabolization are changed and thus fish embryo cellular transporters could be induced or inhibited via the metabolites [102,103,104,105]. Furthermore, a time-dependent chemical analysis of the metabolites formed by the zebrafish embryos or S9 homogenates might help to gain further insights into the metabolism capacities of the different systems.
Overall, this study showed that the FET with metabolization may be able to compensate for strong differences between FET and AFT data. Using this pre-metabolisation method, the predictive ability of the FET for toxicity to juvenile fish could be improved, if there is evidence of strong biotransformation of the sample. This may reduce or eliminate the need to use juvenile or adult vertebrates for acute ecotoxicological data in future risk assessment.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. Pre-experimental data including the removal of s9 mixes via dialysis or heat denaturation are available from the corresponding author on reasonable request.
Abbreviations
- AFT:
-
Acute Fish Test
- FET:
-
Fish embryo acute toxicity test
- B[a]P:
-
Benzo[a]pyrene
- CP:
-
Chlorpyrifos
- TDCPP:
-
Tris(1,3-dichloro-2-propyl) phosphate
- CYP:
-
Cytochrome P450 monooxygenases
- NADPH:
-
β-Dihydronicotinamide–adenine dinucleotide phosphate
- DMSO:
-
Dimethyl sulfoxide
- hpf:
-
Hours post-fertilization
References
OECD TG 203. Test no. 203: fish acute toxicity test.: OECD guideline for testing of chemicals. OECD 2019.
Burden N, Benstead R, Benyon K, Clook M, Green C, Handley J et al (2020) Key opportunities to replace, reduce, and refine regulatory fish acute toxicity tests. Environ Toxicol Chem 39:2076–2089. https://doi.org/10.1002/etc.4824
Paparella M, Scholz S, Belanger S, Braunbeck T, Bicherel P, Connors K et al (2021) Limitations and uncertainties of acute fish toxicity assessments can be reduced using alternative methods. Altex 38:20–32. https://doi.org/10.14573/altex.2006051
EC No 1907. Regulation (EC) No 1907/2006 of the european parliament and of the council: concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a european chemicals agency, amending directive 1999/45/EC and repealing council regulation (EEC) No 793/93 and commission regulation (EC) No 1488/94 as well as council directive 76/769/EEC and commission directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Official Journal of the European Union. 2006.
ECHA Guidance R.7b. Guidance on information requirements and chemical safety assessment: Chapter R.7b: endpoint specific guidance. ECHA Guidance. 2017;4:30.
EU No 284. Comission regulation (EU) no 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with regulation (EC) No 1107/2009 of the european parliament and of the council concerning the placing of plant protection products on the market. Official Journal of the European Union. 2013.
CVMP/VICH/790/03. Committee for medicinal products for veterinary use (CVMP): Guideline on environmental impact assessment for veterinary medicinal products phase II. International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. 2004.
EU No 528. Regulation (EU) No 528/2012 of the european paliament and of the council of 22 May 2012 concerning the making available on the market and use of biocidal products. Official Journal of the European Union. 2012.
EC No 429. Comission regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of regulation (EC) No 1831/2003 of the european parliament and of the council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. Official Journal of the European Union. 2008.
Water Framework Directive 2000/60/EC. Directive 2000/60/EC of the european parliament and of the council of 23 (2000) establishing a framework for community action in the field of water policy. Off J Eur Communities 2000:1–73
Russell W, Burch RL (1959) The principles of humane experimental technique. Methuen & Co LTD, London
Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S et al (2012) Zebrafish embryos as an alternative to animal experiments - a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33:128–132. https://doi.org/10.1016/j.reprotox.2011.06.121
OECD TG 236. Test no. 236: fish embryo acute toxicity (FET) test. Paris: OECD Publishing; OECD 2013.
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T (2009) Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C Toxicol Pharmacol 149:196–209. https://doi.org/10.1016/j.cbpc.2008.11.006
Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE et al (2010) The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat Toxicol 97:79–87. https://doi.org/10.1016/j.aquatox.2009.12.008
Halder M, Léonard M, Iguchi T, Oris JT, Ryder K, Belanger SE et al (2010) Regulatory aspects on the use of fish embryos in environmental toxicology. Integr Environ Assess Manag 6:484–491. https://doi.org/10.1002/ieam.48
Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R (2015) The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res Int 22:16247–16261. https://doi.org/10.1007/s11356-014-3814-7
Belanger SE, Rawlings JM, Carr GJ (2013) Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ Toxicol Chem 32:1768–1783. https://doi.org/10.1002/etc.2244
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ et al (2014) OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69:496–511. https://doi.org/10.1016/j.yrtph.2014.05.018
Opinion EFSA (2005) Opinion of the scientific panel on animal health and welfare (AHAW) on a request from the commission related to the aspects of the biology and welfare of animals used for experimental and other scientific purposes. EFSA J 3:292. https://doi.org/10.2903/j.efsa.2005.292
Directive 2010/63/EU. Directive 2010/63/EU of the european parliament and of the council of 22 september 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union. 2010.
Nagel R (2002) DarT: The embryo test with the Zebrafish Danio rerio-a general model in ecotoxicology and toxicology. Altex 19(Suppl 1):38–48
Su T, Lian D, Bai Y, Wang YYL, Zhang D, Wang Z, You J (2021) The feasibility of the zebrafish embryo as a promising alternative for acute toxicity test using various fish species: a critical review. STOTEN 787:147705. https://doi.org/10.1016/j.scitotenv.2021.147705
Birke A, Scholz S (2019) Zebrafish embryo and acute fish toxicity test show similar sensitivity for narcotic compounds. Altex 36:131–135. https://doi.org/10.14573/altex.1808101
Sobanska M, Scholz S, Nyman A-M, Cesnaitis R, Gutierrez Alonso S, Klüver N et al (2018) Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of registration, evaluation, authorisation, and restriction of chemicals (REACH). Environ Toxicol Chem 37:657–670. https://doi.org/10.1002/etc.4055
ISO 15088. Water quality - Determination of the acute toxicity of waste water to zebrafish eggs (Danio rerio): European Standard. International Organization for Standardization ISO 2007. doi:https://doi.org/10.31030/1495364.
Braunbeck T, Böhler S, Knörr S, Lörracher A-K, Pelka K, Kais B. Development of an OECD guidance document for the application of OECD test guideline 236 (acute fish embryo toxicity test): final report. Umweltbundesamt Dessau-Roßlau. 2020.
Scholz S, Klüver N, Kühne R. Analysis of the relevance and adequateness of using Fish Embryo Acute Toxicity (FET) Test Guidance (OECD 236) to fulfil the information requirements and addressing concerns under REACH.: Report ECHA-UFZ contract ECHA/2014/341. European Chemicals Agency. 2016.
Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM (2011) Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci 120(Suppl 1):S49-75. https://doi.org/10.1093/toxsci/kfq338
Ioannides C, Lewis DFV. Cytochromes P450 in the bioactivation of chemicals: Bentham Science Publishers; 2004.
Loerracher A-K, Braunbeck T (2021) Cytochrome P450-dependent biotransformation capacities in embryonic, juvenile and adult stages of zebrafish (Danio rerio)-a state-of-the-art review. Arch Toxicol 95:2299–2334. https://doi.org/10.1007/s00204-021-03071-7
Bräunig J, Schiwy S, Broedel O, Müller Y, Frohme M, Hollert H, Keiter SH (2015) Time-dependent expression and activity of cytochrome P450 1s in early life-stages of the zebrafish (Danio rerio). Environ Sci Pollut Res Int 22:16319–16328. https://doi.org/10.1007/s11356-015-4673-6
Kais B, Schiwy S, Hollert H, Keiter SH, Braunbeck T (2017) In vivo EROD assays with the zebrafish (Danio rerio) as rapid screening tools for the detection of dioxin-like activity. STOTEN 590–591:269–280. https://doi.org/10.1016/j.scitotenv.2017.02.236
Meyer-Alert H, Ladermann K, Larsson M, Schiwy S, Hollert H, Keiter SH (2018) A temporal high-resolution investigation of the Ah-receptor pathway during early development of zebrafish (Danio rerio). Aquat Toxicol 204:117–129. https://doi.org/10.1016/j.aquatox.2018.09.007
Schiwy S, Bräunig J, Alert H, Hollert H, Keiter SH (2015) A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res Int 22:16305–16318. https://doi.org/10.1007/s11356-014-3185-0
Otte JC, Schultz B, Fruth D, Fabian E, van Ravenzwaay B, Hidding B, Salinas ER (2017) Intrinsic xenobiotic metabolizing enzyme activities in early life stages of zebrafish (Danio rerio). Toxicol Sci 159:86–93. https://doi.org/10.1093/toxsci/kfx116
Verbueken E, Bars C, Ball JS, Periz-Stanacev J, Marei WFA, Tochwin A et al (2018) From mRNA expression of drug disposition genes to in vivo assessment of CYP-mediated biotransformation during zebrafish embryonic and larval development. Int J Mol Sci. https://doi.org/10.3390/ijms19123976
Otte JC, Schmidt AD, Hollert H, Braunbeck T (2010) Spatio-temporal development of CYP1 activity in early life-stages of zebrafish (Danio rerio). Aquat Toxicol 100:38–50. https://doi.org/10.1016/j.aquatox.2010.07.006
Klüver N, Ortmann J, Paschke H, Renner P, Ritter AP, Scholz S (2014) Transient overexpression of adh8a increases allyl alcohol toxicity in zebrafish embryos. PLoS ONE. https://doi.org/10.1371/journal.pone.0090619
Mattsson A, Ullerås E, Patring J, Oskarsson A (2012) Albendazole causes stage-dependent developmental toxicity and is deactivated by a mammalian metabolization system in a modified zebrafish embryotoxicity test. Reprod Toxicol. https://doi.org/10.1016/j.reprotox.2012.02.007
Busquet F, Nagel R, von Landenberg F, Mueller SO, Huebler N, Broschard TH (2008) Development of a new screening assay to identify proteratogenic substances using zebrafish Danio rerio embryo combined with an exogenous mammalian metabolic activation system (mDarT). Toxicol Sci 104:177–188. https://doi.org/10.1093/toxsci/kfn065
Weigt S, Huebler N, Braunbeck T, von Landenberg F, Broschard TH (2010) Zebrafish teratogenicity test with metabolic activation (mDarT): effects of phase I activation of acetaminophen on zebrafish Danio rerio embryos. Toxicology 275:36–49. https://doi.org/10.1016/j.tox.2010.05.012
Pype C, Verbueken E, Saad MA, Bars C, van Ginneken CJ, Knapen D, van Cruchten SJ (2017) Antioxidants reduce reactive oxygen species but not embryotoxicity in the metabolic Danio rerio test (mDarT). Reprod Toxicol. https://doi.org/10.1016/j.reprotox.2017.06.132
Richardson SJ, Bai A, Kulkarni AA, Moghaddam MF (2016) Efficiency in drug discovery: liver S9 fraction assay as a screen for metabolic stability. Drug Metab Lett 10:83–90. https://doi.org/10.2174/1872312810666160223121836
OECD TG 471. Bacterial reverse mutation test: OECD guideline for testing of chemicals. OECD 1997:1–11.
OECD TG 487. In vitro mammalian cell micronucleus test.: Guideline for the testing of chemicals. OECD 2016:1–29.
Brendt J, Crawford SE, Velki M, Xiao H, Thalmann B, Hollert H, Schiwy A (2021) Is a liver comparable to a liver? A comparison of different rat-derived S9-fractions with a biotechnological animal-free alternative in the Ames fluctuation assay. STOTEN 759:143522. https://doi.org/10.1016/j.scitotenv.2020.143522
Atzori L, Dore M, Congiu L (1989) Aspects of allyl alcohol toxicity. Drug Metabol Drug Interact 7:295–319
Auerbach SS, Mahler J, Travlos GS, Irwin RD (2008) A comparative 90-day toxicity study of allyl acetate, allyl alcohol and acrolein. Toxicology 253:79–88. https://doi.org/10.1016/j.tox.2008.08.014
Gelboin HV (1980) Benzoalphapyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev 60:1107–1166. https://doi.org/10.1152/physrev.1980.60.4.1107
Miller KP, Ramos KS (2001) Impact of cellular metabolism on the biological effects of benzoapyrene and related hydrocarbons. Drug Metab Rev 33:1–35. https://doi.org/10.1081/DMR-100000138
Bukowska B, Mokra K, Michałowicz J (2022) Benzoapyrene-environmental occurrence, human exposure, and mechanisms of toxicity. Int J Mol Sci. https://doi.org/10.3390/ijms23116348
Reichstein IS, König M, Wojtysiak N, Escher BI, Henneberger L, Behnisch P et al (2023) Replacing animal-derived components in in vitro test guidelines OECD 455 and 487. STOTEN 868:161454. https://doi.org/10.1016/j.scitotenv.2023.161454
Croom EL, Wallace AD, Hodgson E (2010) Human variation in CYP-specific chlorpyrifos metabolism. Toxicology 276:184–191. https://doi.org/10.1016/j.tox.2010.08.005
Choi K, Joo H, Rose RL, Hodgson E (2006) Metabolism of chlorpyrifos and chlorpyrifos oxon by human hepatocytes. J Biochem Mol Toxicol 20:279–291. https://doi.org/10.1002/jbt.20145
Crane AL, Klein K, Olson JR (2012) Bioactivation of chlorpyrifos by CYP2B6 variants. Xenobiotica 42:1255–1262. https://doi.org/10.3109/00498254.2012.702246
Sams C, Cocker J, Lennard MS (2004) Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP). Xenobiotica 34:861–873. https://doi.org/10.1080/00498250400017273
Chen M-H, Zhang S-H, Jia S-M, Wang L-J, Ma W-L (2022) In vitro biotransformation of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate by mouse liver microsomes: kinetics and key CYP isoforms. Chemosphere 288:132504. https://doi.org/10.1016/j.chemosphere.2021.132504
Nomeir AA, Kato S, Matthews HB (1981) The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (fyrol FR-2) in the rat. Toxicol Appl Pharmacol 57:401–413. https://doi.org/10.1016/0041-008x(81)90238-6
van den Eede N, Maho W, Erratico C, Neels H, Covaci A (2013) First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett 223:9–15. https://doi.org/10.1016/j.toxlet.2013.08.012
Gold MD, Blum A, Ames BN (1978) Another flame retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science 200:785–787. https://doi.org/10.1126/science.347576
Niwa T, Fujimoto M, Kishimoto K, Yabusaki Y, Ishibashi F, Katagiri M (2001) Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol Pharm Bull 24:1064–1067. https://doi.org/10.1248/bpb.24.1064
Nakamura S, Tezuka Y, Ushiyama A, Kawashima C, Kitagawara Y, Takahashi K et al (2011) Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol Lett 203:92–95. https://doi.org/10.1016/j.toxlet.2011.03.010
Schmidt J, Kotnik P, Trontelj J, Knez Ž, Mašič LP (2013) Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes. Toxicol In Vitro 27:1267–1276. https://doi.org/10.1016/j.tiv.2013.02.016
Yoshihara S, Makishima M, Suzuki N, Ohta S (2001) Metabolic activation of bisphenol A by rat liver S9 fraction. Toxicol Sci 62:221–227. https://doi.org/10.1093/toxsci/62.2.221
Jaeg JP, Perdu E, Dolo L, Debrauwer L, Cravedi J-P, Zalko D (2004) Characterization of new bisphenol a metabolites produced by CD1 mice liver microsomes and S9 fractions. J Agric Food Chem 52:4935–4942. https://doi.org/10.1021/jf049762u
Kang J-H, Katayama Y, Kondo F (2006) Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicology 217:81–90. https://doi.org/10.1016/j.tox.2005.10.001
Ike M, Chen M-Y, Jin C-S, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of biodegradation products of bisphenol-A. Environ Toxicol 17:457–461. https://doi.org/10.1002/tox.10079
Guengerich FP (2018) Mechanisms of cytochrome P450-catalyzed oxidations. ACS Catal 8:10964–10976. https://doi.org/10.1021/acscatal.8b03401
Burke M, Thompson S, Weaver RJ, Wolf C, Mayers RT (1994) Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem Pharmacol 48:923–936. https://doi.org/10.1016/0006-2952(94)90363-8
Donato MT, Gómez-Lechón MJ. Fluorescence-based screening of cytochrome P450 activities in intact cells. In: Cytochrome P450 Protocols: Humana Press, Totowa, NJ; 2013. p. 135–148. https://doi.org/10.1007/978-1-62703-321-3_12.
Montellano PRO de. Cytochrome P450. Structure, mechanism, and biochemistry. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2005.
Ghosal A, Hapangama N, Yuan Y, Lu X, Horne D, Patrick JE, Zbaida S (2003) Rapid determination of enzyme activities of recombinant human cytochromes P450, human liver microsomes and hepatocytes. Biopharm Drug Dispos 24:375–384. https://doi.org/10.1002/bdd.374
Schiwy S, Herber A-K, Hollert H, Brinkmann M (2020) New insights into the toxicokinetics of 3,4-dichloroaniline in early life stages of zebrafish (Danio rerio). Toxics. https://doi.org/10.3390/toxics8010016
Gurumurthy P, Mannering GJ (1985) Membrane bound cytochrome P-450 determines the optimal temperatures of NADPH-cytochrome P-450 reductase and cytochrome P-450-linked monooxygenase reactions in rat and trout hepatic microsomes. Biochem Biophys Res Commun 127:571–577. https://doi.org/10.1016/S0006-291X(85)80198-4
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Long Y, Li L, Li Q, He X, Cui Z (2012) Transcriptomic characterization of temperature stress responses in larval zebrafish. PLoS ONE 7:e37209. https://doi.org/10.1371/journal.pone.0037209
Nabb DL, Mingoia RT, Yang C-H, Han X (2006) Comparison of basal level metabolic enzyme activities of freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat. Aquat Toxicol 80:52–59. https://doi.org/10.1016/j.aquatox.2006.07.012
OECD TG 319B. Determination of in vitro intrinsic clearance using rainbow trout liver S9 subcellular fraction (RT-S9): OECD GUIDELINE FOR TESTING OF CHEMICALS. OECD 2018.
Johanning K, Hancock G, Escher B, Adekola A, Bernhard MJ, Cowan-Ellsberry C et al (2012) Assessment of metabolic stability using the rainbow trout (Oncorhynchus mykiss) liver S9 fraction. Curr Protoc Toxicol 53:14. https://doi.org/10.1002/0471140856.tx1410s53
Kropf C, Begnaud F, Gimeno S, Berthaud F, Debonneville C, Segner H (2020) In vitro biotransformation assays using liver S9 fractions and hepatocytes from rainbow trout (Oncorhynchus mykiss): overcoming challenges with difficult to test fragrance chemicals. Environ Toxicol Chem 39:2396–2408. https://doi.org/10.1002/etc.4872
Nichols J, Fay K, Bernhard MJ, Bischof I, Davis J, Halder M et al (2018) Reliability of in vitro methods used to measure intrinsic clearance of hydrophobic organic chemicals by rainbow trout: results of an international ring trial. Toxicol Sci 164:563–575. https://doi.org/10.1093/toxsci/kfy113
Braunbeck T, Böttcher M, Hollert H, Kosmehl T, Lammer E, Leist E et al (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: The fish embryo toxicity test goes multi-species–an update. Altex 22:87–102
Serafini-Cessi F (1972) Conversion of allyl alcohol into acrolein by rat liver. Biochem J 128:1103–1107. https://doi.org/10.1042/bj1281103
Patel JM, Wood JC, Leibman KC (1980) The biotransformation of allyl alcohol and acrolein in rat liver and lung preparations. Drug Metab Dispos 8:305–308
ECHA Guidance. Guidance on information requirements and chemical safety assessment: Chapter R.10: characterisation of dose [concentration]-response for environment; 2008.
Wang G, Shi H, Du Z, Chen H, Peng J, Gao S (2017) Bioaccumulation mechanism of organophosphate esters in adult zebrafish (Danio rerio). Environ Pollut 229:177–187. https://doi.org/10.1016/j.envpol.2017.05.075
Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T (2013) DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquat Toxicol 140–141:229–238. https://doi.org/10.1016/j.aquatox.2013.05.022
Scopel CFV, Sousa C, Machado MRF, Santos WGD (2021) BPA toxicity during development of zebrafish embryo. Braz J Biol 81:437–447. https://doi.org/10.1590/1519-6984.230562
Gülden M, Mörchel S, Tahan S, Seibert H (2002) Impact of protein binding on the availability and cytotoxic potency of organochlorine pesticides and chlorophenols in vitro. Toxicology 175:201–213. https://doi.org/10.1016/S0300-483X(02)00085-9
Weigt S, Huebler N, Strecker R, Braunbeck T, Broschard TH (2011) Zebrafish (Danio rerio) embryos as a model for testing proteratogens. Toxicology 281:25–36. https://doi.org/10.1016/j.tox.2011.01.004
Jeon H-J, Lee Y-H, Kim M-J, Choi S-D, Park B-J, Lee S-E (2016) Integrated biomarkers induced by chlorpyrifos in two different life stages of zebrafish (Danio rerio) for environmental risk assessment. Environ Toxicol Pharmacol 43:166–174. https://doi.org/10.1016/j.etap.2016.03.010
Zhang J, Liu L, Ren L, Feng W, Lv P, Wu W, Yan Y (2017) The single and joint toxicity effects of chlorpyrifos and beta-cypermethrin in zebrafish (Danio rerio) early life stages. J Hazard Mater 334:121–131. https://doi.org/10.1016/j.jhazmat.2017.03.055
Hui TJ, Ariffin MM, Tahir NM (2010) Adsorption of formulated chlorpyrifos on selected agricultural soils of Terengganu. Malaysian J Analy Sci. 14:76–81
Godfrey A, Abdel-Moneim A, Sepúlveda MS (2017) Acute mixture toxicity of halogenated chemicals and their next generation counterparts on zebrafish embryos. Chemosphere 181:710–712. https://doi.org/10.1016/j.chemosphere.2017.04.146
Chow WS, Chan WK-L, Chan KM (2012) Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J Appl Toxicol 33:670–678. https://doi.org/10.1002/jat.2723
ECHA dossier. Registration dossier 4,4'-isopropylidenediphenol: Short-term toxicity to fish. https://echa.europa.eu/registration-dossier/-/registered-dossier/15752/6/2/2#. Accessed 13 Jun 2023.
Mohanty R, Das SK, Singh NR, Patri M (2016) Withania somnifera leaf extract ameliorates benzoapyrene-induced behavioral and neuromorphological alterations by improving brain antioxidant status in zebrafish (Danio rerio). Zebrafish 13:188–196. https://doi.org/10.1089/zeb.2015.1215
Irons TD, MacPhail RC, Hunter DL, Padilla S (2010) Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol 32:84–90. https://doi.org/10.1016/j.ntt.2009.04.066
Richardson RJ (1995) Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J Toxicol Environ Health 44:135–165. https://doi.org/10.1080/15287399509531952
Iyanagi T (2007) Molecular mechanism of phase I and phase II drug-metabolizing enzymes: implications for detoxification. Int Rev Cytol 260:35–112. https://doi.org/10.1016/S0074-7696(06)60002-8
Luckenbach T, Fischer S, Sturm A (2014) Current advances on ABC drug transporters in fish. Comparative biochemistry and physiology. Toxicol Pharmacol 165:28–52. https://doi.org/10.1016/j.cbpc.2014.05.002
Fischer S, Klüver N, Burkhardt-Medicke K, Pietsch M, Schmidt A-M, Wellner P et al (2013) Abcb4 acts as multixenobiotic transporter and active barrier against chemical uptake in zebrafish (Danio rerio) embryos. BMC Biol 11:69. https://doi.org/10.1186/1741-7007-11-69
Döring B, Petzinger E (2014) Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug Metab Rev 46:261–282. https://doi.org/10.3109/03602532.2014.882353
Matthee C, Brown AR, Lange A, Tyler CR (2023) Factors determining the susceptibility of fish to effects of human pharmaceuticals. Environ Sci Technol 57:8845–8862. https://doi.org/10.1021/acs.est.2c09576
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was funded by the German Federal Institute for Risk Assessment Grant Agreement Number 60–0102-01.P608.
Author information
Authors and Affiliations
Contributions
IR and AB conducted the experiments and visualized, analyzed and interpreted the resulting data. Additionally, IR was major contributor in writing the first draft of the manuscript. SJ, TB, SS and AS were contributing to the methodology and the review and editing process of the manuscript. HH contributed to the supervision and resources important for the conduction of the project. AS, SS and HH did the conceptualization of the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that might influence the work or interpretation of the data reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of chemicals. Table S2. List of materials. Table S3. List of lethal-equivalent and sublethal endpoints in the Fish Embryo Acute Toxicity Test (FET). Table S4. EC50, EC10 and hill slope values derived from FET data in zebrafish (Danio rerio) embryos at 120 h post fertilization (hpf) for the five model pro-toxicants. EC50, EC10 and hill slope values were calculated with (w/) or without (w/o) addition of S9 homogenates (0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R combined with 0.5 mM NADPH); 95% profile likelihood confidence limits in brackets. n.d. = not determinable. Figure S1. Range-finding experiments for the identification of optimal pre-metabolization conditions. Effects [%] of two S9 homogenates and NADPH zebrafish (Danio rerio) embryos at 120 h post fertilization (hpf) in the FET. Model for nonlinear regression (GraphPad Prism 9): [agonist] vs. response, variable slope (four parameters), bottom = 0%, top = 100%. Dotted lines indicate 10% effect (considered the non-effect threshold according to OECD TG 236). Data given as mean values ± standard deviation from independent biological replicates (n = 3 – 4). Figure S2. EROD activity of S9 homogenates respresented as nmol formed resorufin/mg proteinS9-homogenate in presence of 0.5 mM NADPH and 100 µM dicoumarol. The amount of resorufin formed was calculated by additionally recording the fluoresence of resorufin at excitation/emission wavelenghts of 588 nm/635 nm (concentration range 0—4 µM) in the presence of 0.5 mM NADPH and 100 µM dicoumarol (see Formula S1). The mean and error bars show the calculated nmol resorufin formed/mg protein (n = 3) with standard deviation. Figure S3. Concentration–response curve (concentration [μM] vs. effects [%] in zebrafish (Danio rerio) embryos at 120 h post fertilization (hpf) after expsoure to bisphenol A without NADPH and with or without addition of S9 homogenates. S9 homogenates were used at the concentrations of 0.01 mg/ml (rat S9) or 0.05 mg/ml (ewoS9R). Process controls contained S9 homogenates only and NADPH at the same concentrations, but no test substance. Data given as mean values ± standard deviatiom from n = 3—4 independent biological replicates. Model for nonlinear regression (GraphPad Prism 9): [agonist] vs. response, variable slope (four parameters), bottom = 0%, top = 100%. * Significant differences of the best-fit calues (hill slope, EC50) with S9 homogenate from runs without addition of metabolization. Figure S4. Overview on the type and quantity ratios of the effects after exposure to model substances at 120 h post fertilization (hpf)) in the FET with Danio rerio. For this, the observed effects of all tested concentrations were summed up to determine the percentage frequency of the individual effects. Blood congestion, no/reduced heartbeat, no/slow blood flow and heart deformations are all considered as “cardio-vascular effects”. The S9-homogenates were used at the concentrations of 0.01 mg/ml (rat S9) or 0.05 mg/ml (ewoS9R) together with or without 0.5 mM NADPH. Figure S5. Biotransformation potential of two metabolization systems within the FET using Danio rerio embroys at 48 h post fertilization (hpf). Allyl alcohol (A), TDCPP (B), bisphenol A (C), benzo[a]pyrene (D), chlorpyrifos (E) were tested with or without the addition of 0.01 mg/ml rat S9 or 0.05 mg/ml ewoS9R together with 0.5 mM NADPH. Process controls contained only S9 homogenates and NADPH at the same concentrations w/o chemicals. Data are given as means ± standard deviation (n = 3–4). Model for nonlinear regression (GraphPad Prism 9): [agonist] vs. response, variable slope (four parameters), bottom = 0%, top = 100%. *Significant differences of the best-fit values (hillslope, EC50) with S9 homogenate compared to no addition of an external metabolization system.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reichstein, I.S., Becker, A.H., Johann, S. et al. Biotechnological metabolization system has the potential to improve the predictive ability of the fish embryo acute toxicity (FET) test with the zebrafish (Danio rerio). Environ Sci Eur 36, 91 (2024). https://doi.org/10.1186/s12302-024-00913-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00913-w