Abstract
Dietary exposure to heavy metals and metalloids [metal(loid)s] deserves increased attention; however, there is a lack of a comprehensive understanding of the risks of dietary exposure from multi-type food intake and multi-metal(loid) exposure using a bioaccessibility-corrected method. Here, we determined the concentration and bioaccessibility of six toxic metal(loid)s in six major food groups in an industrial city with intensive smelting activity. By incorporating children’s dietary patterns, the probability distribution of health risks from dietary metal(loid)s exposure was assessed. Marked differences were observed in the dietary exposure characteristics within metal(loid)s and major food groups. Cereal and vegetables were the major food groups local children intake. Cereals, meat, beans, and aquatic products had the highest concentrations of Cu and Cd, Pb and Cr, Ni, and As, respectively. Generally, meat, eggs, and aquatic products exhibited higher bioaccessibility than cereals, beans, and vegetables. The bioaccessibility of metal(loid)s decreased in the following order: Pb > Cr > Cu > Ni > As > Cd. For non-carcinogenic risks, the average hazard index was 7.3, with 99.6% exceeding acceptable levels. Arsenic was the dominant risk element and aquatic product was the main risk source. The aggregated carcinogenic risk was 3.4 × 10–3, with Cr the major risk contributor and cereals and meat the dominant food sources. The findings demonstrated that (1) the metal(loid) dietary exposure risk of children from areas with intensive smelting activities should be taken seriously, and (2) it is necessary to identify the specific food category and metal(loid) based on a comprehensive analysis of the metal(loid) concentration and bioaccessibility, and dietary habits of the population to effectively reduce risk.
Similar content being viewed by others
Introduction
The health risks associated with heavy metal and metalloid [metal(loid)] exposure have received considerable attention worldwide, with food ingestion being the major exposure route [1, 2]. Dietary metal(loid) exposure could cause a series of adverse effects on human health, especially for children due to their underdeveloped immune systems and immature behaviors [3,4,5]. For instance, Tsuji et al. [6] found an association between low-level As ingestion and developmental neurotoxicity in children. Yan et al. [7] found that diet contributed approximately 87% of Pb exposure in children, and the burden of mild intellectual disability resulting from dietary Pb exposure was 36.64 disability-adjusted life years per 1000 children in China. Shen et al. [8] found that multi-metal (Pb, Mn, Sb, Sn, Ti) ingestion exposure could lead to a decline in the IQ scores of children. Therefore, the health hazards associated with dietary exposure to metal(loid)s in children deserves attention.
To assess these health risks, different types of food were sampled, dietary habits were investigated, and health risks were generally obtained by combining metal(loid) concentrations in food and the corresponding consumption rates in previous studies [9, 10]. However, most studies have focused on the health risks of consuming a single food category, such as rice [11, 12], aquatic products [13, 14], and vegetables [15,16,17,18]. Therefore, there is still a knowledge gap in the comprehensive understanding of dietary metal(loid) exposure via multiple types of food, which may hinder the precise identification and management of the exposure risks, especially for people with diverse dietary patterns.
In addition, whether a metal(loid) in food affects human health also depends on the dissolution and release characteristics of the element in the human digestive system. Thus, the introduction of bioaccessibility can notably improve the accuracy of health risk assessments. Xu et al. [19] have shown that bioaccessibility-adjusted dietary Cd exposure could predict internal exposure more accurately. However, research on the bioaccessibility of metal(loid)s in various food categories is still lacking, and a few studies on limited food types have found that bioaccessibility varied widely among food categories and metal(loid)s. For example, Cr, Pb, Cu, Zn, and Cd bioaccessibility in shellfish ranged from 25.0 to 61.9% [13], and As bioaccessibility was 82.3% in fish [20], 26.7% in rice [20], 60.1% in radish [21], and 76.8% in shrimp [13]. Thus, it is necessary to introduce bioaccessibility into dietary metal(loid) exposure risk assessments via the intake of diverse food categories, rendering it possible to accurately identify the preferentially controlled metal(loid) and food categories.
Here, both the concentrations and bioaccessibility of Pb, Cr, Cd, Ni, Cu, and As in six major food groups from an industrial city with intensive smelting activities in Northwest China were determined, and a dietary questionnaire survey of local children was conducted. The Monte Carlo Simulation method was used to assess the probability distribution of the health risk. This study aimed to (i) investigate the distribution patterns of metal(loid)s in six major food groups; (ii) explore the discrepancy in bioaccessibility across metal(loid)s and food groups; (iii) estimate the health risk resulting from the intake of various food groups; and (iv) discuss the health risk contribution of each food group and metal(loid). In addition, these findings will provide a scientific basis for effective risk reduction measures.
Method
Study area
The study site was in Baiyin, Gansu Province, Northwest China, in the middle and temperate continental arid and semi-desert climatic zones (Fig. 1). The annual average temperature is 6–9 ℃ and annual rainfall 180–450 mm, mostly concentrated in July, August, and September and accounting for > 60% of the annual precipitation. The annual evaporation reaches 1500–1600 mm, 4.5 times the average precipitation. Baiyin is rich in mineral resources and known as “China's Copper City”. Smelting forms the backbone of this region, and the soil is mainly loess and anthropogenic alluvial. This could represent the characteristics of food metal(loid) exposure in this type of polluted area.
Questionnaire survey
The dietary questionnaires were answered by 200 children, aged 6–11 years, who were randomly recruited from three local primary schools. The information collected included: (1) sociodemographics, including age, height, weight, and sex; and (2) food intake frequency (the unit is times per week or times per day), including the frequency of commonly consumed food categories—classified into six categories: cereals, vegetables, beans, meat, eggs, and aquatic products—and the corresponding consumption (the unit is gram per time). The food categories considered in this study were a subset of food groups from the six China Total Diet Surveys [22, 23]. The intake rate of each food category for each child was calculated by multiplying the intake frequency by the corresponding consumption. In addition, zero was assigned to the intake rate of a specific food category for child who reported to have no eating behavior for that food category. The total dietary intake was calculated as the sum of cereals, vegetables, beans, meat, eggs, and aquatic products, and the contribution rate was calculated by dividing the individual food category intake by the total dietary intake.
Sample collection and preparation
Based on the children’s dietary habits from the questionnaire survey, one to three food types that local children commonly consumed were sampled for each food group. In particular, rice and steamed bun were collected as cereals; Chinese cabbage and potatoes were sampled as vegetables; soybeans were collected as beans; pork, beef, and mutton were sampled as meat; carps were collected as aquatic products; and chicken eggs were sampled as eggs. Moreover, eight parallel samples were collected from eight local markets for each food type to ensure sampling representativeness. Thus, a total of 80 food samples were obtained.
The wet weight of each food sample was measured after removing inedible parts, cleaning with deionized water, and wiping with filter paper. All samples were then freeze-dried to a constant weight using a vacuum freeze dryer, and the dry weights were measured again. Finally, the freeze-dried food samples were ground and passed through a 2-mm sieve.
Sample pretreatment
A 0.25 g subsample was placed in an acid-washed Teflon tube and digested with 3 mL HNO3 and 2 mL H2O2 (both chromatographic grade, Sinopharm Chemical Reagent Co., Ltd, Beijing, China) using a microwave digestion system (CEM, MARS-6, USA). The digestion program was as follows: first the temperature was gradually increased to 120 ℃ and maintained for 10 min, then increased to 160 ℃ and maintained for 10 min, finally increased to 180 ℃ and maintained for 15 min. After digestion, the solution was diluted to 25 mL with deionized water and stored at 4 °C prior to analysis.
In vitro digestion method
The physiologically based extraction test (PBET) method was selected to determine bioaccessibility through the oral route. This method, developed by Ruby [24], was the earliest in vitro digestion method based on the principle of human physiology, involving the simulation of the gastric and intestinal stages. Moreover, the PBET method can better predict metal(loid) bioaccessibility in food than other in vitro assays [17].
This model comprised a two-step procedure involving the gastric and intestinal stages. The simulated gastric fluid (1 L) consisted of 1.25 g pepsin (Sigma-Aldrich, St. Louis, USA), 0.5 g sodium malate (Aladdin, Shanghai, China), 0.5 g sodium citrate, and 420 μL lactic acid (Aladdin, Shanghai, China), with the pH adjusted to 2.5 ± 0.1 by HCl (chromatographic grade, Sinopharm Chemical Reagent Co., Ltd, Beijing, China), while the simulated intestinal fluid (1 L) contained 0.5 g trypsin (Sigma-Aldrich, St. Louis, USA), 0.5 g pancreatin (Sigma-Aldrich, St. Louis, USA), and 1.75 g bile salts (Sigma-Aldrich, St. Louis, USA), with the pH adjusted to 5.50 ± 0.05 using a saturated NaHCO3 (chromatographic grade, Aladdin, Shanghai, China) solution.
The food sample (0.5 g) was placed in a centrifuge tube containing 50 mL of simulated gastric fluid. The mixture was then placed in a constant temperature water bath shaker (SHZ-C, Shanghai, China) for 1 h, with the temperature and speed of the shaker controlled at 37 ℃ and 150 rpm, respectively. The mixture pH was adjusted to 2.5 ± 0.1. After 1 h, 5 mL of the gastric digestion solution was collected for metal(loid) analysis.
The remaining solution was then modified to 50 mL simulated intestinal fluid. The mixture was placed in a constant temperature water bath shaker for 4 h, with the temperature and speed of the shaker controlled at 37 ℃ and 150 rpm, respectively. During the reaction, mixture pH was adjusted to 5.5 ± 0.05. After 4 h, 5 mL of the intestinal digestion solution was collected for metal(loid) analysis.
The liquid collected from the gastric and intestinal stage was centrifuged at 4000 rpm for 10 min. The supernatant was collected, filtered through a 0.22-μm membrane, and then stored at 4 ℃ prior to analysis.
Sample determination
The Cr, Cd, Pb, Ni, Cu, and As concentrations in the food samples were determined using high-resolution inductively coupled plasma mass spectrometry (ICP-MS) (Agilent-7500a, Agilent Scientific Technology Ltd., USA) under optimized condition. The main ICP-MS operating parameters for the determination of metal(loid) concentrations are listed in Additional file 1: Table S2.
We used a series of quality control measures to guarantee the accuracy and reliability of sample pretreatment and determination. First, each digestion batch included 10% reagent blank, parallel, and spiked samples. During the testing procedure, metal(loid) concentrations were also determined in certified reference materials of spinach (GBW10015), cabbage (GBW10014), wheat (GBW10011a), and chicken (GBW10018), obtained from the National Research Center for Certified Reference Materials, Beijing, China. Certified reference materials (10%) were run for each testing batch to maintain instrument calibration. In addition, replicated analyses (accounting for 10% of each sample batch) were also determined to calculate the coefficient of variation and validate the analytical precision. The detection limits for Cr, Cd, Pb, Ni, Cu, and As ranged from 4.0 to 20 μg kg−1. The coefficient of variation for parallel measurements was < 5.0%, and the recovery rates of the spiked samples ranged from 94.8 to 111.4% (Additional file 1: Table S1).
Exposure assessment
Human exposure to food primarily occurs through oral ingestion. For the non-carcinogenic contaminant, the average daily dose (ADD, mg kg−1 day−1) via food intake was calculated referring to the model first put forward by the US Environmental Protection Agency [25]:
where C is the metal(loid) concentration in a specific food group (mg kg−1); IR is the ingestion rate of a specific food group in mg day−1; BA is the bioaccessibility for a specific metal(loid) in a specific food group; EF is the exposure frequency in day year−1; ED is the exposure duration in year; BW is children’s body weight in kg; and AT is the average exposure time in days.
For the carcinogenic contaminant, the lifetime average daily dose (LADD, mg·kg−1 day−1) was calculated using the following formula [25]:
where LT is the lifetime in days. The exposure factors obtained from the questionnaire survey here and from the US Environmental Protection Agency [26] are presented in Additional file 1: Table S3.
Risk calculation
The hazard quotient (HQ) was calculated to assess the non-carcinogenic risk using the following equation [25]:
where RfD is the estimated maximum allowable daily intake dose of a specific metal(loid) for humans that is likely to be without appreciable risk of deleterious effects in mg·kg−1 day−1. If the HQ value is greater than 1, adverse health effects are likely to occur; otherwise, potential health risks would not occur. In addition, the hazard index (HI) was used to assess the cumulative non-carcinogenic risk posed by multiple metal(loid)s:
For food category j, the risk contribution rate of metal(loid) i (CR) was calculated by dividing the non-carcinogenic risk of metal(loid) i exposure through food category j intake [HQ(i,j)] by the accumulated metal(loid) exposure risk via food category j intake (HIj) using Eq. (5):
The incremental lifetime cancer risk (ILCR) was calculated to assess the carcinogenic risk over a lifetime exposure using Eq. (6):
where SF is the cancer slope factor of a specific metal(loid) in [mg kg−1 day−1]−1. Risk in the range of 10–6 to 10–4 was regarded as acceptable. According to the classification group orders defined by the International Agency for Research on Cancer, Cu, Ni, and As are regarded as non-cancer effect elements, whereas Pb, Cr, and Cd are treated as potential carcinogenic effect elements. The oral SF and RfDs of each metal(loid), derived from the Integrated Risk Information System of the U.S. EPA based on extensive epidemiological and toxicological data, are listed in Additional file 1: Table S4.
Statistical analysis
The probability distributions of non-carcinogenic and carcinogenic risks were obtained using Monte Carlo simulations with 10,000 iterations by repeatedly sampling the probability distributions of the risk equation inputs and using these inputs to calculate a range of risk values. The Oracle Crystal Ball® (11.1.2.4.850) loaded in Microsoft Excel was used to perform the Monte Carlo simulation. The probability distribution type of each parameter is listed in Additional file 1: Table S3. Based on the results of the Shapiro–Wilk tests on the field-measured data, we assumed that the body weight and food intake rate could be described by a lognormal distribution. In addition, quantitative sensitivity analysis was conducted using Monte Carlo simulations to evaluate the influence of the model input variabilities on non-carcinogenic and carcinogenic risks. Since most parameters do not fit the normal distribution, Spearman correlation coefficients were calculated. All statistical analyses were performed using SPSS 20.0, with a significance level of 0.05, for two-tailed testing. The metal(loid) concentrations in each food group were exhibited as mean ± standard deviation and minimum to maximum. The Shapiro–Wilk method was used to test whether the data fit a normal distribution, and difference analysis was carried out using a T-test. Correlation analysis was performed using Spearman’s method.
Results and discussion
Dietary patterns of the children
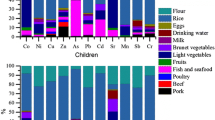
The food intake rate could reflect local children’s dietary habits and play an important role in dietary exposure risk assessment. The local children consumed an average of 342 g cereal, accounting for 42.6% of their total intake. The second most consumed food group was vegetables, with an average daily ingestion rate of 221 g, contributing 30.2% of the total consumption. Meat ranked third, accounting for 11.9%. In addition, the daily consumption rate of beans, aquatic products, and eggs was less at 35, 47, and 30 g/d, respectively. This dietary pattern is similar to that observed in Thailand [27]. However, compared to children from America, meat ingestion rate was much lower, whereas cereal and egg consumption was dramatically higher [28].
Metal(loid) levels in six major food groups
The metal(loid) levels in various food groups are shown in Table 1. Across all food groups, the concentrations of Pb, Cr, Cd, Ni, Cu, and As were 114.1–203.8, 115.7–315.7, 0.4–56.7, 13.2–416.1, 789.3–1653.3, and 31.5–880.4 μg kg−1, respectively. The levels of Pb, Cr, Cd, and As here were greater than those collected from markets in five regions of China (Pb: 5–80, Cr: 10–200, Cd: 0.2–20, and As: 0.2–750 μg kg−1) [29, 30], and were comparable to those from Ghana in areas impacted by mining activity [31]. Based on the latest national food safety standards in China, most metal(loid)s were within the threshold, except for Pb. Pb in all mutton samples, with an average level of 295 μg kg−1, exceeded the limit of 200 μg kg−1.
Metal(loid) levels vary widely among food groups. The highest concentrations of Pb and Cr were found in meat, especially in mutton, which contained 1.6–1.9 times more Pb and Cr than other kinds of meat. In addition, when the terrestrial food types were classified into animal-origin foods (including meat and eggs) and plant-origin foods (including cereals, legumes, and vegetables)—based on a previous study by Pipoyan et al. [32]—we observed that the levels of Pb and Cr in terrestrial animal-origin foods were significantly higher than those in terrestrial plant-origin foods (p < 0.05). Considering the lower trophic level of terrestrial plant-origin food and the higher trophic level of terrestrial animal-origin food, this indicates the potential biomagnification of these two elements [33, 34]. Cereals had the highest Cd content, whereas beans had the highest Ni concentrations. Moreover, higher levels of Cd and Ni were found in cereals, legumes, and vegetables than in meat and eggs, implying a biodilution effect with increasing trophic levels, consistent with the findings of Sun et al. [30]. Similar distribution characteristics of Pb, Ni, and Cd across food groups were also reported by Pipoyan et al. [32], who revealed that meat products, vegetables, and bread products contained the highest Pb, Ni, and Cd levels, respectively.
Cereals and beans had the highest Cu levels, followed by meat, vegetables, eggs, and aquatic products. For As, aquatic products contained the highest level, with an average of 880.4 μg kg−1, 14 to 28 times that in other food groups. Consistent with previous studies, due to biotransformation and bioaccumulation, fish were identified to be the major food source of As exposure for humans [35].
Metal(loid) bioaccessibility in six major food groups
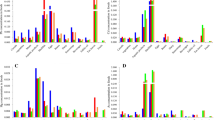
The bioaccessibility of each target metal(loid) in the six major food groups determined in the gastric and intestinal phases is shown in Fig. 2. The bioaccessibility in the gastric phase for Pb, Cr, Cd, Ni, Cu, and As was 55–88, 28–94, 14–48, 31–59, 34–58, and 30–60%, respectively, while in the intestinal phase it was 12–67%, 16–56%, 10–41%, 18–52%, 41–84, and 35–84%, respectively. These values are all within the ranges reported in previous studies [15, 36, 37]. Further, it was found that the bioaccessibility differed widely among the meta(loid)s. Among the six metal(loid)s, Cu—an essential trace element for the human body—showed higher levels of bioaccessibility in comparison with other metal(loid)s, similar to the findings of a previous study by Tian et al. [16]. Cd exhibited the lowest bioaccessibility, two-thirds lower than Cu. The metal(loid) bioaccessibility in the gastric phase decreased in the order of Cu > As > Pb > Cr > Ni > Cd, whereas that in the intestinal phase was Pb > Cr > Cu > Ni > As > Cd.
Metal(loid) bioaccessibility also differed greatly between the food groups. In general, meat, eggs, and aquatic products (carp were sampled in this study), which can be classified as animal-origin products, showed higher bioaccessibility (1.7 to 2.4 times) than plant-origin products (cereals, beans, and vegetables). This difference could be attributed to the discrepancies in food composition, metal(loid) speciation, and metal(loid) enrichment capacity among the different food groups. First, eggs, aquatic products, cereals, vegetables, and legumes contain higher contents of dietary fibers in comparison to meat [38], while high levels of fibers can partially encapsulate metal-binding proteins and inhibit their release into gastrointestinal digestive fluids, further decreasing the bioaccessibility of metal(loid)s [16, 36]. Second, the distribution patterns of metal(loid) speciation differ among food categories, and different metal(loid) speciation leads to different chemical behavior during gastrointestinal digestion, resulting in great discrepancies in bioavailability. Take As as an example, organic As is mostly found in fish, including dimethylarsine (DMA), monomethylarsine (MMA), and arsenobetaine (AsB), while plant products contain predominantly inorganic As [36]. During digestion, organic As can first be transformed into inorganic As, which increases the dissolution amount of organic As in gastric and intestinal fluids and leads to increased bioavailability of organic As in food. Finally, bioaccessibility is affected by metal(loid) contamination levels [39]. Here, the metal(loid) enrichment capacity varied widely within the food groups, and most metal(loid)s showed significant correlations between concentration and bioaccessibility (Cr: R = 0.342, p < 0.05; Pb: R = 0.403, p < 0.05; Cd: R = 0.496, p < 0.05; Cu: R = 0.315, p < 0.05).
In addition, most metal(loid)s exhibit decreased bioaccessibility from the gastric to the intestinal phase. For example, Pb, Cr, Cd, and Ni had an average reduction in bioaccessibility of 40.1, 35.9, 23.8, and 22.0%, respectively. This can be largely attributed to the dramatic increase in pH during the intestinal phase, during which a series of chemical reactions, including complexation, adsorption, and sedimentation, inevitably occur with metal(loid) ions [15]. However, exceptions were observed for Cu and As, which exhibited an average 14.9 and 15.8% increase from the gastric to intestinal phase, respectively. An increasing trend was also observed in previous studies. For instance, a 23% increase of Cu bioaccessibility in wheat kernels in the intestinal compared to the gastric phase was reported by Wang et al. [40] who also found that As bioaccessibility in the intestinal phase in vegetables was 1.2 times that in the gastric phase. The higher bioaccessibility of Cu in the intestinal phase can be largely attributed to the good solubility of Cu-phytate complexes [15]. Organic As can be demethylated into soluble inorganic As with the introduction of sodium cholate and pancreatic enzymes in the intestinal phase, which do not occur in the gastric phase [41]. Moreover, because animal food contains mainly organic As, whereas plant products are dominated by inorganic As [36], our study also found that the discrepancy in the bioaccessibility of As between the gastric and intestinal phases was higher in meat, aquatic products, and eggs (20 to 47%) than in cereals, vegetables, and beans (5 to 20%).
Non-carcinogenic risk
Figure 3 shows the cumulative probability distribution of the non-carcinogenic risk originating from each food group and metal(loid) exposure. The mean accumulated non-carcinogenic risk was 7.3, with 99.6% risk exceeding the acceptable level, implying a potential threat to the local children. The risk of exposure to individual metal(loids) was ranked as follows: As (HI = 5.4) > Pb (HI = 0.8) > Cr (HI = 0.5) > Cu (HI = 0.4) > Cd (HI = 0.1) > Ni (HI = 0.03) (Additional file 1: Fig. S1). As was identified to be the dominant risk element, contributing 73.7% to the total risk and 99.2% to the independent risk, surpassing the threshold. Pb was another important risk element, with the percentile values of HI > 1 at approximately 24%. For Cr, Cu, Cd, and Ni, almost all values were below the acceptable limit. In addition, each metal(loid) contributed differently to dietary exposure in the different food groups (Fig. 4). It was found that As was the dominant risk element for most food groups, with a contribution rate within the range of 47 to 95%, whereas Pb was an important risk source for meat, accounting for 43% of the total risk.
Probability distribution of non-carcinogenic risk deriving from a Ni, Cd, Cu, Cr, Pb, and As exposure via dietary intake and the accumulated risk and b metal(loid) exposure via beans, eggs, vegetables, meat, cereals, aquatic food intake and the accumulated risk. Monte Carlo simulations of 10,000 iterations were used. The black vertical dashed lines, light blue areas, and pink areas reflect the acceptable risk threshold, the area of negligible non-carcinogenic risks, and unacceptable non-carcinogenic risks, respectively
Contribution of each metal(loid) exposure to the accumulative non-carcinogenic risk deriving from each food group. For each food category, the risk contribution rate of an individual metal(loid) was calculated by dividing the non-carcinogenic risk of the individual metal(loid) exposure through that food category intake by the accumulated metal(loid) exposure risk
In addition, wide variation was observed in the metal(loid) exposure risk across different food groups. Aquatic products showed the highest non-carcinogenic risk (HI = 4.1), with an approximate value of 3.4% within the acceptable level. Aquatic products were the dominant risk source accounting for 56% of the total risk. Cereals were another important risk source, with an average contribution rate of 23.3% and 93.6% values exceeding the threshold. The risks derived from the ingestion of meat and vegetables also could not be ignored, with 12.7 and 4.3% risks higher than the acceptable levels, respectively. However, the risks resulting from the ingestion of eggs and beans were acceptable.
In addition, it can be found that there was a significant decrease (with bioaccessibility: HI = 7.3; without bioaccessibility: HI = 13.9) in the non-cancer risk value when bioaccessibility was introduced. Moreover, the contribution rate of each food category to the total risk changed when bioaccessibility was considered (Additional file 1: Fig. S2). For non-carcinogenic risk, when bioaccessibility was not considered, the contribution rates of cereals (33%) and aquatic food (36%) to the total non-carcinogenic risk were equally important. When bioaccessibility was considered, the contribution of aquatic food increased to 56%, whereas that of cereals decreased to 21%. This suggests that the identification of priority food types can be affected by differences in the bioaccessibility of metal(loid)s in different food types. Thus, the introduction of bioaccessibility not only improves the accuracy of health risk assessments but also identifies high-risk foods more precisely. In addition, it is noteworthy that even in a single food category, bioaccessibility could differ among food types. For example, different vegetables will also have different metal(loid) bioaccessibilities due to different phytic acid contents [31]. However, the inter-category differences in bioaccessibility are lower than the intra-category differences [29, 39]. Thus, an alternative method is to introduce bioaccessibility by food category when there are multiple types of foods that need to be risk accessed.
Carcinogenic risk
Three metal(loid)s were involved in the carcinogenic risk assessment (Fig. 5). The aggregated carcinogenic risk for all types of food consumption was 2.4 × 10–4. A large variation was also observed in the carcinogenic risk across food groups, with the risk decreasing in the order of cereals > meat > vegetables > aquatic products > eggs > beans. Cereals and meat were the dominant metal(loid) food type exposure risk sources, at 40.5 and 34.8% of the total risk, respectively. In terms of metal(loid)s, Cr was the major risk contributor to the local children (77.1%). Notably, the cumulative lifetime incremental cancer risk for each metal(loid) exposure was greater than 10–4, indicating carcinogenic health effects in local children.
Probability distribution of carcinogenic risk deriving from a Pb, Cr, and Cd exposure via dietary intake and the accumulated risk and b metal(loid) exposure via beans, eggs, vegetables, meat, cereals, aquatic food intake and the accumulated risk. Monte Carlo simulations of 10,000 iterations were used. The blue areas reflect unacceptable carcinogenic risks
Sensitivity analysis and study limitations
To effectively reduce the health risks from metal(loid) dietary exposure, a sensitivity analysis was carried out to rank the variables according to their Spearman rank-order correlation coefficients. For non-carcinogenic risks, As concentration in aquatic food was the most sensitive parameter, followed by aquatic food intake rate and body weight, and As bioaccessibility in aquatic food (Additional file 1: Fig. S3). However, cereal intake rate had the greatest influence on carcinogenic risk, followed by Cr concentration in cereals and body weight (Additional file 1: Fig. S4).
In this study, some limitations inevitably existed in the health risk assessment of dietary metal(loid) exposure. First, only one to three food types were collected from each food group. However, considering that these were the food types most frequently consumed by local children, based on the survey, the sampling might represent the dietary exposure characteristics of most local children. Second, bioavailability, which was obtained by in vivo experiments, refers to the proportion of ingested metal(loid)s that could pass through the gastrointestinal tract and eventually enter the circulatory system of the human body, whereas bioaccessibility only reflects the proportion that could be released in the simulated gastrointestinal environment [42]. However, owing to the high cost, long cycle time, and ethical limitations of animal experiments, only bioaccessibility was assessed here. Therefore, the risks assessed may have been overestimated. Third, only raw food was sampled and analyzed; therefore, the effect of cooking on metal(loid) levels and bioaccessibility in food was not considered, which might have introduced bias in the risk assessment results. For example, cooking can facilitate the release of As from rice into the gastrointestinal fluid and subsequently increase its bioaccessibility [22, 23]. Finally, the total metal(loid) concentrations were analyzed, and like most health risk assessment studies, owing to the lack of a toxicity coefficient for the total concentration of some metal(loid)s, the toxicity coefficient for species of metal(loid)s was used [43]. For example, the toxicity coefficient of inorganic As was used, and because organic As compounds are less harmful than inorganic As, the health risk might be overestimated to some extent. In addition, a specific toxicity coefficient for children is not available.
Conclusions
As far as we know, this research is the first to comprehensively assess the probabilistic risks of dietary exposure deriving from multi-type food intake and multi-metal(loid) exposure using a bioaccessibility-corrected method. Significant differences were observed in the dietary exposure to metal(loid)s and food categories. Cereals and vegetables were the major food groups consumed by local children. Cereals, meat, beans, and aquatic products contained the highest levels of Cu and Cd, Pb and Cr, Ni, and As, respectively. In general, meat, eggs, and aquatic products have a higher bioaccessibility than cereals, beans, and vegetables. The bioaccessibility of metal(loid)s decreased in the following order: Pb > Cr > Cu > Ni > As > Cd. For non-carcinogenic risk, the average hazard index was 7.3, with 99.6% exceeding the acceptable level. As was the dominant risk element, while aquatic products were the main risk source, accounting for 56% of the total risk. The aggregated carcinogenic risk was 3.4 × 10−3, with Cr being the major risk contributor and cereals and meat the dominant food sources.
The findings demonstrated that (1) the metal(loid) dietary exposure risk of children from areas with intensive smelting activities should be taken seriously and (2) it is necessary to identify the specific food category and metal(loid) based on a comprehensive analysis of metal(loid) concentration and bioaccessibility, and dietary habits of the population to effectively reduce risk.
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Kumari M, Bhattacharya T (2023) A review on bioaccessibility and the associated health risks due to heavy metal pollution in coal mines: content and trend analysis. Environ Dev. https://doi.org/10.1016/j.envdev.2023.100859
Qin G, Niu Z, Yu J, Li Z, Ma J, Xiang P (2021) Soil heavy metal pollution and food safety in China: effects, sources and removing technology. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129205
Kordas K, Cantoral A, Desai G, Halabicky O, Signes-Pastor AJ, Tellez-Rojo MM, Peterson KE, Karagas MR (2022) Dietary exposure to toxic elements and the health of young children: methodological considerations and data needs. J Nutr 152(11):2572–2581. https://doi.org/10.1093/jn/nxac185
Shah S, Jeong KS, Park H, Hong YC, Kim Y, Kim B, Chang N, Kim S, Kim Y, Kim BN, Kwon H, Bae S, Kim HC, Leem JH, Park EK, Joo H, Park B, Ha M, Ha E (2020) Environmental pollutants affecting children’s growth and development: collective results from the MOCEH study, a multi-centric prospective birth cohort in Korea. Environ Int. https://doi.org/10.1016/j.envint.2020.105547
Zheng K, Zeng Z, Tian Q, Huang J, Zhong Q, Huo X (2023) Epidemiological evidence for the effect of environmental heavy metal exposure on the immune system in children. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2023.161691
Tsuji JS, Garry MR, Perez V, Chang ET (2015) Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology 337:91–107. https://doi.org/10.1016/j.tox.2015.09.002
Yan Y, Yang S, Zhou Y, Song Y, Huang J, Liu Z, Wang Y, Wei S (2020) Estimating the national burden of mild intellectual disability in children exposed to dietary lead in China. Environ Int. https://doi.org/10.1016/j.envint.2020.105553
Shen M, Zhang C, Yi X, Guo J, Xu S, Huang Z, He M, Chen X, Luo D, Yang F (2021) Association of multi-metals exposure with intelligence quotient score of children: a prospective cohort study. Environ Int. https://doi.org/10.1016/j.envint.2021.106692
Allen DS, Kolok AS, Snow DD, Satybaldiyev B, Uralbekov B, Nystrom GS, Thornton Hampton LM, Bartelt-Hunt SL, Sellin Jeffries MK (2023) Predicted aquatic and human health risks associated with the presence of metals in the Syr Darya and Shardara Reservoir, Kazakhstan. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.159827
Wei JX, Gao JQ, Cen K (2019) Levels of eight heavy metals and health risk assessment considering food consumption by China’s residents based on the 5th China total diet study. Sci Total Environ 689:1141–1148. https://doi.org/10.1016/j.scitotenv.2019.06.502
Ortiz ML, Moreno-Ortega A, Trulls HE, Cámara-Martos F (2021) Evaluation of in vitro bioaccessibility of Cr, Ni and Pb in rice varieties: effect of cooking, dietary components and risk assessment. J Cereal Sci. https://doi.org/10.1016/j.jcs.2021.103332
Zhuang P, Sun S, Zhou X, Mao P, McBride MB, Zhang C, Li Y, Xia H, Li Z (2020) Bioavailability and bioaccessibility of cadmium in contaminated rice by in vivo and in vitro bioassays. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.137453
Chai M, Li R, Gong Y, Shen X, Yu L (2021) Bioaccessibility-corrected health risk of heavy metal exposure via shellfish consumption in coastal region of China. Environ Pollut. https://doi.org/10.1016/j.envpol.2021.116529
Yu Y, Liu L, Chen X, Xiang M, Li Z, Liu Y, Zeng Y, Han Y, Yu Z (2021) Brominated flame retardants and heavy metals in common aquatic products from the pearl river delta, south china: bioaccessibility assessment and human health implications. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124036
Hiller E, Pilková Z, Filová L, Mihaljevič M, Špirová V, Jurkovič Ľ (2022) Metal(loid) concentrations, bioaccessibility and stable lead isotopes in soils and vegetables from urban community gardens. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.135499
Tian W, Zhang M, Zong D, Li W, Li X, Wang Z, Zhang Y, Niu Y, Xiang P (2023) Are high-risk heavy metal(loid)s contaminated vegetables detrimental to human health? A study of incorporating bioaccessibility and toxicity into accurate health risk assessment. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2023.165514
Zheng X, Zhang Z, Chen J, Liang H, Chen X, Qin Y, Shohag MJI, Wei Y, Gu M (2022) Comparative evaluation of in vivo relative bioavailability and in vitro bioaccessibility of arsenic in leafy vegetables and its implication in human exposure assessment. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.126909
Zhou F, Li Y, Ma Y, Peng Q, Cui Z, Liu Y, Wang M, Zhai H, Zhang N, Liang D (2021) Selenium bioaccessibility in native seleniferous soil and associated plants: comparison between in vitro assays and chemical extraction methods. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.143119
Xu FF, Song J, Li YQ, Lai YF, Lin J, Pan JL, Chi HQ, Wang Y, Li ZY, Zhang GQ, Cai ZF, Liang XX, Ma AD, Tan CT, Wu WL, Yang XF (2021) Bioaccessibility and bioavailability adjusted dietary exposure of cadmium for local residents from a high-level environmental cadmium region. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.126550
Wang W, Gong Y, Greenfield BK, Nunes LM, Yang Q, Lei P, Bu W, Wang B, Zhao X, Huang L, Zhong H (2021) Relative contribution of rice and fish consumption to bioaccessibility-corrected health risks for urban residents in eastern China. Environ Int. https://doi.org/10.1016/j.envint.2021.106682
Hu J, Wu F, Wu S, Cao Z, Lin X, Wong MH (2013) Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere 91(4):455–461. https://doi.org/10.1016/j.chemosphere.2012.11.066
Li H, Li HL, Wu X, Wu YG, Zhang J, Niu YM, Wu YQ, Li JQ, Zhao YF, Lyu B, Shao B (2022) Human dietary exposure to bisphenol-diglycidyl ethers in China: comprehensive assessment through a total diet study. Environ Int 170:107578. https://doi.org/10.1016/j.envint.2022.107578
Li MH, Guo XY, Wen NH, Gan ZJ, Huang YR, Zhang YB, Kang Y, Zeng LX (2022) Speciation and bioaccessibility of arsenic in rice under different cooking methods and its implication in risk assessment. Environ Sci Pollut Res 29:87938–87949. https://doi.org/10.1007/s11356-022-21895-3
Ruby MV, Davis A, Schoo R, Eberle S, Sellstone CM (1996) Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol 30:422–430. https://doi.org/10.1021/es950057z
U.S.EPA (1989) Risk assessment guidance for superfund volume I human health evaluation manual (Part A). U.S. Environmental Protection Agency, Washington, DC. EPA/540/I-89/002
U.S. EPA (2014) Child-specific exposure scenarios examples. National Center for Environmental Assessment, Washington, D.C.; EPA/600/R-14/217F
Sunya S, Yenuthok A, Paengphua P, Sangsuay S (2023) Estimation of dietary intake of polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls from the Thai total diet study in 2019. Food Chem Toxicol 182:114154. https://doi.org/10.1016/j.fct.2023.114154
Jenkins M, Jefferds MED, Aburto NJ, Ramakrishnan U, Martorell R, Addo OY (2023) What do United States adolescents eat? Food group consumption patterns and dietary diversity from a decade of nationally representative data. Curr Dev Nutr 7(8):101968. https://doi.org/10.1016/j.cdnut.2023.101968
Jin J, Zhao X, Zhang L, Hu Y, Zhao J, Tian J, Ren J, Lin K, Cui C (2023) Heavy metals in daily meals and food ingredients in the Yangtze River Delta and their probabilistic health risk assessment. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.158713
Sun S, Zhang H, Luo Y, Guo C, Ma X, Fan J, Chen J, Geng N (2022) Occurrence, accumulation, and health risks of heavy metals in Chinese market baskets. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.154597
Afriyie RZ, Arthur EK, Gikunoo E, Baah DS, Dziafa E (2023) Potential health risk of heavy metals in some selected vegetable crops at an artisanal gold mining site: a case study at Moseaso in the Wassa Amenfi West District of Ghana. J Trace Elem Miner 4:100075. https://doi.org/10.1016/j.jtemin.2023.100075
Pipoyan D, Stepanyan S, Beglaryan M, Stepanyan S, Mendelsohn R, Deziel NC (2023) Health risks of heavy metals in food and their economic burden in Armenia. Environ Int. https://doi.org/10.1016/j.envint.2023.107794
Goswami V, Deepika S, Diwakar S, Kothamasi D (2023) Arbuscular mycorrhizas amplify the risk of heavy metal transfer to human food chain from fly ash ameliorated agricultural soils. Environ Pollut 329:121733. https://doi.org/10.1016/j.envpol.2023.121733
Mwelwa S, Chungu D, Tailoka F, Beesigamukama D, Tanga C (2023) Biotransfer of heavy metals along the soil-plant-edible insect-human food chain in Africa. Sci Total Environ 881:163150. https://doi.org/10.1016/j.scitotenv.2023.163150
Lescord GL, Johnston TA, Ponton DE, Amyot M, Lock A, Gunn JM (2022) The speciation of arsenic in the muscle tissue of inland and coastal freshwater fish from a remote boreal region. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.136140
Fu YQ, Du HL, Wang PF, Yin NY, Cai XL, Geng ZQ, Li YP, Cui YS (2023) Effects of foods and food components on the in vitro bioaccessibility of total arsenic and arsenic species from Hizikia fusiforme seaweed. Sci Total Environ 900:165775. https://doi.org/10.1016/j.scitotenv.2023.165775
Shentu J, Fang Y, Wang Y, Cui Y, Zhu M (2023) Bioaccessibility and reliable human health risk assessment of heavy metals in typical abandoned industrial sites of southeastern China. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2023.114870
Khorasaniha R, Olof H, Voisin A, Armstrong K, Wine E, Vasanthan T, Armstrong H (2023) Diversity of fibers in common foods: key to advancing dietary research. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2023.108495
Da Silva Junior EC, Babaahmadifooladi M, Folens K, dos Reis AR, Guilherme LRG, Van de Wiele T, Jacxsens L, Du Laing G (2023) Content, speciation and in vitro bioaccessibility of trace elements in seaweeds and derived food products. J Food Compos Anals. https://doi.org/10.1016/j.jfca.2023.105162
Wang L, Yin X, Gao S, Jiang T, Ma C (2020) In vitro oral bioaccessibility investigation and human health risk assessment of heavy metals in wheat grains grown near the mines in North China. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126522
Lin CQ, Wang WQ, Hu GR, Yu RL, Huang HB, Liao D (2023) Incorporating source apportionment and bioaccessibility into human health risk assessment of heavy metals in a soil-rice system in the Jiulong River basin, southeast China. J Food Compos Anal 124:105692. https://doi.org/10.1016/j.jfca.2023.105692
Raffy G, Mercier F, Glorennec P, Mandin C, Le Bot B (2018) Oral bioaccessibility of semi-volatile organic compounds (SVOCs) in settled dust: a review of measurement methods, data and influencing factors. J Hazard Mater 352:215–227. https://doi.org/10.1016/j.jhazmat.2018.03.035
Feng W, Wang T, Zhu Y, Sun F, Giesy JP, Wu F (2023) Chemical composition, sources, and ecological effect of organic phosphorus in water ecosystems: a review. Carbon Res 2:12. https://doi.org/10.1007/s44246-023-00038-4
Acknowledgements
We appreciate the contributions of all participants.
Funding
This study was financially supported by the National Key Research and Development Program of China (No. 2019YFC1804602), Fundamental Research Funds for the Central Universities (No. FRF-IDRY-22-027), and the National Natural Science Foundation of China (No. 42007386).
Author information
Authors and Affiliations
Contributions
BBW developed the methodology, conducted the literature search, and organized and wrote the first draft of the manuscript. LWZ, WYF and HZ investigated and curated the data. XLD and NQ wrote, reviewed, edited, and supervised the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of Nankai University (NKVIRB2020066). Prior to the survey, written informed consent was obtained from the participants and their guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Instrument and method detection limits for different metal(loid) in the study. Table S2. The main operating parameters of ICP-MS for the determination of element concentrations. Table S3. Exposure parameters obtained from the questionnaire survey of the current study to estimate the dietary exposure risks by Monte Carlo simulation. Table S4. Toxicity parameters for each metal(loid). Figure S1. The average HQ of individual metal(loid) dietary exposure risk (a) and the average HQ of metal(loid)s exposure risk via an individual food category intake (b). Figure S2. Contribution rate of each food category to the total non-cancer risk when risk assessment with and without the introduction of bioaccessibility. Figure S3. Parameter sensitivity for metal(loid)s dietary exposure non-cancer risk. Figure S4. Parameter sensitivity for metal(loid)s dietary exposure cancer risk.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Zhang, L., Feng, W. et al. Bioaccessibility-corrected probabilistic health risk assessment of dietary metal(loid) exposure in six major food groups in children from Northwest China. Environ Sci Eur 36, 6 (2024). https://doi.org/10.1186/s12302-023-00830-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00830-4