Abstract
Background
Worldwide observations point to a two-stage theory of disease called Toxicant-Induced Loss of Tolerance (TILT): Stage I, Initiation by an acute high-level or repeated lower-level chemical exposures, followed by Stage II, Triggering of multisystem symptoms by previously tolerated, structurally diverse chemical inhalants, foods/food additives and drugs. Until recently, there was no known biological mechanism that could explain these observations. In 2021, we published a plausible and researchable two-stage biomechanism for TILT involving mast cells: Stage I, Initiation via mast cell sensitization; Stage II, Triggering of mast cell degranulation by previously tolerated exposures, resulting in the release of thousands of mediators, including histamine and a host of inflammatory molecules. The objective of this study was to identify common TILT initiators.
Methods
A randomized, population-based sample of 10,981 U.S. adults responded to a survey which included items concerning medical diagnoses, personal exposures, antibiotic use, and several possible initiators of Chemical Intolerance (CI). CI was assessed using the internationally validated Quick Environmental Exposure and Sensitivity Inventory (QEESI). Participants identified as chemically intolerant were asked to recall when their intolerances began and what they felt had initiated their condition.
Results
Twenty percent met QEESI criteria for TILT, approximately half of whom identified one or more initiating exposures. Initiators in order of frequency were mold (15.6%), pesticides (11.5%), remodeling/new construction (10.7%), medical/surgical procedures (11.3%), fires/combustion products (6.4%), and implants (1.6%). Protracted antibiotic use for infections involving the prostate, skin, tonsils, gastrointestinal tract, and sinuses were strongly associated with TILT/CI (OR > 2).
Discussion
Participants identified two broad classes of TILT initiators: 1) fossil fuel-derived toxicants (i.e., from coal, oil, natural gas), their combustion products, and/or synthetic organic chemical derivatives, e.g., pesticides, implants, drugs/antibiotics, volatile organic compounds (VOCs); and 2) biogenic toxicants, e.g., particles and VOCs from mold or algal blooms. One in four primary care patients suffers from Medically Unexplained Symptoms (MUS). Doctors in primary care, neurology, psychiatry, psychology, occupational medicine, and allergy/immunology would be well-advised to include TILT in their differential diagnosis of patients with so-called MUS. Because 20% of U.S. adults meet QEESI criteria for CI, the role of contemporary exposures in initiating and exacerbating these conditions via mast cells needs our immediate attention. There is a concomitant need for policies and practices that reduce initiating exposures as well as ubiquitous and often unavoidable triggers such as fragranced personal care, cleaning, and laundry products in multi-occupant housing, workplaces, medical settings, schools, places of worship, and all public buildings—literally anywhere air is shared. Fossil fuels are assaulting humans and other animal species both from within via mast cell sensitization, and from without via climate change.
Similar content being viewed by others
Introduction
Chemical Intolerance (CI) is characterized by multisystem symptoms triggered by everyday exposures to chemicals, foods, and drugs [2, 4]. Symptoms often include fatigue, headaches, weakness, rash, mood changes, musculoskeletal pain, gastrointestinal and respiratory problems, as well as difficulties with attention and concentration often described as “brain fog” [2, 4, 38, 76, 117]. CI is a rapidly rising international public health concern. Prevalence estimates range from 8 to 33% in population-based surveys [5, 20, 27, 66, 87]. In both Japan [53] and the U.S. [103], surveys conducted a decade apart revealed substantial increases in CI. This paper builds upon our earlier report for this cohort which documented that 20% of U.S. adults fulfill criteria for CI as measured by the Quick Environmental Exposure and Sensitivity Inventory (QEESI) [89].
Over the past 35 years, despite numerous proposed case definitions for the condition, no consensus has emerged [2, 4, 28, 29]. The published literature often refers to CI as multiple chemical sensitivity (MCS) or idiopathic environmental intolerance (IEI). We no longer use MCS or IEI, because they are too limiting and overlook the two-stage TILT process. As we have shown [72] and show once again in this paper, there are well-documented and well-characterized exposures which initiate illness in large groups of individuals exposed to chemicals, e.g., during the Gulf War, breast implants, pesticides, and VOCs during new construction or remodeling. A recent comprehensive epidemiologic and diagnostic review indicates that assessing CI most often involves the QEESI [97]. First published in 1999 [82], the QEESI is now widely used in lieu of a case definition and is considered the reference standard for assessing CI. To date, researchers in more than 16 countries on five continents have used the QEESI in their studies [43, 46, 52, 60, 101]. The QEESI is a validated questionnaire derived by factor analysis from symptoms and intolerances to chemicals, foods, and/or drugs reported by individuals who said they became ill following exposure to either an organophosphate pesticide or new construction/remodeling [81, 98]. Subsequent studies of groups who reported developing chronic illness following exposures to other toxicants, including Gulf War veterans and breast implant recipients, further demonstrated the QEESI’s utility as a measure of CI. The QEESI offers high sensitivity and specificity in differentiating CI individuals from the general population, making it useful for clinical and research applications [52, 81, 82].
The present paper builds on our two prior publications that focused on TILT as a global phenomenon [80], and mast cell activation and mediator release as a plausible underlying biomechanism for TILT [80]. Here we attempt to differentiate between TILT initiators (Stage I of TILT) and TILT triggers (Stage II), the latter being more readily observable both by affected individuals and their medical providers. Patients and clinicians who are unaware of the two-stage nature of the condition often mistake the myriad triggers in Stage II of TILT as causal and overlook Stage I (relating to what initiated TILT). There is great value in using self-reported questionnaires to better understand the types of events or exposures which affected individuals recall as having preceded CI. Their recollections may help guide future research and help predict and prevent TILT in the future.
TILT as an underlying mechanism for CI
Individuals with CI often attribute onset of their illness to specific exposure events such as the Gulf War, the World Trade Center collapse, or exposures to pesticides, VOCs associated with new construction or remodeling, implants, and/or mold [72, 77, 91]. The fact that those who share the same initial exposure frequently exhibit different manifestations complicates diagnosis. For example, an entire family may be exposed to mold in their home. Some members may experience headaches, others nausea, while still others have cognitive difficulties. Some may report no symptoms at all. Moreover, if family members see different doctors, a pattern of new-onset, environmentally initiated illnesses may be missed.
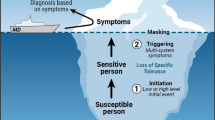
First proposed by [75, 76], TILT is a two-stage disease process: Stage I, called initiation, and Stage 2, triggering [2, 4, 76]. Initiation begins with exposure to a particular chemical or combination of chemicals, often at levels below so-called “safe” occupational or environmental exposure limits. TILT can develop rapidly (e.g., after a pesticide exposure), or gradually over a period of months (e.g., in a “sick” building) (see Fig. 1). Initiating events commonly go unrecognized and therefore unreported, leaving triggers and symptoms as the only documented components. This has thwarted our understanding of the etiology of TILT. Further, our failure to ask patients about possible initiating events has caused confusion concerning the origins of other comorbid conditions such as ADHD, autism, asthma, irritable bowel syndrome, migraine headaches, depression, anxiety, brain fog and other cognitive and mood difficulties. In addition, it has led to a slew of non-etiologic diagnoses including MCS and IEI.
Masking often obscures awareness of both initiators and triggers. Masking results from the overlapping of symptoms triggered by multiple ongoing exposures [2, 4, 78]. Routine use of nicotine-containing products; xanthines (e.g., chocolate, coffee, tea); alcoholic beverages; certain medications; scented personal care; cleaning or laundry products; and exposure to combustion products from a gas stove or heating system often mask or hide the relationship between exposures and symptoms. A 10-item Masking Index (which is not a scale) is therefore included in the QEESI to assess ongoing exposures that may otherwise be difficult for patients to recognize. For a detailed discussion of masking, see [2, 4] and [78]. A person with a high masking score on the QEESI is less able to recognize symptom triggers [53, 81]. A very low masking score suggests that a person may have been avoiding triggering exposures for such a long time that they no longer recognize specific triggers.
Following TILT initiation, affected individuals report an inability to tolerate everyday exposures to a wide range of chemically diverse substances (including but not limited to the initiator itself) at levels that never bothered them previously and do not bother most people. Triggering exposures can include structurally unrelated ingestants, inhalants, and skin contactants. Common triggers include fragrances, nail polish/remover, hairspray, pesticides, mothballs, cleaning products, fresh paint, tobacco smoke, organic solvents, diesel or gas engine exhaust, as well as foods/food additives and medications (see Fig. 2) [2, 4, 39, 40, 117].
Understanding TILT and initiating events
Our understanding that chemical exposures can cause new-onset intolerances (often perceived as “allergies” for chemicals, foods, and drugs) evolved from interviews with physicians in nine European countries who reported seeing patients with heightened multisystem symptoms along with sensitivities to odors, solvents, and sometimes foods, often following a single major chemical exposure or repeated lower level exposures [3, 79]. In both Europe and the United States, commonly reported initiators included pesticides, paints and lacquers/organic solvents, formaldehyde, anesthetic agents, and hairdressing chemicals.
The first systematic study of initiating events was published in 1995 by [77]. They compared two groups who reported developing intolerances following distinctly different exposures: a well-characterized organophosphate pesticide exposure (n = 37) and an exposure related to new construction or remodeling (n = 75). Although these exposures involved entirely different chemical classes, individuals in both groups reported strikingly similar patterns of chemical and food intolerances [77]. Subsequently, [81] studied symptoms and intolerances reported by Gulf War veterans (n = 72), breast implant recipients (n = 87), and individuals suffering from MCS who attributed onset of their illness to various exposures (n = 96) [81].
Recently we reviewed and summarized initiating exposure events reported by eight well-documented groups of individuals who had shared the same initial exposures and subsequently developed CI [72]. These groups included: (1) EPA workers exposed following new carpet installation; (2) Gulf War veterans; (3) casino workers exposed to organophosphate pesticides; (4) pilots and cabin crews exposed to aircraft oil fumes (“fume events”); (5) World Trade Center first responders and others in close proximity to the disaster; (6) breast and other implant recipients; (7) individuals exposed to mold in their homes; and 8) tunnel workers exposed to gasoline vapors (benzene) in a confined space.
These studies have helped elucidate the phenomenology of TILT (see Fig. 1), but until now, there have been no population-based data to help us understand which exposures people most often view as having initiated their intolerances. In the present study, we draw upon our previously described population-based sample of more than 10,000 U.S. adults to answer the following key research questions:
-
A)
What percentage of U.S. adults meet the QEESI criteria for CI?
-
B)
Among those with CI, which initiators do they most commonly implicate?
-
C)
Does exposure to multiple initiators increase the risk of CI?
-
D)
Do repeated or protracted courses of antibiotics over the lifespan increase the risk of CI?
While pesticides are frequently reported CI initiators, both antibiotics and pesticides are known to disrupt the microbiome [22, 104], hence our fourth research question. Digestive difficulties and food intolerances are common in all TILT exposure groups [77, 81]. Further, as we previously reported, our gastrointestinal tracts are densely populated with mast cells, which are our ancient immune systems’ first responders to foreign substances [80]. In the GI tract, mast cells protect us against the largest quantity of xenobiotics we encounter—the food we eat. We reasoned that disruption of the microbiome might play a prominent role in the development of CI. Consequently, we included exploratory questions concerning antibiotic use. Finally, it should be noted that the majority of antibiotics today are themselves synthetic chemical derivatives of petroleum [47].
Methods
Sample population
We conducted a population-based survey of U.S. adults aged 18 years and older. The survey was deployed between June 1–2, 2020, using the SurveyMonkey audience platform (2020). SurveyMonkey recruitment procedures are available here: www.surveymonkey.com/mp/audience. 10,981 respondents were randomly selected from nearly 3 million online users of the SurveyMonkey platform. The survey had an abandonment rate of 10.1% and took an average of approximately 5 min to complete. The modeled error estimate for this survey was ± 1.4%. Data were weighted based on the population sizes of all 50 states plus the District of Columbia, as well as by gender, age, race, and education within each census region to match the U.S. Census Bureau’s 2015 American Community Survey (ACS) targets.
Survey
Respondents answered an 80-item survey we called the Personal Exposure Inventory which included several items concerning individuals’ medical diagnoses and personal exposures including antibiotic use and several possible initiating exposures. CI was assessed using the QEESI Chemical Intolerance and Symptom Scales [81] (see supplement section for list of worldwide studies using the QEESI, now considered the reference standard for screening CI). Scores greater than or equal to 40 on both scales are considered to be very suggestive of CI. Scores from 20 to 39 on one or both scales are suggestive of CI. Scores less than 20 on both scales are not suggestive of CI [81]. Of the total sample, 281 (2.5%) could not be classified for CI and were excluded from the analysis because they did not complete both QEESI scales.
All respondents were queried concerning protracted antibiotic use: “Over your lifetime, have you taken a prolonged course of antibiotics for any persistent, difficult-to-treat infection(s)? (Check all that apply).” A series of “yes” or “no” check boxes followed, inquiring about specific sites or types of infections: ear, tonsils, sinus, dental, lungs, gastrointestinal, skin, genitourinary, wound, fungal; plus, two gender-specific sites, vagina and prostate.
The Brief Environmental Exposure Sensitivity Inventory (BREESI) is a validated three-question screening survey that has shown excellent predictive value against the QEESI’s CI categories [87]. Individuals with a positive screen on the BREESI (i.e., those reporting adverse responses to chemicals, foods, and/or drugs) were asked what they thought had initiated their CI (see the flowchart in Fig. 3). Participants responded “yes” or “no” to a series of check-box items: “Was there a particular exposure(s) that initiated your chemical intolerances/sensitivities?” Respondents who answered affirmatively were asked to select which of the following potential initiators applied to them: Medical/Aesthetic Implants, Pesticides, Combustion Products, Mold, Surgical/Medical Procedures, or New Construction/Remodeling. We included the question about surgical/medical procedures because we wanted to learn whether procedures other than implants might be implicated.
Statistical analysis
Data quality measure | Data quality dimension | Definition |
|---|---|---|
Any NULL chemical Score | Completeness | Any record having at least 1 NULL value in the QEESI chemical score |
Any NULL symptom Score | Completeness | Any record having at least 1 NULL value in the QEESI symptom score |
Same non-0 chemical Score | Accuracy | Any record having the same non-0 QEESI chemical score |
Same non-0 symptom Score | Accuracy | Any record having the same non-0 QEESI symptom score |
Gender mismatch | Validity | Any record submitted with the gender questions not matching the survey monkey panel gender |
Male & vaginitis | Validity | Any record indicating male gender and vaginitis |
Female & prostate | Validity | Any record indicating female gender and prostate cancer |
Male & breast Implants | Validity | Any record indicating male gender and breast implants |
Too Fast | Accuracy | Any record indicating survey completion in 2 min or less |
The 10,981 survey records were assessed for data quality (DQ). A list of data conditions that could pose completeness, validity, or accuracy concerns was created. Any record with these DQ concerns was excluded from the analytic data set. Some of the measures could technically be accurate (e.g., “Male & Breast Implants”), but out of an abundance of caution were excluded. The same could be said for the “Too Fast” measure: with a survey length of between 15 and 22 questions, it is very unlikely that a respondent could read and respond accurately to all questions in under two minutes. By omitting any records that violated one or more DQ measures, 2985 records were excluded (27.2%). The largest single DQ measure contributing to exclusion was “Too Fast” with 1,616 records, and the second largest was “Gender Mismatch” with 614 records. We have taken this approach to help ameliorate some well-known DQ issues associated with web-based surveys, including response probabilities and biases [11, 25]. Our final analytic sample was N = 7997.
Binary logistic regressions were conducted to determine the extent to which initiating events and protracted antibiotic use were predictive of CI risk. The binary outcome variable compared not suggestive to very suggestive QEESI categories. We first used initiating exposure events and protracted antibiotic use as continuous variables (based on the number of identified exposures) in separate models which included age, gender, and household income as covariates (Table 4, Models 1 and 2). In Models 3 and 4 (Table 4), initiating events and antibiotics were used as independent dichotomous (individual yes/no) predictors of CI. A final model combined both the initiating event and antibiotics items (Table 4, Model 5) in the form of a multivariate model. All models were adjusted for gender, age, and household income. In this study, we used a p-value 0.05 threshold to determine statistical significance. Analyses were conducted using JMP (1989–2019) and SAS software (2014) [61, 98].
Results
Descriptive statistics
Table 1 provides descriptive statistics for the entire survey population. The number and percentages of QEESI scores, initiating events, and protracted antibiotic courses are shown. Figure 3 depicts respondent flow and shows the numbers and percentages who completed survey items for initiating events. Only individuals with a positive BREESI screen (79.2%) were asked about initiating exposure events. Approximately half of those with a positive BREESI screen (54.5%) did not identify an initiating event. In order of frequency, initiators specified by participants who identified any initiating event were Mold (15.6%), Pesticides (11.5%), Medical/Surgical Procedures (11.3%), Remodeling/New Construction (10.7%), other (6.7%), Combustion Products (6.4%), and Implants (1.6%). Table 2 shows the overlapping responses for the initiator items. The initiators most frequently selected together were Mold, Pesticides, and Remodeling/Construction.
Table 3 shows the distribution of the total numbers and percentages reported for initiating events and protracted antibiotic use.
Figure 4 shows the initiating exposure items. In descending order, the initiating events with the highest average QEESI scores were Implants, Pesticides, Combustion Products and Medical/Surgical Exposures. Figure 5 breaks down the initiator responses by QEESI category. As might be expected, the very suggestive CI group identified the most initiators, followed by the suggestive group which in turn identified more than the not suggestive group. Figure 6 depicts the mean number of initiating events by QEESI category, suggesting a linear relationship.
Statistical modeling results
Model 1: Initiating exposure events as a continuous predictor variable
Results from the logistic regression model that used initiating events as a count variable are presented in Table 4. The model R2 was 0.16. Initiator count, gender, and age were significant contributors to the model (p < 0.01). To assess a non-linear association, a squared term for Initiating Exposures count was added and was not significant (p = 0.31). Interactions between gender and age were also evaluated and were not significant, nor was household income (p = 0.07). Females were twice as likely to have QEESI scores very suggestive of CI. Respondents older than 60 years were approximately half as likely to have scores very suggestive of CI compared to respondents under 60. Notably, for every initiating exposure event reported by respondents, the odds of their belonging to the very suggestive category nearly tripled, increasing by 2.9 on average.
Model 2: Antibiotics as a continuous predictor variable
Results from the logistic regression model that used the number of protracted antibiotic exposures appear in Table 4. To assess a non-linear association, a squared term for antibiotic exposure count was added. Both the linear and non-linear terms for antibiotic count, as well as gender, income, and age were all significant contributors to the model (p < 0.01). The interaction between gender and age was significant (p = 0.01), but the overall effect estimate was small, indicating that females over 60 years of age were slightly less likely to have scores very suggestive of CI. The model reported an R2 of 0.21. Importantly, for every initiating exposure reported by a respondent, the odds of having scores very suggestive of CI increased by 1.9 on average. When including the significant non-linear trend, the odds ratio (OR) showed a 2.4 increase for each initiator.
Model 3: Initiating exposure events as binary predictors
Results from the logistic regression model that included Initiating Exposures as individual binary exposure variables are also presented in Table 4 (Model 3). The model reported an R2 of 0.16. Gender and age were significant contributors (p < 0.01), unlike household income (p = 0.06). Each of the six initiating exposures contributed significantly to the model (p < 0.01). Ranked in order are the ORs for each class of initiating exposures: Breast Implants (OR = 7.5), Pesticides (OR = 4.4), Combustion Products (OR = 3.6), Mold (OR = 2.9), Surgical/Medical Procedures (OR = 2.4), New Construction/Remodeling (OR = 2.2).
Model 4: Antibiotics as binary predictors
Results from the logistic regression model that included antibiotic exposures as individual binary exposure variables are presented in Table 4. The model reported an R2 of 0.20. Gender, income, and age were significant contributors to the model (p < 0.01). All protracted antibiotic exposures contributed significantly to the model (p < 0.04). Ranked in order are the ORs for protracted antibiotic use by infection site/type: prostate (OR = 3.0), skin (OR = 2.8), tonsils (OR = 2.7), gastrointestinal tract (OR = 2.5), sinuses (OR = 2.2), wounds (OR = 1.9), fungal (OR = 1.8), pneumonia (OR = 1.7), ear (OR = 1.7), dental (OR = 1.7), vagina (OR = 1.6), urinary tract (OR = 1.6).
Model 5: Initiating exposures and antibiotics as binary predictors
Results from the logistic regression model that considered Initiating Exposures and Protracted Antibiotic Use as individual binary exposure variables are presented in Table 4. The model reported an R2 of 0.22 with good fit. As in the other models, gender and age remained significant contributors to the model (p < 0.01), while household income did not. All six initiating exposures significantly contributed to this model (p < 0.01). Nine of the 12 antibiotic exposures contributed significantly to this model (p < 0.05). Model 5 fit the data well (p = 1.0 for lack of fit).
Ranked in order, the ORs for both protracted antibiotic use and exposure events are as follows: Pesticides (OR = 4.8), Breast Implants (OR = 2.9), Combustion Products (OR = 2.3), Mold (OR = 2.3), Remodeling/New Construction (OR = 2.2), and Surgical/Medical Procedure (OR = 1.9). For antibiotics, the ranked order was: Gastrointestinal (OR = 1.9), Sinus Infection (OR = 1.8), Wound (OR = 1.8), Skin (OR = 1.7), Urinary Tract (OR = 1.7), Tonsils (OR = 1.6), Pneumonia (OR = 1.5), Dental (OR = 1.4), Fungal (OR = 1.1).
Discussion
Identifying initiators
Approximately two-thirds of our sample (n = 5576/7997, 70%) had a positive BREESI screen (answered “yes” to one or more of the three BREESI items), indicative of at least some degree of chemical, food, and/or drug intolerance. Approximately 40% of these individuals attributed onset of their illness to an initiating exposure event or multiple events. Further, a lifetime history of protracted antibiotic use was associated with chemical intolerance. Specifically, we found that with every additional initiating exposure event, the odds of reporting CI nearly tripled. We also demonstrated that prolonged courses of antibiotics were associated with increased risk of CI, and with every additional course of antibiotics the odds of CI nearly doubled. That discrete exposure events were associated with CI is consistent with findings from of our prior published study showing that CI is frequently preceded by identifiable toxicant exposures such as new carpet installation, pesticide use, combustion/pyrolysis emissions, occupying a moldy home, occupational exposures to VOCs, and breast implants [72]).
Women tended to have higher scores on the QEESI, a finding consistent with other studies which have shown the prevalence of CI among females across various populations ranged from 69 to 80% [32, 54, 66, 103]. This difference may be biologically based or stem from differences in exposures, e.g., women repeatedly exposed to fragranced cosmetics, soaps, sprays and personal care products, as well as fragranced cleaning and laundry products, all of which commonly are used in poorly ventilated spaces. In addition, it is well-established that males and females differ in their immune responses to foreign and self-antigens. For instance, elevated humoral immunity (immunoglobulins) in females compared to males is physiologically conserved, perhaps imparting an adaptive advantage for transferring protective antibodies in utero to a fetus [33]. Anatomic differences between males and females also may affect vulnerability to CI [88]. For example, mast cells in the nose can be sensitized by inhaled VOCs, mold, or combustion products. Subsequent exposures can trigger mast cell degranulation releasing a cascade of mediators causing swelling of the nasal mucosa and occluding sinus openings, which are smaller on average in females. Cutting off air to the sinuses results in an anaerobic condition and chronic infections, likely leading to repeated or prolonged courses of antibiotics.
In general, the strength of the association between antibiotic use and CI suggests a potential causal role of antibiotics in CI initiation. Although this question cannot be answered by this single study, in part due to the limitations to be discussed later, it nonetheless supports the hypothesis that alterations in the gut microbiome may be associated with the development of CI [12, 26], suggesting the need to restore normal gut flora. Importantly, however, it is not clear from this analysis nor from prior literature in which direction a potential causal association may lie. That is, it is unclear how antibiotic use may contribute to the development of CI: Do antibiotics compromise the gut microbiome? And/or do CI individuals take more antibiotics?
Regarding protracted antibiotic use for specific types of infections among those with scores very suggestive of CI, antibiotics prescribed for infections categorized as Skin, Tonsils, Gastrointestinal, Prostate, Sinuses, Wound, and Pneumonia were most strongly associated with CI (OR > 1.5). Early evidence of an association between the gut microbiome and CI was previously documented by [77] who showed a direct relationship between the number of intolerances for chemical inhalants and the number of food intolerances reported by people who said they became ill after organophosphate or remodeling exposures [77]. Further, organophosphate exposures, such as exposures to the common agricultural pesticide chlorpyrifos (now banned for household use) are known to disrupt the gut microbiome [31, 67, 121, 123]. Similarly, antibiotics alter the gut microbiome [94]. Adding further weight to this association is evidence of reduced food intolerances among individuals following treatment with probiotics [70, 90, 109, 110].
In the present analysis, the initiating exposures reported most frequently (> 10% of respondents who identified an initiating event) were Mold, Remodeling/New Construction, Medical/Surgical Procedures, and Pesticides, while exposures to Combustion Products, Implants, and others were less frequently reported. The ranking of initiating events based on odds ratios differed from that of their frequencies, with Pesticides, Breast Implants, Mold, and Combustion Products showing the highest odds ratios (OR > 2), followed in order by Surgical/Medical Procedures (OR = 1.9). It is important to note that the rankings of initiating events may be influenced by, and therefore partially reflect, the obviousness of an exposure event from the perspective of a survey participant. For instance, since occupants are apt to see and/or smell mold indoors, as opposed to invisible or non-odorous airborne VOCs and pesticides, participants may have implicated mold disproportionately.
Evidence and implications of initiating exposures
The concept that the exposures and exposure events reported in this analysis have the potential to initiate CI is supported by well-documented reports in peer-reviewed papers describing the initiation of CI among groups of individuals who shared the same initial exposure events, several of which are summarized in our prior work [72, 81], as well as reports of CI-related symptoms that abate following the removal of certain exposures, e.g., breast implants. [114]. Notwithstanding, mold was the most frequently implicated exposure (17.1%) in our study. Mold spores, mold VOCs and debris can concentrate in the air where fresh air ventilation is poor, giving rise to adverse health effects [65]. Evidence of mold-related CI was well-documented in the case of nine Finnish family members who moved into a moisture-damaged house where they subsequently developed a range of symptoms including eye irritation, cough, congestion, shortness of breath, and chemical intolerances. Their symptoms abated only when the family moved to a different home [106].
Finland is located in a subarctic region where snow melt, leaking roofs, and winter storms lead to water intrusion, mold growth and mold-related health problems in homes, workplaces, and schools. Based upon clinical experience with more than 1000 patients with “Dampness and Mold Hypersensitivity Syndrome”, Finnish physician Ville Valtonen reported that approximately half of such patients ultimately developed CI and related symptoms [108]. Likewise, researchers in Tampere, Finland [85] used the QEESI to assess patients referred for respiratory and/or voice symptoms associated with workplace moisture damage. Compared to randomly selected controls from the Finnish Population Information System, the patients had significantly higher scores on the Chemical Intolerance (39% vs. 23%, p < 0.001), Symptom Severity (60% vs. 27%, p < 0.001) and Life Impact scales (53% vs. 20%, p < 0.001). Scandinavian researchers have also identified exposures to both electromagnetic fields (EMF) and mold as potentially altering mast cells and underlying CI [37, 62, 63, 92, 93, 112, 113].
Similarly, Kilburn [64] compared symptomatic adults living in moldy homes to individuals who became ill following exposure to various chemicals (e.g., diesel exhaust, organophosphate insecticides, glutaraldehyde, and cleaning agents) to asymptomatic controls. Applying a comprehensive battery of neurobehavioral tests, he found a more than fivefold higher incidence of abnormalities in the two exposed groups relative to controls [64]. Interestingly, the 1995 publication concerning CI in European countries conducted by one of the present authors did not identify mold as an initiator [3], nor was mold mentioned in the 1989 New Jersey Report or subsequent book by [2, 4]. In recent years, global warming has led to more rainfall, floods, hurricanes, roof leaks, and water intrusion, resulting in increased mold growth indoors.
While mold spores and particles can be toxic via ingestion or inhalation and can irritate any exposed part of the body, inhaling low molecular weight mold VOCs (mVOCs) may constitute an important initiating exposure that has been largely overlooked [10]. Supporting this possibility is an experiment in which developing fruit flies exposed to mVOCs at levels comparable to those reported in moldy buildings [65] exhibited Parkinson’s disease-like symptoms [55, 56]. Importantly, when wet, any organic materials such as carpets, fabrics, paper, plywood, compressed wood, and gypsum board can grow mold within 48–72 h. Careless removal of moldy materials and/or applying chemicals such as cleaning agents, bleach, or disinfectants further exposes workers and occupants. MVOCs vary greatly in toxicity as demonstrated by [122] who compared the toxicities of mVOCs from 24 fungal species isolated from mold colonies cultured following Hurricane Sandy on fruit fly larvae in a shared test tube atmosphere [122].
In the present study, exposure to new construction/remodeling was implicated by 12.0% of those fulfilling QEESI criteria for CI. Strong evidence for a causal role for new construction/remodeling-related chemical exposures and CI arose in 1987 when approximately 27,000 square yards of new carpet were installed in the U.S. EPA headquarters building in Washington, D.C., leading to an estimated 124 of 2000 employees subsequently falling ill, eight of whom acquired CI, most often reacting to fragrances, traffic exhaust, and tobacco smoke. Though not the first evidence of CI, this event represents the first widely acknowledged episode involving CI acquired in a sizable group, many of whom were federal indoor air scientists. The substance most implicated in these illnesses was 4-phenylcyclohexene (4-PCH), an undesirable byproduct with an odor of new carpet from the manufacture of styrene-butadiene rubber (SBR) latex—an adhesive used to attach carpets to their backing. A description of this event and related indoor air testing can be found elsewhere [2, 4, 51, 84].
Although initiation by medical/surgical procedures was reported third most often, by 12.6% of those with CI, diverse scenarios were involved, making it challenging to isolate causal associations. However, potentially relevant exposures included anesthetics, intravenous tubing, chemotherapy and other cancer therapies. The best documentation of CI in the context of medical or surgical procedures relates to breast implant recipients [114]. The present findings, and the fact that our survey included questions concerning both implant- and non-implant-related medical/surgical procedures, suggests the need for further research to understand the potential relationship between non-implant-related medical/surgical procedures and the development of CI. Studies of CI related to anesthesia and chemotherapy may provide useful insights, for example, by using the QEESI to evaluate patients pre- and post-surgery or chemotherapy.
Surgical implants were identified as an initiating event by just 1.8% of our CI participants. Following such procedures, many physicians have reported multisystem symptoms in patients closely resembling chronic fatigue syndrome and CI [15, 111]. Importantly, Wee et al. (2020) reported significant improvements in symptoms such as fatigue, memory, and, most notably food intolerances among 750 breast implant patients whose implants were removed. Similarly, Campbell et al. (1994) reported that implant removal reversed symptoms in 40–60% of breast implant patients. Potential exposures include silicone which may leach slowly from intact breast implant membranes [14, 16], producing inflammatory and immunological responses [16, 111], as well as metals which can migrate into surrounding tissue [42] and processing aids and peroxides used to facilitate the curing process for implant gels. At increased risk were individuals with autoimmune antibodies [111, 120], whose symptoms may involve CI [14, 100]. Additional reported associations between chemical intolerance and breast implants appear elsewhere [2, 4, 57, 81].
Pesticides, implicated by 12% of our participants with CI, have long been known to initiate CI, particularly exposures to organophosphates and/or carbamates [2, 4, 77]. Pesticide exposures arise from domestic pesticide use, commercial extermination, occupational use/production, agricultural use, or community-wide spraying [23]. Described in detail casino workers who developed a “mystery illness” coinciding with the application of carbamate and pyrethroid pesticides in the employee café and basement walls [23]. Subsequently, 12 of 19 workers who were referred for medical evaluation developed CI, manifesting as new-onset “sensitivities” to perfumes, gasoline, newsprint, cleaning materials, pesticides, and solvents.
In many communities, aerial pesticide spraying is a regular occurrence, particularly during peak mosquito seasons and in the wake of hurricanes and floods [30, 69, 71]. TILT may represent yet another adverse impact related to climate change as global temperatures rise and the populations and habitat ranges of pests such as ticks and mosquitos increase [58, 73]. Safer approaches to pest management, including organic gardening and farming, the use of beneficial insects, sophisticated modeling to better understand pest populations, and methods that reduce pesticide resistance, can help protect the public from pesticide-initiated CI.
Pesticides were widely applied to prevent vector-borne disease among Gulf War troops. The U.S. Department of Defense estimated that at least 40,000 service members may have been overexposed to organophosphates [50, 96]. Following the war, over 200,000 individuals developed so-called “Gulf War Illness”, characterized by multiple symptoms which often included chemical, food, and drug intolerances [35, 102, 107, 115]. Importantly, research on wartime exposures has also implicated chemical weapons released or present near military personnel during the Gulf War as risk factors for CI, including the organophosphate (OP) nerve agents sarin and cyclosarin, which similarly inhibit the enzyme acetylcholinesterase (AChE). Further, pyridostigmine bromide, also a carbamate, was administered in pill form to an estimated 250,000 U.S. soldiers as a pre-treatment against possible nerve agent exposure [41]. Researchers have thoroughly explored OP toxicity in terms of cholinesterase inhibition. With TILT, we appear to be dealing with an entirely new mechanism for OP toxicity, that is, sensitization of mast cells. This offers a new view of the multisystem, often disabling symptoms and chemical, food, and drug intolerances reported by OP-exposed individuals [80]. As early as 1996, physician and researcher Gunnar Heuser postulated that chemical exposures can trigger a mast cell disorder, which he felt could explain the underlying mechanism of TILT [48, 49].
Miller and Mitzel [77] described 37 individuals who reported multisystem symptoms and chemical and food intolerances following OP pesticide extermination. Theirs was also the first paper to implicate OPs as likely initiators of Gulf War Illness. Other researchers investigating Gulf War Illness have shown that troops exposed to petroleum fires, as well as chemical weapons containing sarin and cyclosarin developed symptoms of CI [41, 50, 115].
Combustion products (including pyrolysis products), which were cited as initiators by over 7% of our CI participants, similarly have been implicated as initiators of CI. This was described by pilots, flight attendants, and frequent flyers exposed to visible smoke/fumes and strong odors followed by CI-like symptoms, a phenomenon known as “aerotoxic syndrome”. Michaelis et al. [74] surveyed British pilots about their experience with contaminated air aboard aircraft and found that 88% were aware of cabin air contamination, with 34% reporting “frequent” exposures, 7% reporting visible smoke or mist, and 53% describing neurological symptoms including “chemical sensitivity” [74]. Similarly, following the collapse of the World Trade Center (WTC), Dr. Steven Levin of the Mount Sinai School of Medicine noted that some of his patients “once away from Lower Manhattan have noticed a general improvement in their symptoms but find that exposure to cigarette smoke, vehicle exhaust, cleaning solutions, perfume, or other airborne irritants provokes reoccurrence of their symptoms in ways they never experienced before 9/11.” The WTC disaster exposed many individuals to high concentrations of complex combustion particles [72].
Possible mechanisms
There is evidence linking CI to autoimmunity through autoantibody production against myelin basic protein, myelin-associated glycoprotein, ganglioside GM1, smooth muscle cells, and antinuclear autoantibodies [1, 19, 44, 105]. Other proposed mechanisms for CI involve olfactory-limbic kindling, that is, amplification of reactivity to inhaled and ingested chemicals resulting in persistent affective, cognitive, and somatic symptoms [7,8,9, 17]. Building on this mechanism, [86] outlined a plausible set of interacting synergistic biomechanisms implicating an excess of N-methyl-D-aspartic acid or N-methyl-D-aspartate (NMDA) activity effecting nitric oxide-mediated stimulation of glutamate, decreased cytochrome P450 metabolism, and ATP depletion, resulting in increased permeability of the blood–brain barrier, thereby allowing organic chemicals to enter the central nervous system and resulting in CI symptomatology [86].
Organophosphate pesticides (OPs), which bind irreversibly to cholinergic receptors, appear to be among the most severe and permanently damaging CI initiators. Organophosphates can trigger degranulation of human and animal mast cells [119]. In mice, repeated oral administration of the OP malathion led to mast cell degranulation at doses below those that inhibit cholinesterase. Malathion is widely used for mosquito control, agriculture, and landscaping. Residues are present in foods [6].
The parasympathetic nervous system also modulates mast cell activity via a cholinergic pathway [34]. Mast cells play pivotal roles in regulating cerebral blood flow [68], directly affecting brain function. Notably, both mast cell activation syndrome (MCAS) and CI patients commonly report cognitive difficulties, including “brain fog”. A study by one of this paper’s authors (Miller) involving low-level VOC (acetone) exposure in ill vs. asymptomatic Gulf War vets demonstrated that cerebral blood flow failed to increase to match the requirements of a difficult cognitive task [18, 81].
Some of the most severely “TILTed” individuals report initiation by exposure to OPs [77]. Groups exposed to OPs and at risk for TILT include agricultural workers, sheep dippers, building occupants exposed to pesticides, Gulf War soldiers, and airline crew members exposed to “fume events” during which engine lubricants bleed into cabin air [2, 4, 116]. OPs irreversibly bind acetylcholinesterase (ACHE). Activity of the enzyme paraoxonase, or PON1, helps determine a person’s ability to detoxify OPs [24, 36, 59] and may explain why certain individuals are particularly susceptible to TILT. However, mast cell sensitization helps explain the long-term illnesses and CI that some individuals develop.
Finally, the role of oxidative stress (OS) must be considered. It has been established that reactive oxygen species (ROS) are involved with intracellular signaling of various proinflammatory cytokines regulating innate immunity, including mast cells [21]. Several studies demonstrate that over-production of ROS triggers proinflammatory processes through activation of several regulatory proteins [95].
OS in known to mediate various allergic disorders, such as asthma, rhinitis, and atopic dermatitis. Understanding the biopathways of OS along with the role that antioxidants play, can help in the development of treatments of many associated diseases [45]. Persistent OS can damage proteins, lipids, and DNA as a result of inflammation initiated from environmental exposures [13]—either from a single high-level toxicant exposure, or from concurrent low-level exposures from multiple sources, such as those initiating exposures reported by the respondents in our survey.
TILT appears to involve alteration/sensitization of our immune systems’ ancient first responders—mast cells—so that individuals no longer tolerate previously endured chemical inhalants, foods and drugs. In this way, TILT resembles both allergy and toxicity. Both stages of TILT—(I) initiation by a wide variety of toxicants and (2) subsequent triggering by previously tolerated xenobiotics—appear to involve oxidative stress leading to the generation of free radicals which can disrupt cell metabolism, gene expression, and signal transduction. TILT initiation, for example, may result from DNA oxidation causing instability of the genome by affecting mast cell DNA, either directly or epigenetically [21].
Taken together, our data support the idea that the person who reports multiple symptoms, multiple intolerances, and recurrent infections as well as a history of exposure events is sharing a cohesive narrative, one that points to physiological (as opposed to psychosomatic) explanations of their oft-confusing complaints. Patients with high QEESI scores may have experienced one or several toxic exposures over time as well as multiple protracted antibiotic courses. Personal histories are complex. For example, a water intrusion event that led to mold growth may have been remediated using phenolic disinfectants or bleach, with fragrances applied in order to mask odors. Subsequent remodeling might include demolition, installing new carpet, painting, applying adhesives, and the introduction of new outgassing finishing materials and furnishings (e.g., particleboard). These exposures irritate the airways and could lead to chronic or recurrent sinus infections, for which doctors may prescribe protracted courses of antibiotics. Both pesticides and antibiotics can disrupt normal gastrointestinal flora resulting in new food intolerances.
Our results suggest that exposures to antibiotics over the life course and certain major environmental exposures are predictive of CI. This is consistent with a recent paper by [99] reporting an association between infection and CI [99]. Although not reported in their manuscript, it may be surmised that those with infections receive antibiotic treatments. From this we might infer that there may be a causal relationship between these exposures and later intolerances that manifest as multisystem symptoms which wax and wane in response to subsequent chemical, food, and drug exposures. Future research should explore the mechanism by which exposures and/or alterations in the gut microbiome may compromise our ancient mast cells and innate cell-mediated tolerance. Allergy and toxicology as currently practiced appear to have overlooked the two steps of TILT and the fact that toxic exposures can sensitize mast cells.
Chemically intolerant individuals have few proven treatment options other than avoiding exposures that initiate and continue to trigger their symptoms. Many remain on restrictive elimination diets for years. Pre- and probiotics offer a potentially attractive option. In our most recent study, we demonstrated that nearly 60% of patients diagnosed with mast cell activation syndrome (MCAS) met QEESI criteria for CI. This suggests that therapies used to treat MCAS may also be useful for treating CI/TILT, e.g., medications like cromolyn which prevent mast cell degranulation and/or H1 and H2 antihistamines which block the action of histamine released by mast cells on tissues [80, 83, 118].
Implications for future research
Our large population-based surveys have identified many of the most frequently cited CI/TILT initiating exposures. All of these exposures except for mold involve fossil fuels, their combustion products and/or their synthetic chemical derivatives. Key culprits in both cases—VOCs from mold and fossil fuels—appear to involve low molecular weight VOCs such as terpenes in fragrances or short chain volatiles released by molds identified by [122]. Further studies should address the following research questions, which we believe to be high yield:
-
(1)
Are fossil fuels exposures sensitizing our ancient immune systems’ first responders, that is, mast cells? (TILT Stage l, Initiation)
-
(2)
Do sensitized mast cells release cascades of mediators (histamine, cytokines) when they subsequently encounter structurally unrelated xenobiotics, for example, nanograms of inhaled VOCs, or foods/food additives in the GI tract? (TILT Stage 2, Triggering).
Practical interventions for patients suspected of having TILT now involve: (1) explaining to them, their families, landlords and employers that they are now sensitive to tiny quantities of particles and VOCs which may arise from biological sources (mold, algae) or from fossil fuels, their combustion products or synthetic chemical derivatives and (2) discussing how these might be eliminated from their home, school, and work environments (e.g., no exposure to fragrances, combustion products, no attached garages, etc.). Living with restrictions like these is a daunting challenge. Consequently, we recommend that public health measures and medical counseling should include recommendations to reduce or avoid initiating exposures as the first order of business.
One in four primary care patients suffers from MUS. Doctors in primary care, neurology, psychiatry, psychology, occupational medicine, and allergy/immunology would be well-advised to incorporate the QEESI and an individualized exposure history in their evaluation of patients with MUS as well as those diagnosed with conditions of unknown etiology such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), asthma, fibromyalgia, autism, ADHD, depression or other psychological conditions. None of these are etiologic diagnoses; CI and TILT are etiologic diagnoses. Practitioners should not presume that any illness is psychosomatic without exploring environmental initiators and triggers. They need to screen all patients using the BREESI/QEESI and learn about CI and TILT. The GI tract is densely populated with mast cells, and digestive difficulties and food intolerances are prevalent in CI. The role of contemporary exposures in initiating and exacerbating these conditions via mast cell sensitization needs our immediate attention. Mast cells evolved over 500 million years ago. In contrast, our exposures to fossil fuels and combustion products are new since the Industrial Revolution (less than 300 years ago), while exposures to fossil fuel-derived synthetic chemicals have grown exponentially in recent generations since WWII—less than 100 years ago. Fossil fuels are assaulting humans and other animal species both from within via mast cell sensitization, and from without via climate change.
Strengths and limitations
The considerable number of randomly sampled survey participants (n > 10,000) is a strength of this study. It improves the generalizability of our findings and enhances our ability to understand the prevalence of CI across the U.S. and across genders and age groups. It also improves our understanding of exposures that may initiate CI/TILT, thus extending our prior work, which was limited to previously published case reports and others’ studies that were not population-based [72].
Also, the application of multivariate statistical methods expands our understanding of the relative roles of various xenobiotics in CI/TILT initiation, representing a step forward in the literature. Lastly, our search for the underlying causes of CI represents a much-needed addition to the CI/TILT literature whose principal focus has been on so-called “triggers” that elicit CI symptoms from day-to-day with no attempt to determine what initiated TILT.
This study also has important limitations. First, we should note that event-driven analyses remain an inherently more reliable indicator of what initiates CI/TILT. However, in the absence of large cohorts who developed TILT following well-characterized exposure events, the use of population-based surveys, as employed in this study, represents a valuable alternative for understanding what initiates or truly underlies CI/TILT. Of note, our findings mirror decades of observations in countries on five continents by many thousands of patients, physicians, and public health practitioners.
An additional limitation is that only about half of those with positive QEESI screens recalled a specific exposure that may have initiated their symptoms. However, given the exponential increase in exposures to toxicants derived from fossil fuels and biological sources, coupled with reduced fresh air in buildings due to energy conservation efforts spurred by the 1973 Arab oil embargo, TILT has become epidemic.
Another potential limitation is the absence of race/ethnicity data for our participants, which prevents any comparison of CI prevalence across different minority populations. Although this analysis includes a diverse survey population and substantial numbers of participants, we cannot rule out the possibility of selection bias. Thus, it is useful to discuss the ways in which such bias may have entered our study as well as its implications for our findings. Given that the completion of our survey on a computer required active engagement and therefore a minimum level of health and wellbeing, it is likely that our survey under-sampled individuals most affected by CI/TILT. Importantly, however, this “healthy participant effect” (analogous to the healthy worker effect) would tend to bias our results in the direction of making our estimates of CI prevalence overly conservative.
Such bias may have been more pronounced among elderly survey participants, which may partly explain the reduced prevalence of CI reported in this age group. Lack of access to the Internet, a computer, or a smartphone, as well as language limitations, also may have reduced the generalizability of our findings across low-income and minority populations. Lastly, a potential limitation inherent in health-related surveys is so-called “recall bias”, in which individuals most afflicted by an illness are more apt to recall and report exposure-related details.
Conclusion
Using the internationally validated QEESI in a survey of over 10,000 U.S. adults, we were able to document a 20% prevalence of CI, half of whom attributed onset of their illness to an initiating exposure event or multiple events. A lifetime history of protracted courses of antibiotics also was associated with CI/TILT, as were specific prostate, skin, tonsil, gastrointestinal tract, and sinus-related antibiotic uses. The initiating exposures most frequently reported to be associated with CI/TILT were mold, remodeling/new construction, medical/surgical procedures, and pesticides, while combustion products, implants, and others were less frequently reported. Initiating events with the highest odds ratios included pesticides, breast implants, mold, and combustion exposures, followed in order by remodeling/new construction and surgical/medical procedures. Overall, woman tended to score higher on the QEESI than did men. This study elucidates the types of exposure events that may initiate CI/TILT, thereby providing useful insights into the ways in which populations, including sensitive subgroups, can avoid TILT in the future. Although certain exposures such as medical/surgical procedures may be difficult to avoid, reducing exposures to contaminants related to pesticide use, new construction/remodeling, and mold is possible and should be the focus of efforts to prevent future CI/TILT. The fact that many patients report TILT initiated by various drugs, implants, and surgical procedures makes it important that all patients be screened for CI using the BREESI/QEESI and whenever feasible, that individual susceptibility be considered prior to medical or surgical interventions.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
References
Abou-donia MB, Lieberman A, Curtis L (2018) Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures. Toxicol Ind Health 34(1):44–53. https://doi.org/10.1177/0748233717733852
Ashford NA, Miller CS (1998) Low-level chemical exposures: a challenge for science and policy. Environ Sci Technol 32(21):508A
Ashford N, Heinzow B, Lütjen K, Marouli C, Mølhave L, Mönch B, Papadopoulos S, Rest K, Rosdahl D, Siskos P, Velonakis E (1995) Chemical sensitivity in selected european countries: an exploratory study. A Report to the European Commission, Athens
Ashford N, Miller C (1998) Chemical exposures: low levels and high stakes. Wiley, Hoboken
Azuma K, Uchiyama I, Katoh T, Ogata H, Arashidani K, Kunugita N (2015) Prevalence and characteristics of chemical intolerance: a Japanese population-based study. Arch Environ Occup Health 70(6):341–353. https://doi.org/10.1080/19338244.2014.926855
Bajwa U, Sandhu KS (2014) Effect of handling and processing on pesticide residues in food- a review. J Food Sci Technol 51(2):201–220. https://doi.org/10.1007/s13197-011-0499-5
Bell IR (1996) Clinically relevant EEG studies and psychophysiological findings: possible neural mechanisms for multiple chemical sensitivity. Toxicology 111(1–3):101–117. https://doi.org/10.1016/0300-483X(96)03395-1
Bell IR, Rossi J, Gilbert ME, Kobal G, Morrow LA, Newlin DB, Sorg BA, Wood RW (1997) Testing the neural sensitization and kindling hypothesis for illness from low levels of environmental chemicals. Environ Health Perspect 105(SUPPL. 2):539–547. https://doi.org/10.1289/ehp.97105s2539
Bell IR, Miller CS, Schwartz GE (1992) An olfactory-limbic model of multiple chemical sensitivity syndrome: possible relationships to kindling and affective spectrum disorders. Biol Psychiatry. 32(3):218–242. https://doi.org/10.1016/0006-3223(92)90105-9
Bennett JW, Inamdar AA (2015) Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins 7(9):3785–3804. https://doi.org/10.3390/toxins7093785
Biffignandi S, Bethlehem J (2021) Handbook of web surveys. Wiley, Hoboken
Borody TJ, Nowak A, Finlayson S (2012) The GI microbiome and its role in chronic fatigue syndrome: a summary of bacteriotherapy. J Australas Coll Nutr Environ Med 31(3):3–8
Bowler RP, Crapo JD (2002) Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol 110(3):349–356. https://doi.org/10.1067/mai.2002.126780
Brautbar N, Campbell A (1995) Silicone implants and immune dysfunction—scientific evidence for causation. Int J Occup Med Tox 4:3–13
Brautbar N, Vojdani A, Campbell AW (1992) Multiple chemical sensitivities—fact or myth. Toxicol Ind Health 8:5–8
Brautbar N, Vojdani A, Campbell A (1994) Silicone breast implants and autoimmunity: causation or myth? Arch Environ Health 49(3):151–153. https://doi.org/10.1080/00039896.1994.9940373
Brown-DeGagne A-M, McGlone J (1999) Multiple chemical sensitivity: a test of the olfactory-limbic model. J Occup Environ Med 41(5):366–377
Bunegin L, Mitzel HC, Miller CS, Gelineau JF (2001) Cognitive performance and cerebrohemodynamics associated with the Persian Gulf Syndrome. Toxicol Ind Health. https://doi.org/10.1191/0748233701th100oa
Andrew W. Campbell, Jack D. Thrasher, Roberta A. Madison, Aristo Vojdani, Michael R. Gray, Al Johnson (2003) Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch Environ Health: Int J 58(8):464–474. https://doi.org/10.3200/AEOH.58.8.464-474
Caress SM, Steinemann AC (2004) Prevalence of multiple chemical sensitivities: a population-based study in the Southeastern United States. Am J Public Health 94(5):746–747. https://doi.org/10.2105/AJPH.94.5.746
Chelombitko MA, Fedorov AVI, Ilyinskaya OP et al (2016) Role of reactive oxygen species in mast cell degranulation. Biochem Moscow 81:1564–1577. https://doi.org/10.1134/S000629791612018X
Chiu K, Warner G, Nowak RA, Flaws JA, Mei W (2020) The impact of environmental chemicals on the gut microbiome. Toxicol Sci 176(2):253–284. https://doi.org/10.1093/toxsci/kfaa065
Cone JE, Sult TA (1992) Acquired intolerance to solvents following pesticide/solvent exposure in a building: a new group of workers at risk for multiple chemical sensitivities? Toxicol Ind Health 8(4):29–39. https://doi.org/10.1177/074823379200800404
Costa LG, Cole TB, Vitalone A, Furlong CE (2005) Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta 352(1–2):37–47
Couper M (2000) Web surveys: a review of issues and approaches. Public Opin Q 64(4):464–494
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19(2):179–194. https://doi.org/10.1016/S1474-4422(19)30356-4
Cui X, Lu X, Hiura M, Oda M, Hisada A, Miyazaki W, Omori H, Katoh T (2014) Prevalence and interannual changes in multiple chemical sensitivity in Japanese workers. Environ Health Prev Med 19(3):215–219. https://doi.org/10.1007/s12199-014-0378-6
Cullen MR (1987) The worker with multiple chemical sensitivities: an overview. Occup Med 2(4):655–662
Damiani G, Alessandrini M, Caccamo D, Cormano A, Guzzi G, Mazzatenta A, Micarelli A, Migliore A, Piroli A, Bianca M, Tapparo O, Pigatto PDM (2021) Italian expert consensus on clinical and therapeutic management of multiple chemical sensitivity (MCS). Int J Environ Res Public Health 18(21):1–19. https://doi.org/10.3390/ijerph182111294
Duprey Z, Rivers S, Luber G, Becker A, Barr D, Weerasekera G, Kieszak S, Dana W, Rubin C, Blackmore C, Barr D, Weerasekera G, Kieszak S, Flanders WD, Rubin C (2008) Community aerial mosquito control and Naled exposure. J Am Mosq Control Assoc 24(1):42–46
Fang B, Li JW, Zhang M, Ren FZ, Pang GF (2018) Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol 111:144–152
Fitzgerald DJ (2008) Studies on self-reported multiple chemical sensitivity in South Australia. Environ Health 8(3):33–39
Fink AL, Klein SL (2018) The evolution of greater humoral immunity in females than males: implications for vaccine efficacy. Curr Opin Physiol. 6–20. https://doi.org/10.1016/j.cophys.2018.03.010. Epub 2018 Mar 29. PMID: 30320243; PMCID: PMC6181235
Forsythe P. (2015) The parasympathetic nervous system as a regulator of mast cell function. Methods Mol Biol;1220:141–154. https://doi.org/10.1007/978-1-4939-1568-2_9MID: 25388249
Fukuda K, Nisenbaum R, Stewart G, Thompson W, Robin L, Washko R, Noah D, Barrett D, Randall B, Herwaldt B, Mawle A, Reeves W (1998) Chronic Multisymptom illness affecting air force veterans of the Gulf War. J Am Med Assoc 280:981–988
Furlong CE (2000) PON1 status and neurologic symptom complexes in Gulf War veterans. Genome Res 10(2):153–155. https://doi.org/10.1101/gr.10.2.153
Gangi S, Johansson O (2000) A theoretical model based upon mast cells and histamine to explain the recently proclaimed sensitivity to electric and/or magnetic fields in humans. Med Hypotheses 54(4):663–671. https://doi.org/10.1054/mehy.1999.0923
Genuis SJ (2010) Sensitivity-related illness: the escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci Total Environ 408(24):6047–6061. https://doi.org/10.1016/j.scitotenv.2010.08.047
Gibson PR, Vogel VM (2008) Sickness-related dysfunction in persons with self-reported multiple chemical sensitivity at four levels of severity. J Clin Nurs 18(1):72–81
Glinton GJ (2005) Multiple-chemical sensitivity. Medsurg Nurs 14(6):365–369
Golomb BA (2008) Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci USA 105(11):4295–4300. https://doi.org/10.1073/pnas.0711986105
Gotman I (1997) Characteristics of metals used in implants. J Endourol 11(6):383–389
Gould Peek G, Wallace Lyon M (2014) Housing and health relationship: multiple chemical sensitivity (MCS). Hous Soc 41(1):31–52. https://doi.org/10.1080/08882746.2014.11430620
Gray MR, Thrasher JD, Crago R, Madison RA, Arnold L, Campbell AW, Vojdani A (2003) Mixed mold mycotoxicosis: Immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health 58:410–420
Han M, Lee D, Lee S, Kim TH (2021) Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants 10(8):1266
Heo Y, Kim SH, Lee SK, Kim HA (2017) Factors contributing to the self-reported prevalence of multiple chemical sensitivity in public facility workers and the general population of Korea. J UOEH 39(4):249–258. https://doi.org/10.7888/juoeh.39.249
Hess J, Bednarz D, Bae J, Pierce J (2011) Petroleum and health care: Evaluating and managing health care’s vulnerability to petroleum supply shifts. Am J Public Health 101(9):1568–1579. https://doi.org/10.2105/AJPH.2011.300233
Heuser G (2000) Mast cell disorder to be ruled out in MCS. Arch Environ Health 55(4):284–285
Heuser G, Kent P (1996) Mast cell disorder after chemical exposure. 124th Annual Meeting. Am Public Health Assoc, New York
Hilborne L, Golomb B, Marshall G, Davis L, Sherbourne C, Augerson W, Spektor D, Harley N, Foulkes E, Hudson A, Anthony C, Cecchine G, Marlowe D, Rettig R, Fricker R, Reardon E, Cotton S, Hawes-Dawson J, Pace J, Hosek S (2020) Examining possible causes of Gulf War illness: RAND policy investigations and reviews of the scientific literature. RAND Corporation, Santa Monica. https://doi.org/10.7249/rb7544
Hirzy JW, Morison R (1991) Carpet/4-phenylcyclohexene toxicity: the EPA headquarters case. Anal Commun Percept Risk. https://doi.org/10.1007/978-1-4899-2370-7
Hojo S, Kumano H, Yoshino H, Kakuta K, Ishikawa S (2003) Application of quick environment exposure sensitivity inventory (QEESI©) for Japanese population: study of reliability and validity of the questionnaire. Toxicol Ind Health 19(6):41–49. https://doi.org/10.1191/0748233703th180oa
Hojo S, Mizukoshi A, Azuma K, Okumura J, Ishikawa S, Miyata M, Mizuki M, Ogura H, Sakabe K (2018) Survey on changes in subjective symptoms, onset-trigger factors, allergic diseases, and chemical exposures in the past decade of Japanese patients with multiple chemical sensitivity. Int J Hyg Environ Health 221:1085–1096
Hutton Carlsen K, Topp AM, Skovbjerg S (2012) Living with a chemically sensitive wife: a “We” situation. ISRN Public Health 2012:1–6. https://doi.org/10.5402/2012/285623
Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW (2013) Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc Natl Acad Sci USA 110(48):19561–19566. https://doi.org/10.1073/pnas.1318830110
Inamdar AA, Masurekar P, Bennett JW (2010) Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. Toxicol Sci 117(2):418–426. https://doi.org/10.1093/toxsci/kfq222
Israeli E, Pardo A (2011) The sick building syndrome as a part of the autoimmune (auto-inflammatory) syndrome induced by adjuvants. Mod Rheumatol 21:235–239. https://doi.org/10.3109/s10165-010-0380-9
Jaenson TGT, Hjertqvist M, Bergström T, Lundkvist Å (2012) Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors 5(1):1–13. https://doi.org/10.1186/1756-3305-5-184
Jansen K, Cole T, Park S, Furlong C, Costa L (2009) Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicol Appl Pharmacol 236(2):1–26. https://doi.org/10.1016/j.taap.2009.02.001.Paraoxonase
Jeon B-H, Lee S-H, Kim H-A (2012) A validation of the Korean version of QEESI© (The quick environmental exposure and sensitivity inventory). Korean J Occup Environ Med 24(1):96. https://doi.org/10.35371/kjoem.2012.24.1.96
JMP® (1989) JMP, Version 15
Johansson O, Gangi S, Liang Y, Yoshimura K, Jing C, Liu P-Y (2002) Cutaneous mast cells are altered in normal healthy volunteers sitting in front of ordinary TVs/PCs—results from open-field provocation experiments. J Cutan Pathol 28(10):513–519
Karvala K, Sainio M, Palmquist E, Nyback MH, Nordin S (2018) Prevalence of various environmental intolerances in a Swedish and Finnish general population. Environ Res 161(July 2017):220–228. https://doi.org/10.1016/j.envres.2017.11.014
Kilburn KH (2009) Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. Toxicol Ind Health 25(10):681–692. https://doi.org/10.1177/0748233709348390
Korpi A, Järnberg J, Pasanen AL (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39(2):139–193. https://doi.org/10.1080/10408440802291497
Kreutzer R, Neutra RR, Lashuay N (1999) Prevalence of people reporting sensitivities to chemicals in a population-based survey. Am J Epidemiol 150(1):1–12. https://doi.org/10.1126/science.4.80.55
Li JW, Fang B, Pang GF, Zhang M, Ren FZ (2019) Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic Biochem Physiol 159:68–79. https://doi.org/10.1016/j.pestbp.2019.05.018
Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML (2010) Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab 30(4):689–702. https://doi.org/10.1038/jcbfm.2009.282
Livingston EH (2017) Safety of aerial pesticide spraying for mosquitoes. J Am Med Assoc 310(3):333
Madden JAJ, Hunter JO (2002) A review of the role of the gut microflora in irritable bowel syndrome and the effects of probiotics. Br J Nutr 88(S1):s67–s72. https://doi.org/10.1079/bjn2002631
Mann ME, Emanuel KA (2006) Atlantic hurricane trends linked to climate change. Eos 87(24):233–241. https://doi.org/10.1029/2006EO240001
Masri S, Miller CS, Palmer RF, Ashford N (2021) Toxicant-induced loss of tolerance for chemicals, foods, and drugs: assessing patterns of exposure behind a global phenomenon. Environ Sci Europe. https://doi.org/10.1186/s12302-021-00504-z
McMichael AJ, Woodruff RE, Hales S (2006) Climate change and human health: present and future risks. Lancet 367(9513):859–869. https://doi.org/10.1016/S0140-6736(06)68079-3
Michaelis S, Burdon J, Howard C (2017) Aerotoxic syndrome: a new occupational disease? Public Health Panorama 03(02):198–211
Miller CS. (1996) Chemical sensitivity: symptom, syndrome or mechanism for disease? Toxicology 111(1-3):69–86. https://doi.org/10.1016/0300-483x(96)03393-8. PMID: 8711750
Miller CS (1997) Toxicant-induced loss of tolerance--an emerging theory of disease? Environ Health Perspect 105 (Suppl 2):445-453. https://doi.org/10.1289/ehp.97105s2445. PMID: 9167978; PMCID: PMC1469811
Miller C, Mitzel H (1995) Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch Environ Health 50(2):119–129
Miller CS (2000) Toxicant-induced loss of tolerance. Addiction. https://doi.org/10.1080/09652140020017003
Miller CS, Ashford N (1995) Chemical sensitivity: perspectives from North America and Europe. 1–19
Miller CS, Palmer RF, Dempsey TT, Ashford NA, Afrin LB (2021) Mast cell activation may explain many cases of chemical intolerance. Environ Sci Europe. https://doi.org/10.1186/s12302-021-00570-3
Miller CS, Prihoda TJ (1999) A controlled comparison of symptoms and chemical intolerances reported by Gulf War veterans, implant recipients and persons with multiple chemical sensitivity. Toxicol Ind Health. 15(3-4):386–397. https://doi.org/10.1177/074823379901500312
Miller CS, Prihoda TJ (1999) The environmental exposure and sensitivity inventory (EESI): a standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Ind Health 15(4):370–385. https://doi.org/10.1177/074823379901500311
Molderings GJ, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL, Panse J, Butterfield J, Afrin LB (2016) Pharmacological treatment options for mast cell activation disease. Naunyn-Schmiedeberg’s Arch Pharmacol 389(7):671–694. https://doi.org/10.1007/s00210-016-1247-1
National Federation of Federal Employees (1989) Indoor Air Quality and Work Environment Study
Nynäs P, Vilpas S, Kankare E, Karjalainen J, Lehtimäki L, Numminen J, Tikkakoski A, Kleemola L, Uitti J (2021) Clinical findings among patients with respiratory symptoms related to moisture damage exposure at the workplace—the samdaw study. Healthcare. https://doi.org/10.3390/healthcare9091112
Pall ML (2002) NMDA sensitization and stimulation by peroxynitrite, nitric oxide, and organic solvents as the mechanism of chemical sensitivity in multiple chemical sensitivity. FASEB J 16(11):1407–1417. https://doi.org/10.1096/fj.01-0861hyp
Palmer RF, Jaén CR, Perales RB, Rincon R, Forster JN, Miller CS (2020) Three questions for identifying chemically intolerant individuals in clinical and epidemiological populations: the brief environmental exposure and sensitivity inventory (BREESI). PLoS ONE 15(9 September):1–10. https://doi.org/10.1371/journal.pone.0238296
Park SW, Choi J, Park HO, Lim YS, Lee KS, Kim NG, Kim JS (2004) Are gender differences in external noses caused by differences in nasal septal growth? J Cranio-Maxillo-Facial Surgery 42:11401147
Palmer RF, Walker T, Kattari D, Rincon R, Perales RB, Jaén CR, Grimes C, Sundblad DR, Miller CS (2021) Validation of a brief screening instrument for chemical intolerance in a large U.S. national sample. Int J Environ Res Public Health 18(16):8714. https://doi.org/10.3390/ijerph18168714. PMID: 34444461; PMCID: PMC8391803
Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC (2017) Diet, gut microbiota and cognition. Metab Brain Dis 32(1):1–17. https://doi.org/10.1007/s11011-016-9917-8
Proctor SP (2000) Chemical sensitivity and gulf war veterans’ illnesses. Occup Med 15(3):587–599
Rajkovic V, Matavulj M, Johansson O (2005) Histological characteristics of cutaneous and thyroid mast cell populations in male rats exposed to power-frequency electromagnetic fields. Int J Radiat Biol 81(7):491–499
Rajkovic V, Matavulj M, Johansson O (2010) Combined exposure of peripubertal male rats to the endocrine-disrupting compound atrazine and power-frequency electromagnetic fields causes degranulation of cutaneous mast cells: a new toxic environmental hazard? Arch Environ Contam Toxicol 59:334–341
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H (2020) Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol 10(November):1–10. https://doi.org/10.3389/fcimb.2020.572912
Ranneh Y, Ali F, Akim AM et al (2017) Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: a review. Appl Biol Chem 60:327–338. https://doi.org/10.1007/s13765-017-0285-9
Research Advisory Committee on Gulf War Veterans’ Illness (2008) Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations
Rossi S, Pitidis A (2018) Multiple chemical sensitivity: review of the state of the art in epidemiology, diagnosis, and future perspectives. J Occup Environ Med 60(2):138–146. https://doi.org/10.1097/JOM.0000000000001215
SAS® (2014) SAS, 9.4, Third Edition
Schovsbo SU, Møllehave LT, Petersen MW, Ahrendt Bjerregaard A, Eliasen M, Pedersen SB, Eplov LF, Kårhus LL, Fink P, Linneberg A, Dantoft TM, Jørgensen T, Benros ME (2022) Association between infections and functional somatic disorders: a cross-sectional population-based cohort study. BMJ Open 12(11):e066037. https://doi.org/10.1136/bmjopen-2022-066037
Shoenfeld Y, Ryabkov VA, Scheibenbogen C, Brinth L, Martinez-Lavin M, Ikeda S, Heidecke H, Watada A, Bragazzi NL, Chapman J, Churilov LP, Amital H (2020) Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune autoimmune dysautonomia and small fiber neuropathy. Clin Immunol 214:1–11
Skovbjerg S, Berg ND, Elberling J, Christensen KB (2012) Evaluation of the quick environmental exposure and sensitivity inventory in a Danish population. J Environ Public Health. https://doi.org/10.1155/2012/304314
Steele L (2000) Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol 152(10):992–1002. https://doi.org/10.1093/aje/152.10.992
Steinemann A (2018) National prevalence and effects of multiple chemical sensitivities. J Occup Environ Med 60(3):e152–e156. https://doi.org/10.1097/JOM.0000000000001272
Syromyatnikov MY, Isuwa MM, Savinkova OV, Derevshchikova MI, Popov VN (2020) The effect of pesticides on the microbiome of animals. Agriculture 10(3):1–14. https://doi.org/10.3390/agriculture10030079
Tuuminen T (2020) The roles of autoimmunity and biotoxicosis in sick building syndrome as a “Starting Point” for irreversible dampness and mold hypersensitivity syndrome. Antibodies 9(2):26. https://doi.org/10.3390/antib9020026
Tuuminen T, Rinne KS (2017) Severe sequelae to mold-related illness as demonstrated in two Finnish cohorts. Front Immunol 8(APR):1–9. https://doi.org/10.3389/fimmu.2017.00382
Unwin C, Blatchley N, Coker W, Ferry S, Hotopf M, Hull L, Ismail K, Palmer I, David A, Wessely S (1999) Health of UK servicemen who served in Persian Gulf War. Lancet 353:169–178
Valtonen V (2017) Clinical diagnosis of the dampness and mold hypersensitivity syndrome: review of the literature and suggested diagnostic criteria. Front Immunol 8(1):1–6. https://doi.org/10.3389/fimmu.2017.00951
Vanderhoof JA, Young RJ (2003) Role of probiotics in the management of patients with food allergy. Ann Allergy Asthma Immonol 90:99–103
der Varrt IB, Heath MD, Guagnini F, Kramer MF (2016) In vitro evidence for efficacy in food intolerance for the multispecies probiotic formulation Ecologic. Beneficial Microbes 7(1):111–118
Vojdani A, Campbell A, Brautbar N (1992) Immune functional impairment in patients with clinical abnormalities and silicone breast implants. Toxicol Ind Health 8(6):415–429. https://doi.org/10.1177/074823379200800606
Vuokko A, Karvala K, Lampi J, Keski-Nisula L, Pasanen M, Voutilainen R, Pekkanen J, Sainio M (2018) Environmental intolerance, symptoms and disability among fertile-aged women. Int J Environ Res Public Health 15(2):1–12. https://doi.org/10.3390/ijerph15020293
Vuokko A, Karvala K, Suojalehto H, Lindholm H, Selinheimo S, Heinonen-Guzejev M, Leppämäki S, Cederström S, Hublin C, Tuisku K, Sainio M (2019) Clinical characteristics of disability in patients with indoor air-related environmental intolerance. Saf Health Work 10(3):362–369. https://doi.org/10.1016/j.shaw.2019.06.003
Wee CE, Younis J, Isbester K, Smith A, Wangler B, Sarode AL, Patil N, Grunzweig K, Boas S, Harvey DJ, Kumar AR, Feng LJ (2020) Understanding breast implant illness, before and after explantation: a patient-reported outcomes study. Ann Plast Surg 85(S1):S82–S86. https://doi.org/10.1097/SAP.0000000000002446
White R, Steele L, O’Callaghan J, Sullivan K, Binns J, Golomb B, Bloom F, Bunker J, Crawford F, Graves J, Hardie A, Klimas N, Knox M, Meggs W, Melling J, Philber M, Grashow R (2016) Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex. https://doi.org/10.1016/j.cortex.2015.08.022.Recent
Winder C (2000) Aerotoxic Syndrome : adverse health effects following exposure to jet oil mist during commercial flights. Towards a safe and civil society. Proceedings of the international congress on occupational health conference, September, 4–6. https://bleedfree.eu/wp-content/uploads/2015/08/07_winder_aerotoxic_syndrome-kopie.pdf
Winder C (2002) Mechanisms of multiple chemical sensitivity. Toxicol Lett 128:85–97
Wirz S, Molderings GJ (2017) A practical guide for treatment of pain in patients with systemic mast cell activation disease. Pain Physician 20(6):E849–E861. https://doi.org/10.36076/ppj.20.5.e849
Xiong S, Rodgers K (1997) Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J Toxicol Environ Health 51(2):159–175. https://doi.org/10.1080/00984109708984019
Zandman-Goddard G, Blank M, Ehrenfeld M, Gilburd B, Peter J, Shoenfeld Y (1999) A comparison of autoantibody production in asymptomatic and symptomatic women with silicone. J Rheumatol 26(1):73–77
Zhang Y, Jia Q, Hu C, Han M, Guo Q, Li S, Bo C, Zhang Y, Qi X, Sai L, Peng C (2021) Effects of chlorpyrifos exposure on liver inflammation and intestinal flora structure in mice. Toxicol Res 10(1):141–149. https://doi.org/10.1093/toxres/tfaa108
Zhao G, Yin G, Inamdar AA, Luo J, Zhang N, Yang I, Buckley B, Bennett JW (2017) Volatile organic compounds emitted by filamentous fungi isolated from flooded homes after Hurricane Sandy show toxicity in a Drosophila bioassay. Indoor Air 27(3):518–528. https://doi.org/10.1111/ina.12350
Zhao Y, Zhang Y, Wang G, Han R, Xie X (2016) Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 153:287–293
Acknowledgements
We thank the Marilyn Brachman Hoffman Foundation for generously funding this study and Marilyn Hoffman for her prescient bequest prioritizing research on Toxicant-Induced Loss of Tolerance. We are deeply grateful to the patients who participated in this groundbreaking study.
Funding
This research was funded by the Marilyn Brachman Hoffman Foundation, Fort Worth, TX.
Author information
Authors and Affiliations
Contributions
CSM and RFP: conception and design of this work. All authors contributed to the survey design and DK was responsible for data acquisition. RFP and DK were responsible for the data analysis and interpretation as well as the first draft. Subsequent editing of the drafts was done by SM, NA, RR, RP, CG and DRS. CSM and RFP were responsible for final revisions of the work. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board Statement: the study was conducted according to the guidelines of the Declaration of Helsinki and approved by the University of Texas Health Science Center San Antonio Institutional Review Board (Approval Number HSC20200718N). Written informed consent was waived due to completely anonymous volunteer participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests associated with this manuscript. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, C.S., Palmer, R.F., Kattari, D. et al. What initiates chemical intolerance? Findings from a large population-based survey of U.S. adults. Environ Sci Eur 35, 65 (2023). https://doi.org/10.1186/s12302-023-00772-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00772-x
Keywords
- Chemical intolerance (CI)
- Toxicant-induced loss of tolerance (TILT)
- Multiple chemical sensitivity (MCS)
- Idiopathic environmental intolerance (IEI)
- Pesticides, mold, antibiotics, combustion products, volatile organic compounds (VOCs)
- Microbiome, prevention, breast implant illness, mast cells, environment, exposures, mast cell activation syndrome (MCAS)
- Toxicity, sensitization, electromagnetic fields (EMF)