Abstract
Background
This paper explores the relationship between chemical intolerance (CI) and mast cell activation syndrome (MCAS). Worldwide observations provide evidence for a two-stage disease process called toxicant-induced loss of tolerance (TILT) as a mechanism for CI. TILT is initiated by a major exposure event or a series of lower-level exposures. Subsequently, affected individuals report that common chemical inhalants, foods, and drugs (i.e., various xenobiotics) trigger multi-system symptoms.
Purpose
To determine whether MCAS provides a plausible biological mechanism for CI/TILT.
Methods
Using the validated Quick Environmental Exposure and Sensitivity Inventory (QEESI), we compared patients diagnosed with MCAS (n = 147) to individuals who reported chemical intolerances (CI/TILT) following various exposures (n = 345) and to healthy controls (n = 76). Using ANOVA, we compared QEESI scores across groups. Clinical scores for the MCAS patient group were used to predict CI status using logistic regression.
Results
More than half (59%) of the MCAS group met criteria for CI. A logistic regression model illustrates that as the likelihood of patients having MCAS increased, their likelihood of having CI/TILT similarly increased, to a near-perfect correspondence at the high ends of the QEESI and clinical MCAS scores. Symptom and intolerance patterns were nearly identical for the CI and MCAS groups.
Discussion
We present data suggesting that xenobiotic activation of mast cells may underlie CI/TILT. The strikingly similar symptom and intolerance patterns for MCAS and TILT suggest that xenobiotics disrupt mast cells, leading to either or both of these challenging conditions. Faced with patients suffering from complex illness affecting multiple organ systems and fluctuating inflammatory, allergic, and dystrophic symptoms, clinicians can now ask themselves two questions: (1) Could MCAS be at the root of these problems? (2) Could environmental exposures be driving MC activation and mediator release? Increasing our understanding of the connection between TILT and MCs has the potential to expose a new link between environmental exposures and illness, offering new opportunities for improving individual and public health.
Conclusion
The close correspondence between QEESI scores and symptom patterns for MCAS and TILT patients supports xenobiotic-driven mast cell activation and mediator release (i.e., MCAS) as a plausible unifying biological mechanism for CI/TILT, with profound implications for medicine, public health, and regulatory toxicology.
Similar content being viewed by others
Introduction
Chemical intolerance
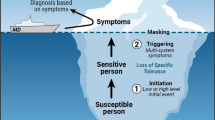
Chemical, food, and drug intolerances are growing international concerns [1,2,3,4,5]. These intolerances may arise following exposures to building construction or remodeling, pesticides, military environments (e.g., Gulf War), combustion products (e.g., World Trade Center disaster, burn pits, wildfires), chemical spills or releases, surgical implants, mold, and many other sources [6, 7]. The exposures may be a one-time acute event; a series of exposures; or long-term, low-level exposures. They often involve particular synthetic chemicals such as an organophosphate pesticide, a combination of synthetic substances, and/or their combustion products. These xenobiotics enter the body via well-known routes: inhalation, ingestion, skin contact, and/or injection/implantation. What remains unclear is why a subset of individuals would subsequently develop multi-system symptoms and persistent intolerances to chemicals, and often foods and drugs, which never bothered them before and do not bother most people. Over the past 70 years, strikingly similar reports have emerged from patients, doctors, and researchers around the world supporting chemical intolerance (CI) as a novel, or at least previously unrecognized, disease.
Many patients attribute onset of their illness and intolerances to a well-defined exposure event [7, 8]. (Readers are directed to this study’s companion paper addressing CI initiating events [7]). Different family members or co-workers who become ill frequently exhibit different manifestations, confounding physicians and investigators [6]. Individuals affected by a particular infectious agent or toxicant often share recognizable constellations of symptoms. This is not the case for CI patients, which has hampered efforts to establish a consensus case definition for CI. It also suggests a mechanism for CI which is distinct from other infectious/toxicant exposures.
There is accumulating evidence for a two-stage causal model linking xenobiotic exposures to subsequent intolerances first described by Miller in 1996 as toxicant-induced loss of tolerance (TILT) [9]. The origins of these intolerances variously have been attributed to classical toxicity, allergy, and psychological factors [10,11,12]. Up to now, an understandable biological mechanism for them has remained elusive.
Mast cells
In the last decade, our understanding of the evolutionarily ancient mast cell (MC) and its ability to effect a host of inflammatory, allergic, and other responses throughout the body has expanded rapidly [13,14,15]. Several factors have resulted in a likely underestimation of the MC’s pivotal role in disease: (1) since the discovery of IgE, allergy’s principal focus has been on the humoral, as opposed to the cellular, immune system; (2) MCs’ typically tiny numbers and their sparse distribution in most tissues have contributed to their anonymity; and (3) MCs are minimally present in the blood, and even where they are present, it has been a challenge to identify and isolate them [16,17,18,19,20].
These sentinel cells guard the perimeters of our skin and other organs, warding off invaders and protecting our internal milieu. They serve as first responders to most bodily invasions and insults. Mast cells originate in the bone marrow and migrate to the interface between our tissues and the external environment [14, 15]. They are highly evolved, critical components of the cellular immune system [15], supporting both innate and adaptive immunity. Largely lying in wait, these warriors spring into action if they perceive a major threat, releasing a vast array of mediators all at once.
Once triggered, MCs can deploy more than 1000 distinct cell-surface mediator receptors [21] resulting in inflammation, allergic-like phenomena, or altered tissue growth and development (dystrophism) [22, 23]. See Additional file 1: Table S1 for representative examples of key mast cell mediators. MCs respond to a wide variety of antigenic triggers and physical forces, causing release of pre-stored and/or newly synthesized mediators particular to the insult and its anatomic location [8, 16, 17]. Appropriate MC mediator release helps tissues resist and recover from insults; aberrant release is harmful in ways specific to the locations and patterns of the released mediators. Preliminary investigations suggest highly heterogeneous, complex profiles of somatic MC regulatory gene mutations drive many cases of MCAS [24].
We have long been aware of MCs’ ability to precipitate anaphylaxis in response to bee stings, peanuts, and other allergens in previously sensitized individuals. MC’s release of histamine into the surrounding tissues and bloodstream leads to immediately recognizable hives, hypotension, syncope, respiratory arrest, and even death [25, 26]. We now understand, however, that there is an extensive array of other mediators that MCs selectively release in response to varying stimuli, including low molecular weight chemicals like formaldehyde and volatile organic compounds [21, 27]. The MC’s enormous repertoire of cell-surface receptors can identify an extraordinary array of signals and effect precise responses [15, 17, 21]. Even while a MC is launching its pre-formed armaments, it signals other cells to join the battle. Meanwhile, behind the frontline, MCs are reloading their weapons and stockpiling new munitions [22, 23, 25, 26]. Thus, our so-called “primitive” immune system is, in fact, quite sophisticated. It was many decades following the discovery of IgE and its relationship to anaphylaxis and classical allergies (such as pollen, animal dander, and dust mites) that we learned of MCs’ capacity to respond to a vast range of stimuli—revealing new, alternative pathways for their activation and degranulation, even in the absence of “classic” binding of antigen with immunoglobulins.
The fact that CI individuals often report immediate symptoms following seemingly insignificant exposures, such as a whiff of fragrance, has led some to speculate that the mechanism underlying CI must be neurological. However, MCs can explosively release, or gradually leak, their mediators. In fact, there is no cellular element of the immune system that reacts faster than mast cells. Lymphocytes take hours to activate, neutrophils require minutes, but MCs can respond to a trigger in sub-second time [16, 17, 28].
A crucial link between our contemporary exposures and our ancient MCs appears to have been missed. The MC’s evolutionary path stretches back to half a billion years [13, 15]. In contrast, emergence of the chemical industry, associated with the industrial revolution, took place only a few hundred years ago (1760–1820). Since WWII, more and more synthetic organic chemicals have crept into our personal environments. In response to the oil embargo and energy conservation efforts in the 1970s, many homes and buildings were sealed more tightly, resulting in insufficient fresh air to dilute contaminants. This has resulted in the accumulation of every sort of indoor air pollutant to levels higher than ever before (e.g., volatile, and semi-volatile organic chemicals outgassing from new construction and remodeling materials, pesticides, mold, disinfectants, and cleaning agents) [6, 7]. Only now are we learning that our contemporary exposures may be provoking MCs to release their inflammatory mediators, resulting in a condition often referred to as “mast cell activation syndrome” (MCAS) [29].
Although proposed diagnostic criteria for MCAS [29, 30] differ in some respects, MCAS diagnosis typically requires: (1) chronic and/or recurrent symptoms consistent with aberrant MC mediator release; (2) exclusion of other conditions which might better explain the patient’s symptoms; and (3) laboratory evidence of MC activation (i.e., MC mediator release). Most MCAS patients respond to MC-targeted treatments, thus providing an important diagnostic clue [31].
By one estimate, 10–17% of the German population may have MCAS [32]. CI prevalence estimates range from 8 to 33% in population-based surveys [33,34,35]. Hojo et al. [2] in Japan and Steinemann [1] in the U.S. each conducted surveys of chemical intolerance in their respective countries on two separate occasions, a decade apart. According to their research, in just 10 years, substantial increases in CI occurred in both countries.
We propose mast cell mediator release, initiated and triggered by xenobiotics, as a plausible biological mechanism underlying many, if not most, cases of CI and TILT. If MCAS and CI are closely related, they should share similar pathophysiologies and exhibit parallel symptoms and intolerances. In this paper, we explore converging lines of evidence supporting MCAS as a plausible unifying explanation for CI/TILT.
Methods
The MCAS group consisted of patients of authors LBA and TTD who were seen between September 2017 and April 2018. Patients were assigned a clinical score reflecting their likelihood of having MCAS using a validated MCAS assessment instrument [36, 37]. Patients also completed the Quick Environmental Exposure and Sensitivity Inventory (QEESI) along with their intake forms [31, 38]. The QEESI is a validated 50-item questionnaire, which is considered the international reference standard for assessing CI (see Palmer et al., for a list of 72 peer-reviewed journal articles using the QEESI in 16 countries with a total of over 32,000 respondents [39]). The QEESI has four scales: Symptom Severity, Chemical Intolerances, Other Intolerances, and Life Impact. Each scale item is scored from 0 to 10 (0 = “not a problem” to 10 = “severe or disabling problem”). Total scale scores range from 0 to 100. There is also a 10-item Masking Index which gauges ongoing exposures (such as to caffeine, tobacco, or drugs) that can reduce, or mask, individuals’ awareness of their intolerances [40]. Responses to the Chemical Exposure Scale explicitly ask participants to respond to how a specific chemical exposure makes them feel. The Symptom Severity Scale asks about common symptoms the person is having, not necessarily associated with the specific exposures on the Chemical Intolerance Scale.
QEESI scores from MCAS patients were compared to QEESI scores derived from earlier published data involving five different groups: CI individuals who identified an initiating exposure event; CI individuals who reported no initiating exposure; implant recipients; Gulf War Veterans; and a control group [38]. Those with QEESI scores of 40 or greater on both the Symptom Severity and Chemical Intolerance Scales were classified as having CI in our predictive model. Mean scores were compared statistically using ANOVA across groups using Tukey post hoc tests.
Clinical scores for the MCAS patient group were used to predict CI status using a logistic regression model. Analyses were performed using SAS software [41]. This study was approved by the University of Texas Health Science Center at San Antonio Internal Review Board (approval number HSC20150821H).
Results
Percentage meeting CI criteria by group
There were 147 patients from the MCAS clinic, ranging in age from 16 to 75 years (mean = 40.7, SD = 13.9). The exposure and control comparison groups were derived from published data by Miller and Prihoda [38]. The number, percent female, age, and percentages meeting CI criteria are presented in Table 1 for all six groups. Fifty-nine percent (59%) of the MCAS clinical group met QEESI criteria for CI, a somewhat higher percentage than among the Gulf War Veterans (49%). Percentages of the other comparison groups meeting CI criteria exceeded 75%, except for controls (7%).
QEESI total scale scores
Figure 1 shows the distribution of total QEESI scores and masking indices by participants in these groups. In every case, controls’ scores were significantly lower than for the other groups (p < 0.001). With few exceptions, the CI groups scored significantly higher than other groups, whether or not they reported an initiating exposure. Regarding the Chemical Intolerance Scale, scores for the MCAS group were not significantly different from the Gulf War Veterans’ scores, but were significantly lower than scores of all other groups. On the Other Intolerance Scale, the MCAS group scored significantly higher than the Gulf War Veterans’ group (p < 0.01); however, the MCAS group’s scores were statistically equivalent to the other groups’ scores. On the Life Impact Scale, the MCAS group’s score did not differ significantly from the implant group’s, and both were significantly higher than the Gulf War Veterans group (p < 0.01). For the Symptom Severity Scale, the Implant group and the CI with known exposure group scored significantly higher than the other groups (p < 0.01). Scores for the CI group without a preceding exposure and the MCAS groups did not differ significantly from each other. The Masking Index (a measure of ongoing exposures) was significantly greater among controls compared to the other groups (p < 0.01), except for the Gulf War Veterans whose Masking Index score was not significantly different from that of controls. The MCAS group and the CI group with known exposures had similarly low masking scores.
Predicted probability of CI with increases in MCAS scores
Logistic regression results appear in Table 2. Compared to the lowest quartile (Q1), those in the 2nd quartile of MCAS scores were 2.6 times more likely to have CI (p = 0.027). Those in the 3rd quartile of MCAS scores were 6.0 times more likely to have CI (p = 0.0001); those in the 4th quartile of MCAS scores were 6.2 times more likely to have CI (p = 0.0001).
Figure 2 shows that the probability of CI increases as MCAS scores increase. There is an exponential increase in the probability of CI with increasing MCAS scores, reaching near-perfect prediction toward the extreme set of MCAS scores.
Distribution of QEESI scores
Figures 3, 4, 5, 6 and 7 show QEESI scale items for TILT, MCAS, and Control groups. Here we merged four groups into one group (TILT group) for purposes of comparison against controls and MCAS patients: CI individuals who reported an initiating exposure (pesticides, remodeling); Gulf War Veterans; implant patients; and the CI individuals who did not report an initiating exposure event but had qualifying QEESI scores.
Symptom Severity Scale (Fig. 3)
There were no significant differences between the TILT and MCAS groups for 8 of the 10 symptom items. For the neuromuscular and affective items, the TILT group’s scores were slightly higher than those of the MCAS group (p < 0.04). Both the TILT and MCAS groups reported significantly more severe symptoms than did controls (p < 0.0001).
Chemical Intolerance Scale (Fig. 4)
TILT and MCAS groups both had significantly higher chemical intolerance scores than did controls (p < 0.0001). The TILT group’s chemical intolerance scores were significantly higher than the MCAS group’s scores for all items (p < 0.01).
Other Intolerance Scale (Fig. 5)
There were no significant differences between the TILT and MCAS groups for 8 of the 10 other intolerance items. Only the chlorinated tap water item was scored significantly higher by the TILT group (p < 0.01). Only the foods/food-additives item was scored significantly higher by the MCAS group (p < 0.03). Both TILT and MCAS groups scored significantly higher than controls (p < 0.0001).
Life Impact Scale (Fig. 6)
Both TILT and MCAS groups scored significantly higher than controls (p < 0.0001) on all Life Impact items. The TILT group consistently scored higher on 9 of the 10 items on this scale than did the MCAS group (p < 0.01), with the exception of the diet item where the MCAS group reported a slightly greater impact of their illness on diet.
Masking Index (Fig. 7)
Both TILT and MCAS groups had significantly lower masking scores than did controls (p < 0.0001), meaning that they had fewer ongoing exposures to tobacco smoke, fragrances, caffeine, or certain drugs which tend to hide (“mask”) the relationship between symptoms and exposures. The MCAS group reported greater use of drugs/medications and gas stoves than did the TILT group (p < 0.05).
Discussion
For decades, both MCAS and CI patients have been misunderstood, marginalized, and often referred for mental health evaluation [6, 42, 43], with practitioners assigning diagnostic labels such as Somatic Symptom Disorder, Multiple Chemical Sensitivity (MCS), or Idiopathic Environmental Intolerances (IEI). Our findings suggest that a vast assortment of chemical exposures may initiate or escalate TILT/CI via chronic, aberrant MC activation.
Similarities between MCAS and TILT
In Figs. 3, 4, 5 and 6, we see that the MCAS and TILT groups had statistically higher scores than did controls on the QEESI scales. We also see that the MCAS and TILT groups share strikingly similar patterns of symptoms and intolerances involving structurally diverse xenobiotics (chemicals, foods, and drugs).
Symptom Severity Scale
For most symptoms, there were significant differences between the MCAS and TILT groups. There was only a slight increase in severity in Affective and Neuromuscular symptoms in the TILT group compared to the MCAS group. Mediators released by MCs in the central nervous system may explain the neuropsychiatric symptoms patients in both groups commonly report.
Chemical Intolerance Scale
The same classes of chemicals appear to trigger symptoms in the MCAS group as in the TILT group, with the TILT group more severely affected. The most problematic triggers for many MCAS patients are fragrances (VOCs at extraordinarily low exposure levels), which also pose major problems for CI individuals [44].
Other Intolerance Scale
There were no significant differences between the TILT and MCAS groups for most of the items. Only the chlorinated tap water item was scored significantly higher by the TILT group.
Life Impact Scale
The TILT group consistently scored higher than the MCAS group on most of the Life Impact items. Individuals with TILT/CI may have greater difficulty tolerating exposures commonly encountered in social activities.
Masking Index
“Masking” can result from overlapping responses to chemicals as well as from an individual’s tendency to habituate to these substances. Masking obscures the relationship between symptoms and chemical, food or drug triggers, literally hiding the cause-and-effect relationship between them from both patients and clinicians [45]. The control group endorsed more items on the Masking Index than did the TILT and MCAS groups, consistent with our prior studies [38, 46]. People without CI or MCAS may be more apt to use alcohol, tobacco products, and caffeine for their stimulatory effects to offset fatigue and brain fog. The MCAS group reported greater use of drugs/medications which could reflect the fact that MCAS is more commonly treated with medications to prevent MC degranulation and/or to block MC mediator effects. Many individuals with CI have experienced so many adverse drug reactions that they avoid most drugs, favoring alternative therapies such as herbs, homeopathy, or acupuncture [47].
Connecting MCAS and TILT
Our understanding of the possible role for MCs in TILT is recent. Both patients with MCAS and those with TILT commonly report symptoms in multiple organ systems and often several systems simultaneously. MCs produce and release scores of chemical signals (generically termed “mediators”) that can affect organs, tissues, and systems throughout the body.
TILT encompasses exposures which may have initiated illness, as well as exposures which continue to trigger symptoms. However, until now, TILT has lacked a clear biological mechanism, which MCAS may provide. An understanding of TILT’s two stages, initiation and triggering suggests practical strategies for prevention and intervention, many of which also appear applicable to MCAS. Knowledge of the MCAS mechanism has the potential to inform new medical interventions and treatments for TILT. Failure to eliminate or reduce initiators such as pesticides or mold can result in chronic, even lifelong, illness in susceptible people, suggesting persistent MC activation and degranulation. The symptoms and findings in TILT patients may be best understood in the context of MCs and the mediators they release.
MCAS, TILT, and the nervous system
Our proposal that MCAS could be the biological mechanism for TILT arises out of recent recognition that the spectrum of MC disease extends beyond clinically recognizable allergic phenomena (e.g., allergy, anaphylaxis, urticaria, angioedema, atopic dermatitis or eczema) and differs from the rare MC malignancy called “mastocytosis”. Mastocytosis, first described in cutaneous form in the latter part of the nineteenth century and then in systemic form in the mid-twentieth century, manifests as chronic MCA resulting from neoplastic proliferation of MCs. Only recently, beginning in the 1980s, did researchers hypothesize the existence of MCAS [48, 49]. In 2007, the first case reports of MCAS appeared, describing patients with heightened release of MC mediators, yet without the excessive numbers of MCs which characterize mastocytosis. Many MC mediators have potent but short-lived effects. They are released locally in sensitized tissues and are exquisitely thermolabile, posing major challenges for measurement. MC’s menagerie of mediators produce multi-system inflammation at minimum, and not uncommonly allergic-like phenomena, and sometimes aberrancies in growth and development (typically benign) in virtually any tissue.
As immunologic "first responders", activated MCs can initiate, amplify, and prolong wide-ranging neuroimmune responses [50]. Several investigators have pointed to neurogenic inflammation as a mechanism for CI [10, 51,52,53]. Rather than being the mechanism for CI, neuroinflammation may be the consequence of MCA and mediator release initiated by xenobiotic/chemical exposures. MCs affect neural function via their released mediators which bind with specific neuronal receptors [18, 54]. Also, MCs physically abut neurons in many tissues. Wherever such dyads are present, there is constant mediator “cross-talk” between the two cell types. Thus, MCA can provoke nearby neurons, inducing their associated symptoms; similarly, neurons can provoke nearby MCs, inducing their associated symptoms.
Correspondingly, quieting of MCs can help reduce neuronal activation, and, again, vice versa. [55]. Additional file 1: Table S1 lists selected MC mediators involved in neuroinflammation (after Theoharides et al. [56,57,58,59]. Many investigators have documented neuroinflammation and inflammatory mediators in CI [53, 60,61,62].
Both MCAS and TILT have prominent neurological features. For example, organophosphate pesticides, which bind irreversibly to cholinergic receptors in the parasympathetic nervous system, appear to be among the most severe and permanently damaging TILT initiators. Correspondingly, organophosphates have been shown to trigger degranulation in human and animal MCs [63]. The parasympathetic nervous system also modulates MC activity via a cholinergic pathway [64]. MCs play pivotal roles in regulating cerebral blood flow [65], directly affecting brain function. Notably, both MCAS and TILT patients commonly report cognitive difficulties which may be the result of reduced cerebral blood flow due to chemical exposures, such as vehicle exhaust or pesticides [66]. Brain MCs lie close to cerebral blood vessels, nerves, and the meninges, and inhabit the area postrema, choroid plexus, thalamus, hypothalamus, and limbic system, thus affecting memory, mood, and concentration. MCs can migrate between nerve tissue and lymphatics and appear to contribute to neuroinflammation in many disorders [67,68,69].
Notably, during stress, corticotropin-releasing factor is secreted by the hypothalamus, and, together with neurotensin, triggers MCs to release inflammatory and neurotoxic mediators, thereby disrupting the blood–brain barrier leading to neuroinflammation [70]. Referring to ADHD, Song et al. [55] cite increasing evidence that MCs are involved in brain inflammation and neuropsychiatric disorders. Selective release of inflammatory mediators by MCs, interacting with glial cells and neurons, may activate the hypothalamic–pituitary–adrenal axis and disrupt blood–brain barrier integrity.
This physiology of MCAS mirrors the two stages of TILT—initiation and triggering, that is, initiation by a single intense exposure, or repeated lower-level exposures (pesticides, implants, drugs, etc.), which immunologically sensitize MCs in the brain and/or other key sites. Thereafter, chemicals structurally related to the initiating event, as well as unrelated xenobiotic exposures, trigger mediator release by these pathologically “twitchy” MCs. Cognitive and mood effects can include sudden rage (e.g., “road rage”); impulsive, violent, or abusive behaviors; addictive tendencies; mental confusion/fatigue; and/or a sense of depersonalization. MC “twitchiness” renders these cells vulnerable to a host of unrelated exposures that never bothered the person before and do not bother most people. Therefore, it seems plausible that MC sensitization and triggering can explain both stages of TILT—initiation and triggering.
Assessing and treating TILT/CI
Trigger identification and avoidance, rather than medications, are mainstays for treating CI. Likewise, these are the first steps for managing MCAS. Medications or desensitization procedures benefit many MCAS patients [31].
Identifying and assessing TILT
A systematic two-step evaluation works well for identifying patients with CI. First, administer the three-item Brief Environmental Exposure and Sensitivity Inventory (BREESI) screener [39, 71] to help identify individuals with significant intolerances for chemicals, foods, and drugs. If one or more BREESI items are endorsed, the full QEESI is administered (http://tiltresearch.org/wp-content/uploads/sites/30/2017/05/qeesi.pdf). These instruments help identify initiators and triggers of CI. A detailed exposure/symptom history and timeline coupled with the QEESI can help identify environments needing specific assessment. Removing initiating exposures appears to be essential for sustained improvement among both TILT and MCAS patients. For both conditions, the QEESI Symptom Star, graphed based upon serial administrations of the QEESI over time, illustrates the dynamics of symptom severity as chronologically related to exposures [72,73,74] (see Additional file 1: S2).
Interestingly, the MCAS group reported greater use of gas stoves than did the TILT group (58% vs 25%, respectively), perhaps suggesting an important source and intervention for MCAS patients who use gas stoves. Historically, as early as the 1960s, removing gas appliances has been a principal recommendation for CI individuals [75].
Dietary interventions
Both TILT and MCAS patients report adverse reactions to foods. Most of these adverse food reactions are food intolerances, as opposed to immunoglobulin-mediated food allergies, e.g., to peanuts, discoverable through skin or blood testing. The gold standard for identifying food intolerances involves the rigorous elimination of suspect foods for 4 to 7 days, followed by judicious reintroduction of single foods, one-at-a-time, under close medical and dietary supervision. We recommend assistance from dieticians who understand food intolerances, food addiction, and elimination diets. Note that foods themselves may be triggers, but food additives and chemical residues on foods also are frequent triggers. Many CI patients opt for organic foods where available and affordable.
Medical interventions
After trigger identification and avoidance strategies are implemented, potential medical interventions for CI may include many of those used to treat MCAS, including agents that prevent MC degranulation like cromolyn and/or reduce tissue inflammation caused by MC mediators, such as H1 and H2 antihistamines administered simultaneously [31, 32, 76, 77]. Patients who respond adversely to excipients in commercially available medications may require compounded formulations. Interestingly, low-dose benzodiazepines help some MCAS patients due to the presence of benzodiazepine receptors on not only neurons, but also MCs [78, 79]. Pharmacotherapy for TILT/CI is by no means simple and requires minimizing exposures to chemicals known to precipitate adverse reactions and monitoring for inadvertent introduction of known triggers into the patient’s regimen, such as when a different formulation is provided as a refill. These same challenges exist for MCAS patients.
Other implications for clinical practice
MC degranulation and mediator release offer an elegant explanation for TILT’s numerous “unexplained” symptoms as well as for a host of so-called “idiopathic” illnesses sharing features of TILT. These include Gulf War Syndrome, breast implant illness, some mold-related illnesses, and various other exposure-induced conditions. Likewise, researchers and clinicians who wish to understand TILT-related or -overlapping conditions including fibromyalgia, chronic fatigue syndrome, depression, irritable bowel syndrome, asthma, eczema, attention deficit/hyperactivity disorder, or autism spectrum disorders [80, 81] need to take exposure histories which include asking when the illness began or was exacerbated, whether an initiating event occurred, and whether other people (or animals) were exposed or affected. Domestic cats for example are particularly sensitive to organophosphate pesticides [82]
Study limitations
To the best of our knowledge, this is the first investigation of the similarities between MCAS and TILT, suggesting MCAS as a plausible mechanism for TILT/CI. However, symptomatic overlap between two study populations is not necessarily proof of a shared pathophysiology. Although an important strength of this study is that the QEESI (the reference standard for identifying CI) was used for all respondents, the MCAS and TILT/CI samples were approximately 20 years apart in data collection. This may introduce unknown historical biases. Additionally, the number of study participants was relatively small and unequal between the groups. Further, only gender and age were assessed and adjusted in the analysis. Other factors, such as medical history (e.g., asthma, obesity, other comorbidities), socioeconomic status, and other lifestyle variables could potentially bias the analysis. As such, these results should be considered preliminary until further studies can be conducted.
Directions for future research and regulation
With this new understanding of the possible role of MCs in TILT, important questions arise concerning individual susceptibility differences that may be influenced by prior exposures, genetics, epigenetics, and nutrition.
Given the close parallels between TILT and MCAS, and the fact that MC activation and mediator release could explain much about TILT, future research should address the following questions: (1) What proportion of the TILT population manifests detectable MC activation as determined by a rigorous diagnostic MCAS work-up? (2) Do patients with TILT have somatic MC regulatory gene mutations as already found in many MCAS patients? (3) If so, are there recurrent mutations reflecting differing clonality patterns (e.g., in KIT) [83] characterizing differing subsets of TILT patients, perhaps even “fingerprinting” particular initiating exposures? and (4) Would specific treatments targeting MCs, or their mediators prove helpful for the TILT population as a whole or for certain subsets? As more research clarifies the role of MCs in TILT, targeted reduction of exposures can be implemented.
Conclusion
Mast cell activation and mediator release appear capable of explaining the increasingly frequent observations by physicians and their patients of chronic multi-system symptoms and new-onset chemical, food and drug intolerances following exposure to a wide variety of xenobiotics. Our logistic regression model demonstrated that as the likelihood of patients having MCAS increases, their likelihood of having CI/TILT similarly increases, to a near-perfect correspondence at the high ends of these scales. Association is, of course, not proof of causation. Nevertheless, the strikingly similar symptom and intolerance patterns for the MCAS and TILT populations suggest that xenobiotics can disrupt mast cells, resulting in either or both of these challenging conditions. Faced with patients suffering from complex illness affecting multiple organ systems and fluctuating inflammatory, allergic, and dystrophic symptoms, researchers and clinicians should now ask themselves two questions: (1) Could MCAS be at the root of these problems? (2) Could xenobiotic exposures be driving MC activation and mediator release? Increasing our understanding of the connection between TILT and MCs has the potential to expose a new link between environmental exposures and illness, offering opportunities for improving individual and public health.
Almost daily, scientists, physicians, journalists, and the public are questioning whether toxic exposures of one sort or another are responsible for persistent symptoms reported following a wide variety of exposures including but not limited to the Gulf War, breast and other implants, the World Trade Center disaster, open burn pits, wildfires, pesticides, mold, and chemical spills and releases. Only in the last decade has knowledge of mast cells expanded to include mast cell activation syndrome (MCAS). MCAS mirrors the two-stage disease mechanism that Miller first described as toxicant-induced loss of tolerance (TILT) in 1996 [9], and we reported in a companion paper in this journal this year [7]—a mechanism we regard as a possible missing link between toxic exposures, multi-system symptoms, and loss of tolerance for chemicals, foods, and drugs.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Steinemann A (2018) National prevalence and effects of multiple chemical sensitivities. J Occup Environ Med 60(3):e152–e156. https://doi.org/10.1097/JOM.0000000000001272
Hojo S, Mizukoshi A, Azuma K, Okumura J, Ishikawa S, Miyata M, Mizuki M, Ogura H, Sakabe K (2018) Survey on changes in subjective symptoms, onset/trigger factors, allergic diseases, and chemical exposures in the past decade of Japanese patients with multiple chemical sensitivity. Int J Hyg Environ Health 221(8):1085–1096. https://doi.org/10.1016/j.ijheh.2018.08.001
Macy E (2018) Chapter 16—Multiple drug intolerance syndrome. In: Khan DA, Banerji A (eds) Drug allergy testing. Elsevier, Amsterdam, pp 165–168, ISBN 9780323485517,
Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R (1994) A population study of food intolerance. Lancet 343(8906):1127–1130. https://doi.org/10.1016/S0140-6736(94)90234-8
Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C (2007) The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol 120(3):638–646. https://doi.org/10.1016/j.jaci.2007.05.026
Ashford N, Miller C (1998) Chemical exposures: low levels and high stakes. Van Nostrand Reinhold, New York
Masri S, Miller CS, Palmer RF, Ashford N (2021) Toxicant-induced loss of tolerance for chemicals, foods, and drugs: assessing patterns of exposure behind a global phenomenon. Environ Sci Eur 33:65. https://doi.org/10.1186/s12302-021-00504-z
Moon TC, Befus AD, Kulka M (2014) Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 5:569. https://doi.org/10.3389/fimmu.2014.00569
Miller CS (1996) Chemical sensitivity: symptom, syndrome or mechanism for disease? Toxicology 111:69–86. https://doi.org/10.1016/0300-483x(96)03393-8
Rossi S, Pitidis A (2018) Multiple chemical sensitivity: review of the state of the art in epidemiology, diagnosis, and future perspectives. J Occup Environ Med 60(2):138–146. https://doi.org/10.1097/JOM.0000000000001215
Dantoft TM, Andersson L, Nordin S, Skovbjerg S (2015) Chemical intolerance. Curr Rheumatol Rev 11(2):167–184. https://doi.org/10.2174/157339711102150702111101
Clauw DJ (2001) Potential mechanisms in chemical intolerance and related conditions. Ann N Y Acad Sci 933:235–253. https://doi.org/10.1111/j.1749-6632.2001.tb05828.x
Wong GW, Zhuo L, Kimata K, Lam BK, Satoh N, Stevens RL (2014) Ancient origin of mast cells. Biochem Biophys Res Commun 451(2):314–318. https://doi.org/10.1016/j.bbrc.2014.07.124
Crivellato E, Travan L, Ribatti D (2015) The phylogenetic profile of mast cells. Methods Mol Biol 1220:11–27. https://doi.org/10.1007/978-1-4939-1568-2_2
Crivellato E, Ribatti D (2010) The mast cell: an evolutionary perspective. Biol Rev Camb Philos Soc 85(2):347–360. https://doi.org/10.1111/j.1469-185X.2009.00105.x
Afrin LB (2016) (2016) Mast cell activation disease and the modern epidemic of chronic inflammatory disease. Transl Res 174:33–59. https://doi.org/10.1016/j.trsl.2016.01.003
Krystel-Whittemore M, Dileepan KN, Wood JG (2016) Mast cell: a multi-functional master cell. Front Immunol. https://doi.org/10.3389/fimmu.2015.00620
Afrin LB, Pöhlau D, Raithel M, Haenisch B, Dumoulin FL, Homann J, Mauer UM, Harzer S, Molderings GJ (2015) Mast cell activation disease: an underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav Immun 50:314–332. https://doi.org/10.1016/j.bbi.2015.07.002
Frieri M (2018) Mast cell activation syndrome. Clin Rev Allergy Immunol 54(3):353–365. https://doi.org/10.1007/s12016-015-8487-6
Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ (2021) Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci 66(4):965–982. https://doi.org/10.1007/s10620-020-06264-9
Ibelgaufts H (2021) "Mast cells" entry in "cytokines on-line pathfinder encyclopedia. http://www.cells-talk.com. Accessed 31 Aug 2021.
Gilfillan AM, Austin SJ, Metcalfe DD (2011) Mast cell biology: introduction and overview. Adv Exp Med Biol 716:2–12. https://doi.org/10.1007/978-1-4419-9533-9_1
Elieh Ali Komi D, Wöhrl S, Bielory L (2020) Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol 58(3):342–365. https://doi.org/10.1007/s12016-019-08769-2
Molderings GJ (2015) The genetic basis of mast cell activation disease—looking through a glass darkly. Crit Rev Oncol Hematol 93(2):75–89. https://doi.org/10.1016/j.critrevonc.2014.09.001
Velez TE, Bryce PJ, Hulse KE (2018) Mast cell interactions and crosstalk in regulating allergic inflammation. Curr Allergy Asthma Rep 18(5):1–7. https://doi.org/10.1007/s11882-018-0786-6
Kalesnikoff J, Galli SJ (2008) New developments in mast cell biology. Nat Immunol 9(11):1215–1223. https://doi.org/10.1038/ni.f.216
Fujimaki H, Imai T, Befus D (1992) Mast cell response to formaldehyde 2 Induction of stress-like proteins. Int Arch Allergy Immunol 98(4):332–338. https://doi.org/10.1159/000236207
Janiszewski J, Bienenstock J, Blennerhassett MG (1994) Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol 267:C138–C145
Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, Buchanan AD, Cuni JR, Davey WP, Dempsey TT, Dorff SR, Dubravec MS, Guggenheim AG, Hindman KJ, Hoffman B, Kaufman DL, Kratzer SJ, Lee TM, Marantz MS, Maxwell AJ, McCann KK, McKee DL, Menk Otto L, Pace LA, Perkins DD, Radovsky L, Raleigh MS, Rapaport SA, Reinhold EJ, Renneker ML, Robinson WA, Roland AM, Rosenbloom ES, Rowe PC, Ruhoy IS, Saperstein DS, Schlosser DA, Schofield JR, Settle JE, Weinstock LB, Wengenroth M, Westaway M, Xi SC, Molderings GJ. (2020) Diagnosis of mast cell activation syndrome: a global "consensus-2". Diagnosis (Berl) 22;8(2):137–152. https://doi.org/10.1515/dx-2020-0005
Valent P, Akin C, Arock M, Brockow K, Butterfield J, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, Nedoszytko B, Orfao A, Schwartz LB, Sotlar K, Sperr WR, Triggiani M, Valenta R, Horny HP, Metcalfe DD (2012) Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol 157:215–225. https://doi.org/10.1159/000328760
Molderings G, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL, Panse J, Butterfield J, Afrin LB (2016) Pharmacological treatment options for mast cell activation disease. Naunyn-Schmiedeberg’s Arch Pharmacol 389:671–694. https://doi.org/10.1007/s00210-016-1247-1
Wirz S, Molderings J (2017) Practical guide for treatment of pain in patients with systemic mast cell activation. Disease Pain Physician 20:E849–E861
Caress SM, Steinemann AC (2004) Prevalence of multiple chemical sensitivities: a population-based study in the southeastern United States. Am J Public Health 94(5):746–747. https://doi.org/10.2105/ajph.94.5.746
Azuma K, Uchiyama I, Katoh T, Ogata H, Arashidani K, Kunugita N (2015) Prevalence and characteristics of chemical intolerance: a Japanese population-based study. Arch Environ Occup Health 70:341–353. https://doi.org/10.1080/19338244.2014.926855
Kreutzer R, Neutra RR, Lashuay N (1999) Prevalence of people reporting sensitivities to chemicals in a population-based survey. Am J Epidemiol 150(1):1–12. https://doi.org/10.1093/oxfordjournals.aje.a009908
Molderings GJ, Brettner S, Homann J, Afrin LB (2011) Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol 4:10. https://doi.org/10.1186/1756-8722-4-10
Afrin LB, Molderings GJ (2014) A concise, practical guide to diagnostic assessment for mast cell activation disease. World J Hematol 3(1):1–17. https://doi.org/10.5315/wjh.v3.i1.1
Miller CS, Prihoda T (1999) The Environmental Exposure and Sensitivity Inventory (EESI): a standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Ind Health 15:370–385. https://doi.org/10.1177/074823379901500311
Palmer RF, Walker T, Kattari D, Rincon R, Perales RB, Jaén CR, Grimes C, Sundblad DR, Miller CS (2021) Validation of a brief screening instrument for chemical intolerance in a large U.S. National Sample. Int Environ Res Public Health 18:8714. https://doi.org/10.3390/ijerph18168714
Miller CS (1999) Are we on the threshold of a new theory of disease? Toxicant-induced loss of tolerance and its relationship to addiction and abdiction. Toxicol Environ Health 15(3–4):284–294. https://doi.org/10.1177/074823379901500302
SAS Institute Inc (2014) SAS® 9.4 statements: reference, 3rd edn. SAS Institute Inc., Cary, NC
Afrin LB, Self S, Menk J, Lazarchick J (2017) Characterization of mast cell activation syndrome. Am J Med Sci 353(3):207–215. https://doi.org/10.1016/j.amjms.2016.12.013
Bornschein S, Hausteiner C, Zilker T, Forst H (2002) Psychiatric and somatic disorders and multiple chemical sensitivity (MCS) in 264 ‘environmental patients.’ Psychol Med 32(8):1387–1394. https://doi.org/10.1017/S0033291702006554
Steinemann A (2019) International prevalence of chemical sensitivity, co-prevalence with asthma and autism, and effects from fragranced consumer products. Air Qual Atmos Health 12(5):519–527. https://doi.org/10.1007/s11869-019-00672-1
Miller CS (2000) Mechanisms of action of addictive stimuli. Addiction 96:115–139
Miller CS, Mitzel HC (1995) Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch Environ Health 50(2):119–129. https://doi.org/10.1080/00039896.1995.9940889
Gibson PR, Nicole-Marie AE, Ruding LA (2003) Perceived treatment efficacy for conventional and alternative therapies reported by persons with multiple chemical sensitivity. Environ Health Perspect 111:12. https://doi.org/10.1289/ehp.5936
Roberts LJ (1988) Carcinoid syndrome and disorders of systemic mast-cell activation including systemic mastocytosis. Endocrinol Metab Clin North Am 17(2):415–436
Roberts LJ (1984) Recurrent syncope due to systemic mastocytosis. Hypertension 6(2, Pt 1):285–294
Li N, Zhang X, Dong H, Hu Y, Qian Y (2017) Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD. Behav Brain Res 322:60–69. https://doi.org/10.1016/j.bbr.2017.01.006
Meggs WJ (2017) The role of neurogenic inflammation in chemical sensitivity. Ecopsychology 9(2):83–89. https://doi.org/10.1089/eco.2016.0045
Meggs WJ (1995) Neurogenic switching: a hypothesis for a mechanism for shifting the site of inflammation in allergy and chemical sensitivity. Environ Health Perspect 103:54–56. https://doi.org/10.1289/ehp.9510354
Dantoft TM, Elberling J, Brix S, Szecsi PB, Vesterhauge S, Skovbjerg S (2014) An elevated pro-inflammatory cytokine profile in multiple chemical sensitivity. Psychoneuroendocrinology 40:140–150. https://doi.org/10.1016/j.psyneuen.2013.11.012
Silverman AJ, Sutherland AK, Wilhelm M, Silver R (2000) Mast cells migrate from blood to brain. J Neurosci 20(1):401–408. https://doi.org/10.1523/JNEUROSCI.20-01-00401.2000
Song Y, Lu M, Yuan H, Chen T, Han X (2020) Mast cell-mediated neuroinflammation may have a role in attention deficit hyperactivity disorder. Exp Ther Med 20:714–726. https://doi.org/10.3892/etm.2020.8789
Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitroset D (2007) Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev 217(1):65–78. https://doi.org/10.1111/j.1600-065X.2007.00519.x
Theoharides TC (2017) Neuroendocrinology of mast cells: challenges and controversies. Exp Dermatol 26(9):751–759. https://doi.org/10.1111/exd.13288
Theoharides TC (2020) The impact of psychological stress on mast cells. Ann Allergy Asthma Immunol. https://doi.org/10.1016/j.anai.2020.07.007
Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC (2015) Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37(5):984–995. https://doi.org/10.1016/j.clinthera.2015.04.002
De Luca C, Scordo MG, Cesareo E, Pastore S, Mariani S, Maiani G, Stancato A, Loreti B, Valacchi G, Lubrano C, Raskovic D, De Padova L, Genovesi G, Korkina LG (2010) Biological definition of multiple chemical sensitivity from redox state and cytokine profiling and not from polymorphisms of xenobiotic-metabolizing enzymes. Toxicol Appl Pharmacol 248:285–292. https://doi.org/10.1016/j.taap.2010.04.017
Belpomme D, Campagnac C, Irigaray P (2015) Reliable disease biomarkers characterizing and identifying electrohypersensitivity and multiple chemical sensitivity as two etiopathogenic aspects of a unique pathological disorder. Rev Environ Health 30:251–271. https://doi.org/10.1515/reveh-2015-0027
Kimata H (2004) Effect of exposure to volatile organic compounds on plasma levels of neuropeptides, nerve growth factor and histamine in patients with self-reported multiple chemical sensitivity. Int J Hyg Environ Health 207:159–163. https://doi.org/10.1078/1438-4639-00262
Xiong S, Rodgers K (1997) Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J Toxicol Environ Health 51(2):159–175. https://doi.org/10.1080/00984109708984019
Forsythe P (2015) The parasympathetic nervous system as a regulator of mast cell function. In: Hughes M, McNagny K (eds) Mast cells. Methods in molecular biology (methods and protocols), vol 1220. Humana Press, New York
Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML (2010) Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab 30:689–702. https://doi.org/10.1038/jcbfm.2009.282
Bunegin L, Mitzel HC, Miller CS, Gelineau JF, Tolstykh GP (2001) Cognitive performance and cerebrohemodynamics associated with the Persian Gulf Syndrome. Toxicol Ind Health 17(4):128–137. https://doi.org/10.1191/0748233701th100oa
Medeiros WLG Junior, Bandeira IP, Franzoi AEA, Brandão WN, Santos Durão ACCD, Gonçalves MVM (2019). Mast cells: A key component in the pathogenesis of Neuromyelitis Optica Spectrum Disorder? Immunobiology. 224(5):706–709. https://doi.org/10.1016/j.imbio.2019.05.010
Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC (1996) Definitive characterization of rat hypothalamic mast cells. Neuroscience 73(3):889–902. https://doi.org/10.1016/0306-4522(95)00606-0
Nautiyal K (2008) Beyond allergy: Mast cells mediate brain-behavior-immune interactions. http://grantome.com/grant/NIH/F31-MH084384-02. Accessed 15 Nov 2019
Theoharides TC, Stewart JM, Hatziagelaki E, Kolaitis G (2015) Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front Neurosci. https://doi.org/10.3389/fnins.2015.00225
Palmer RF, Jaén CR, Perales RB, Rincon R, Forster JN, Miller CS (2020) Three questions for identifying chemically intolerant individuals in clinical and epidemiological populations: The Brief Environmental Exposure and Sensitivity Inventory (BREESI). PLoS ONE. https://doi.org/10.1371/journal.pone.0238296
Hojo S, Sakabe K, Ishikawa S, Miyata M, Kumano H (2009) Evaluation of subjective symptoms of Japanese patients with multiple chemical sensitivity using QEESI©. Environ Health Prevent Med 14:267–275. https://doi.org/10.1007/s12199-009-0095-8
Yun MJ, Kang DM, Lee K-H, Kim YK, Kim JE (2013) Multiple chemical sensitivity caused by exposure to ignition coal fumes: a case report. Ann Occup Environ Med 25:32. https://doi.org/10.1186/2052-4374-25-32
Imai N, Imai Y (2011) Necessity of counseling institutions for sick building syndrome patients. In: Abdul-Wahab SA (ed) Sick building syndrome: in public buildings and workplaces, p 365. Springer, Berlin
Randolph TG (1962) Human ecology and susceptibility to the chemical environment. Springfield, Illinois: Charles C. Thomas.
Shaik Y, Caraffa A, Ronconi G, Lessiani G, Conti P (2018) Impact of polyphenols on mast cells with special emphasis on the effect of quercetin and luteolin. Central-Eur J Immunol 43(4):476–481. https://doi.org/10.5114/ceji.2018.81347
Oksaharju A, Kankainen M, Kekkonen RA, Lindstedt KA, Kovanen PT, Korpela R, Miettinen M (2011) Probiotic Lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J Gastroenterol 17(6):750–759. https://doi.org/10.3748/wjg.v17.i6.750
Taniguchi T, Wang JK, Spector S (1980) Properties of (3H) diazepam binding to rat peritoneal mast cells. Life Sci 27(2):171–178. https://doi.org/10.1016/0024-3205(80)90460-9
Haenisch B, Huber M, Wilhelm T, Steffens M, Molderings GJ (2013) Investigation into mechanisms mediating the inhibitory effect of 1,4-benzodiazepines on mast cells by gene expression profiling. Life Sci 92(6–7):345–351. https://doi.org/10.1016/j.lfs.2013.01.010
Heilbrun LP, Palmer RF, Jaen CR, Svoboda MD, Miller CS, Perkins J (2015) Maternal chemical and drug intolerances: potential risk factors for autism and attention deficit hyperactivity disorder (ADHD). J Am Board Fam Med 28(4):461–470. https://doi.org/10.3122/jabfm.2015.04.140192
Theoharides TC (2009) Autism spectrum disorders and mastocytosis. Int J Immunopathol Pharmacol 22(4):859–865. https://doi.org/10.1177/039463200902200401
Minton NA, Murray VSG (1988) A review of organophosphate poisoning. Med Toxicol Adverse Drug Exp 3:350–375. https://doi.org/10.1007/BF03259890
Molderings GJ, Meis K, Kolck UW, Homann J, Frieling T (2010) Comparative analysis of mutation of tyrosine kinase kit in mast cells from patients with systemic mast cell activation syndrome and healthy subjects. Immunogenetics 62(11–12):721–727. https://doi.org/10.1007/s00251-010-0474-8
Acknowledgements
The authors thank Dr. Carlos Jaén, Chairman of the Department of Family and Community Medicine, University of Texas Health Science Center at San Antonio, for his visionary support and encouragement. This research was made possible by generous funding from the Marilyn Brachman Hoffman Foundation.
Funding
This work was funded by a grant from the Marilyn Brachman Hoffman Foundation, Dallas, Texas (TX).
Author information
Authors and Affiliations
Contributions
CSM conceived and designed this work. LA and TD provided insight and guidance regarding mast cells, and were responsible for the acquisition of the clinical data. RFP acquired the archived data, and analyzed and interpreted the statistical results. All authors contributed substantially to the drafting and revisions of the manuscript and approved the submitted version. All authors are responsible for the accuracy and integrity of the manuscript and data. All authors read and approved the final manuscript.
Authors' information
All authors have approved the manuscript before submission and have agreed to the order of authorship. We verify that all data and figures are compliant with the transparency and reproducibility standards of both the field and journal.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the University of Texas Health Science Center San Antonio Internal Review Board (approval number HSC20150821H).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Representative examples of key mast cell mediators. S2. QEESI Symptom Star. Source: Miller and Prihoda [38].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, C.S., Palmer, R.F., Dempsey, T.T. et al. Mast cell activation may explain many cases of chemical intolerance. Environ Sci Eur 33, 129 (2021). https://doi.org/10.1186/s12302-021-00570-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-021-00570-3