Abstract
Background
Despite specific restrictions on their production and use, per- and polyfluoralkyl substances (PFAS) are still omnipresent in the environment, including aquatic ecosystems. Most biomonitoring studies have investigated the PFAS concentrations in indigenous organisms, whereas active biomonitoring has only been used sporadically. In the present study, accumulated PFAS concentrations were measured in indigenous fish, European perch (Perca fluviatilis) and European eel (Anguilla anguilla), and in translocated freshwater mussels (Dreissena bugensis and Corbicula fluminea) at 44 sampling locations within the main water basins of Flanders, the northern part of Belgium. Finally, both human health risk and ecological risk were assessed based on accumulated concentrations in fish muscle.
Results
Among locations, ΣPFAS concentrations ranged from 8.56–157 ng/g ww (median: 22.4 ng/g ww) in mussels, 5.22–67.8 ng/g ww (median: 20.8 ng/g ww) in perch, and 5.73–68.8 ng/g ww (median: 22.1 ng/g ww) in eel. Concentrations of PFOA and PFTeDA were higher in mussels compared to fish, whereas for PFDA and PFUnDA the opposite was true. A comparison of concentrations on a wet weight basis between both fish species showed significantly higher PFDoDA, PFTrDA, PFTeDA and PFOA concentrations in eel compared to perch and significantly higher concentrations of PFDA and PFOS in perch. In mussels, PFAS profiles were dominated by PFOA and showed a higher relative contribution of short-chained PFAS, while PFAS profiles in fish were dominated by PFOS. Furthermore, all mussel species clearly occupied a lower trophic level than both fish species, based on a stable isotope analysis.
Conclusions
Biomagnification of PFDA, PFUnDA and PFOS and biodilution of PFOA and PFTeDA were observed. Translocated mussels have been proven suitable to determine which PFAS are present in indigenous fish, since similar PFAS profiles were measured in all biota. Finally, mean PFAS concentrations in fish did pose a human health risk for eel, although tolerable daily intake values for perch were close to the reported daily consumption rates in Belgium and exceeded them in highly contaminated locations. Based on the ecological risk of PFOS, the standard was exceeded at about half of the sampling locations (44% for perch and 58% for eel).
Similar content being viewed by others
Background
Since the beginning of the twentieth century, the increased emission of anthropogenic chemicals has led to a dramatic environmental impact [18]. Per- and polyfluoroalkyl substances (PFAS) have been produced at large scale for more than 60 years. Their lipophobic and hydrophobic properties make them suitable for a wide range of applications, as surfactants in surface coatings for textiles, soil repellents, food contact paper, cleaning products, and fire-fighting foams [14]. The manufacturing and use of PFAS has resulted in a global contamination of these chemicals in the environment, wildlife and humans [15, 33, 38, 46].
Due to their persistence, potential health effects and global distribution, multiple manufacturers decided to phase-out the production of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) [28, 70]. In addition, other regulatory measures have been taken, such as the inclusion of both of these PFAS in the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009 and 2019, respectively [67, 68]. Nonetheless, as PFAS are highly resistant to degradation, high environmental concentrations of some PFAS persist [52].
In the Belgian terrestrial environment among the highest concentrations of multiple PFAS have been reported [37, 38]. However, in the Belgian aquatic environment the spatial distribution of PFAS has been studied less frequently and targeted only PFOS [42, 43]. As fish consumption is an important route of PFAS pollution in humans in Flanders [16], it is important to investigate the spatial distribution of PFAS in the Belgian aquatic environment in order to determine potential human and ecological health risks. Furthermore, these studies used passive biomonitoring (PBM) on indigenous organisms, and were performed on a limited number of sampling sites. Studies measuring PFAS using active biomonitoring (ABM) with translocated individuals, on the other hand, have only sporadically been done [4]. This technique allows for the exposure of the same species with a controlled background condition in every sampling location, creating a more standardized sample collection. In addition, individuals of similar size can be exposed during the same pre-defined time [11].
Therefore, as a baseline study the aim of this study was to investigate the current spatial distribution of PFAS in the aquatic environment of Flanders, Belgium, using both ABM (translocated mussels) and PBM (indigenous fish). Furthermore, to test for biomagnification of individual PFAS compounds, a comparison of accumulated concentrations between primary consumers and top predators was made. Thirdly, we examined the suitability of mussels in ABM, by comparing accumulation profiles in fish species with those in the mussels. Finally, we investigated the potential environmental risk and health risks to human through the consumption of PFAS-contaminated fish.
Materials and method
Sampling locations and sample collection
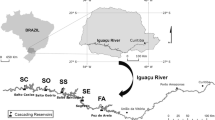
A total of 44 sampling locations were selected within the main water basins of Flanders, the northern part of Belgium (Fig. 1). These locations were characterized as canals, rivers and streams. The nature and number of biota samples are indicated in Table 1. All sampling locations were selected within the existing monitoring network implemented for the Water Framework Directive and showed a variation with respect to anthropogenic pressure (e.g. urban, rural, industrial areas).
Overview of the sampling sites. A more detailed overview of sampling locations can be found in Table 1
Fish
Fish collection was performed by the Research Institute for Nature and Forest (INBO) between 2015 and 2018. European perch (Perca fluviatilis) and European eel (Anguilla anguilla) in its yellow eel stage were caught using electrofishing, with a maximum of 4 eels and 20 perches per location. However, we were not able to catch both species at every sampling point. Both are sedentary, predatory fish with a diet mainly consisting of invertebrates and small fish [63, 76]. Juvenile yellow eels were collected targeting a length class of 45–55 cm. In deeper water bodies, additionally fykes (90 cm diameter and a total length of 22 m) were installed and harvested after 48 h. For a more detailed overview of the fishing procedure and equipment, we refer to Belpaire et al. [8]. The fish were sorted on the field and bycatch was released. The perch and eel were frozen for transport and stored at − 20 °C until further processing.
Mussels
Non-native quagga mussels (Dreissena bugensis) were collected in the recreational lake the Nekker in Mechelen between 2017 and 2019. This area was selected based on the absence of any known pollution sources and hence, low concentrations of PFAS were expected. Furthermore, low concentrations of organic micropollutants (polychlorinated biphenyl’s (PCBs), polybrominated diphenylethers (PBDEs), organochlorine pesticides (OCPs)) measured by Bervoets et al. [11] reflect the general absence of industrial and household influences.
At least 2 weeks prior to exposure, the mussels were acclimated to the current climate in a semi-natural pond (mesocosm structure, University of Antwerp, Belgium), filled with dechlorinated tap water. A subset of 5–10 randomly selected mussels was analysed before exposure as to determine background concentrations. In order to reduce undesired spread of this exotic species to the sampling locations, the exposure took place during autumn and winter, since quagga mussel reproduction declines at low water temperatures [75]. At locations with high salinity (N = 5, mean EC20: > 2.4 mS/cm), Asiatic clams (Corbicula fluminea) were exposed, as quagga mussels would not survive the higher salinity. These clams were collected from the recreational lake the Blaarmeerse in Ghent (2017 and 2018) and in the Nekker in Mechelen (2019). Unfortunately, insufficient individuals could be collected from the Blaarmeerse in order to determine background concentrations. However, we expected little difference with previously measured concentrations in zebra mussels (Dreissena polymorpha) which showed very low ∑PFAS concentrations, namely 8.2 ng/g ww (unpublished data; Additional file 1: Table S1). Due to the very high and fluctuating salinity (EC20: 22.9 ± 14.4 mS/cm) in the harbour channel of the IJzer, local populations of blue mussel (Mytilus edulis) attached to a wharf were collected and analysed. For an overview of the used species per location and exposure year, we refer to Table 1.
A total of 70 to 75 quagga mussels per location were exposed during 6 weeks in two polyethylene cages, each consisting of two attached pond baskets (11 × 11 × 22 cm; mesh size of 2 × 4 mm), allowing free water circulation [5, 11, 66]. When Asiatic clams were used, 25 to 30 individuals were exposed, because of their larger size. Cages were attached to bridges or solid structures on the bank using metal chains and locks, at a depth of at least 1 m below the water surface. After recollection, mussels were depurated for at least 15 h in particle-free water from the respective sampling location at 15–20 °C. Mussels were frozen at − 20 °C until further processing. Per location, three to five mussels were randomly selected for PFAS analysis on each individual. Lipid content and polyaromatic hydrocarbon (PAHs) concentrations were determined in the remaining (pooled) mussels as part of a large monitoring study [69].
Sample preparation
A total of 515 perch and 132 eel were collected. Fish in poor conditions or visibly damaged, were discarded. Muscle samples of ± 1 g per individual were taken from the mid dorsal part of the body, opposite to the anus. Fish were pooled per species per location and homogenized using a stainless steel kitchen mixer (Bosch, MSM65PER). This resulted in 33 perch pools and 41 eel pools. The composition of the pools is presented in Table 1.
Although often higher PFAS concentrations are measured in liver tissue [46, 71], in the present study we chose to measure them in muscle tissue. This facilitates the calculation of human health risk and environmental monitoring (of hydrophobic compounds) in terms of secondary poisoning by top predators.
Mussel soft tissue was removed from the shell and weighed (up to 0.0001 g; Mettler AT261 DeltaRange, Mettler-Toledo). Furthermore, the tissue condition index (CI), as a measure of health, of individual mussels and clams was calculated as CI = tissue wet weight (TWT in gram)/shell dry weight [60] and is displayed in Additional file 1: Table S1. For fish, no CI was calculated since no individual samples were included in the present study and condition range would be very dependent on the size of collected fish. The mussel tissue was further homogenized using a TissueLyer LT (Qiagen, GmbH, Germany) with stainless steel beads (5 mm; Qiagen GmbH, Germany).
Chemical extraction and analyses
All used PFAS abbreviations are according to Buck et al. [14]. Fifteen PFAS were selected as target analytes, including 4 perfluoroalkyl sulfonic acids (PFSAs) and 11 perfluoroalkyl carboxylic acids (PFCAs). The target analytes and isotopically mass-labelled internal standards (ISTDs, MPFAC-MXA, Wellington Laboratories, Guelph, Canada), used in the quantification of these analytes, are illustrated in Additional file 1: Table S2. During the chemical extractions, HPLC grade acetonitrile (ACN; LiChrosolv, Merck Chemicals, Belgium), Milli-Q (MQ; 18.2 mΩ; TOC: 2.0 ppb; Merck Millipore, Belgium) and ammonium hydroxide (Filter Service N.V., Belgium) were used.
The extraction procedure followed the method described by Powley et al. [61] with modifications. The homogenized fish muscle (0.80 ± 0.27 g) and mussel soft tissue (0.23 ± 0.07 g) samples were weighed into 50 mL polypropylene (PP) tubes and spiked with 10 ng of the ISTD mixture. After addition of 10 mL of ACN, the samples were vortex-mixed thoroughly and sonicated for 3 × 10 min (Branson 2510), with vortexing in-between the time periods. Hereafter, the samples were left overnight on a shaking plate (135 rpm, room temperature, GFL 3020, VWR International, Belgium). After centrifugation (4 °C, 2400 rpm, 10 min, Eppendorf centrifuge 5804R, rotor A-4-44), the supernatant was transferred to a 15-mL PP tube and dried to 0.5 mL using a rotational-vacuum-concentrator (Eppendorf concentrator 5301, Hamburg, Germany). The concentrated extract was transferred to a PP Eppendorf tube containing 50 mg of graphitized carbon powder (Supelclean ENVI-Carb, Sigma-Aldrich, Belgium) and 50 μL of glacial acetic acid, to eliminate pigments. In addition, 2 × 250 μL of ACN, used to rinse the 15-mL PP tubes, was added to these Eppendorf tubes. After vortex-mixing, the extracts were centrifuged (4 °C, 10,000 rpm, 10 min, Eppendorf centrifuge 5415R; Rotor F45-24-11). The supernatant was dried completely using the rotational-vacuum-concentrator, and reconstituted with 200 μL of 2% ammonium hydroxide in ACN. The samples were vortex-mixed for at least 1 min and filtrated through an Ion Chromatography Acrodisc 13 mm Syringe Filter with 0.2 μm Supor polyethersulfone Membrane (VWR International, Belgium) attached to a PP auto-injector vial.
UPLC-TQD analysis
Ultra-performance liquid chromatography coupled tandem ES(−) mass spectrometry (UPLC–MS/MS, ACQUITY, TQD, Waters, Milford, MA, USA) was used to analyse the PFAS. The target analytes were separated using an ACQUITY BEH C18 column (2.1 × 50 mm; 1.7 μm, Waters, USA). An ACQUITY BEH C18 pre-column (2.1 × 30 mm; 1.7 μm, Waters USA) was inserted between the solvent mixer and injector to retain PFAS contamination originating from the system. The injection volume was set at 10 μL with a flow rate of 450 μL/min. As mobile phase solvents, 0.1% formic acid in water and 0.1% formic acid in ACN were used. The solvent gradient started at 65% of 0.1% formic acid in water, decreased to 0% in 3.4 min and returned to 65% at 4.7 min. To identify and quantify the target analytes, multiple reaction monitoring (MRM) of two diagnostic transitions per target analytes (Additional file 1: Table S2) was used. This allowed us to confirm the absence of false-positives in the samples.
Quality control and assurance
As quality control for the PFAS analyses, one procedural blank (10 mL of ACN) was analysed per batch of 10–20 samples. Additionally, per 11 samples, one reference sample of sterilized fish muscle tissue (pike-perch (Sander lucioperca), QUASIMEME Laboratory Performance Studies; [72]) was included. All measurements were within ranges of the interlaboratory study results of the reference material (Additional file 1: Table S7). To prevent cross-over contamination between samples during the UPLC-TQD analyses, ACN was injected on a regular basis to rinse the column. The concentrations in the blanks were all below the limit of quantification (LOQ). Individual LOQs were determined in the actual samples, hence taking into account possible matrix effects, based on the signal-to-noise (S/N) ratio of 10 and are displayed in Table 2. LOQs for PFBS and PFHxS were much higher compared to the other compounds, probably due to suboptimal extraction conditions. PFAS profiles might therefore not be accurate, as bioaccumulation of PFHxS was expected in fish [58]. Due to the high LOQ values, these data are possibly lacking. The target analytes were quantified using their corresponding ISTD (Additional file 1: Table S2), with exception of PFPeA, PFHpA, PFTrDA, PFTeDA, PFBS and PFDS for which no ISTD was present. These analytes were quantified using the ISTD closest in terms of functional group and carbon chain length (Additional file 1: Table S2), as has been validated by Groffen et al. [39]. Method recoveries for the fish samples varied between 41% (PFBA) and 96% (PFOA). In mussel tissue, the recoveries varied between 53% (PFHxS) and 115% (PFNA).
Stable isotope analysis
Stable isotope analyses were performed on the pooled fish muscle and mussel samples per species and per location. After freeze-drying at − 55 °C, between 0.5 and 1 mg of homogenized tissue samples were encapsulated in pre-weighted 5 × 8 mm tin (Sn) capsules to determine nitrogen (N) and carbon (C) concentrations, as well as δ15 N and δ13C [73]. Stable isotopes were measured using an EA1110 elemental analyser coupled to a Thermo DeltaV Advantage IRMS with a Conflo IV interface at the Department of Earth and Environmental Sciences, KULeuven (Belgium). For the calibration, a combination of IAEA-600 (caffeine), a leucine and a freeze-dried tuna muscle tissue standard were used. These latter two standards were previously calibrated with certified reference standards and the estimated precisions for δ15N and δ13C were better than 0.05‰ and 0.13‰, respectively.
The stable isotope results are expressed in the standard notation as defined by:
with R = 15N/14N or 13C/12C, for nitrogen and carbon isotopes, respectively.
The δ15N was divided by 3.4, the mean trophic fractionation of δ15N [12], to estimate the trophic level (TL) of the organisms (Additional file 1: Table S3). A side note should be made that, using this method, a relative rather than an absolute TL was calculated, not taking into account site-specific baseline levels and food chain. However, since δ15N of the lower trophic levels (i.e. mussels) showed limited variation between locations, indicating comparable food chain structures, we believed the used method is justified. Relations between TL and levels of bioaccumulated substances could only be assessed for PFOA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA and PFOS, as these were the only compounds that were detected in more than 50% of the samples ( furhter motivation in the statistical analyses section). Trophic magnification factors (TMFs) (i.e. the change in contaminant concentrations per trophic level) for the target analytes were determined based on the TLs and the logarithmically transformed concentrations of the analytes (more details in the statistical analyses section).
Human health and ecological risk assessment
The maximum edible amount of fish, which a person of 70 kg could consume per day without potential health risks, was calculated for PFOA and PFOS based on the minimal risk levels (MRLs) proposed by the ATSDR [2] and based on the MRL levels of the EFSA Panel on Contaminants in the Food Chain (CONTAM) [27]. The EFSA MRL value was determined on the sum of PFOA, PFOS, PFHxS and PFNA. In the present study, however, PFHxS measurements from 2016 were missing (11 sample locations) and both PFHxS and PFNA had > 97% of measurements below LOQ. Due to the high LOQ values, especially for PFHxS, using ½ LOQ would probably give an overestimation of the risk. Therefore, the EFSA 2020 guideline was compared against the sum of PFOS and PFOA. The MRL values are proposed for oral, intermediate intake. The maximum edible amount of fish which can be consumed per day without potential health risks was calculated based on Formula 2 and is displayed in Table 3:
where M is the MRL for oral intake of the substance (ng/kg body weight/day), W is the weight of a person (kg), Q is the maximum amount of contaminated organisms that a person can consume per day without risking health effects (g/days) and C is the observed concentrations of a substance in an organism (ng/g ww).
Furthermore, accumulated PFOS concentrations in fish were tested for compliance against the European Environmental Quality Standards for biota (EQSbiota), namely 9.1 µg/kg ww [24].
Statistical analyses
The statistical analyses were performed using R Studio (version 3.2.2) and the level of significance was set at p ≤ 0.05 (adjusted p-values). The normality assumptions of the residuals were examined using the Shapiro–Wilk test. Concentrations below the LOQ were given a value of LOQ/2 [10]. Whenever the quantified concentrations of an analyte were below the LOQ in more than 50% of the samples at a certain location, or in a certain species, these data were excluded from the statistical analyses in order to minimize left-skewing of the data due to overleverage by left-censored data. Significant differences in CI of the mussels among locations were investigated using a one-way ANOVA followed by Tukey’s honestly significant difference test for post hoc analysis. These tests were also used to investigate differences in stable isotope concentrations, as well as in TLs, among species. Spearman rank correlation tests were used to investigate correlations between the CI of the mussels and the accumulated PFAS concentrations as well as between the PFAS concentrations in the organisms and the δ13C and δ15N concentrations. For this analysis, mean values per location were determined. Paired two-sample T-tests, or Paired Wilcoxon signed rank tests in case of non-normality, were used to test for differences in PFAS concentrations between both fish species and between the fish species and mussels. We used Stable Isotope Bayesian Ellipses in R (SIBER) to compare the isotopic niche area of each species as well as isotopic niche overlap among the species. This technique has been proven useful to compare isotopic niche widths among and within communities [48]. Trophic magnification factors (TMFs) were determined based on a linear regression model between the TLs and the logarithmically transformed concentrations of the target analytes. The TMFs were calculated as 10b, where b is the slope of the linear model [12].
Results
PFAS concentrations and profiles
Mean background ΣPFAS (i.e. the sum concentration of all the target analytes, with values < LOQ replaced by LOQ/2) concentrations for mussels collected from the Nekker (reference location) were 21.88 ng/g ww for quagga mussels and 20.79 ng/g ww for Asian clams. The spatial distributions of ΣPFAS concentrations in the mussel and fish species are displayed in Figs. 2, 3, 4. The ΣPFAS concentrations ranged from 8.56 to 157 ng/g ww in mussels (median ΣPFAS concentration of 22.4 ng/g ww), 5.22–67.8 ng/g ww in perch (median ΣPFAS concentrations of 20.8 ng/g ww), and between 5.73–68.8 ng/g ww in eel (median ΣPFAS concentration of 22.1 ng/g ww). Detailed information on PFAS concentrations in the mussels and fish at each individual location is reported in Additional file 1: Table S1 for mussel and Additional file 1: Table S4 for perch and eel.
The mean and median PFAS concentrations over the sites are displayed for each species in Table 2 and compared in Fig. 5. As PFOA, PFDA, PFUnDA, PFDoDA and PFTeDA were detected in more than 50% of both the mussels and fish muscle tissue (of both species), only these compounds were compared among mussels and fish. In addition, PFTrDA and PFOS were detected in more than 50% of the muscle samples of both fish species and hence we also compared the concentrations of these analytes between eel and perch. Significant differences in PFAS concentrations between mussels and perch were observed for PFOA (p < 0.001), PFDA (t13 = − 4.187, p = 0.001), PFUnDA (p = 0.030) and PFTeDA (p = 0.002). The PFOA and PFTeDA concentrations were higher in the mussels, whereas the concentrations of PFDA and PFUnDA were significantly higher in perch. The PFDoDA concentrations did not significantly differ between perch and mussels (p = 0.326). Similarly to the perch, the PFOA concentrations in eel were also significantly lower than those in the mussels (p < 0.001), whereas PFDA (t17 = − 2.244, p = 0.038) and PFUnDA (p = 0.021) concentrations were higher in the eel. No differences between eel and mussels were observed for PFDoDA (p = 0.265) and PFTeDA (0.167). Between the fish species, significant differences were observed for PFOA (p < 0.001), PFDA (t28 = 3.17, p = 0.004), PFDoDA (p < 0.001), PFTrDA (p < 0.001), PFTeDA (p < 0.001) and PFOS (p = 0.016). The PFOA, PFDoDA, PFTrDA, PFTeDA concentrations were higher in eel than in perch, while PFDA and PFOS concentrations were higher in perch. The PFUnDA concentrations did not differ between the fish species (p = 0.629).
Comparison of PFAS muscle concentrations in ng/g wet weight among eel (red bars; N = 41), perch (green bars; N = 33) and mussels (blue bars; N = 178). Differences in concentrations among the organisms are depicted by different letters for a specific compound. Only compounds with a detection frequency > 50%, in at least two of the three organisms, are included. Hence, the PFTrDA and PFOS concentrations in mussels are excluded from the Figure
The PFAS profiles in the mussel, perch and eel are displayed in Fig. 6. Regarding the mussels, we did not distinguish among the different species and grouped them all together in Fig. 6, as no large differences in PFAS profiles were observed among the three mussel species (Additional file 1: Figure S1). The PFOA contribution in the blue mussels was slightly larger than those in the quagga mussel and Asiatic clam, but this is likely the result of a smaller sample size (due to the collection in only one location) of this species compared to the others. The PFAS profile of the mussels was dominated by PFOA, whereas in both fish species PFOS was the dominant compound. Furthermore, the relative contribution of short-chained PFAS, i.e. PFBA, PFPeA, PFHxA and PFBS was higher in the mussels than in the fish species. Between the fish species, no major differences in detected PFAS compounds were observed, although PFHxA was only detected in eel. However, the relative contribution of the detected compounds did sometimes differ between species. The PFAS profiles of the mussels contained a higher contribution of PFBA (F2,252 = 5.57, p = 0.004), PFOA (F2,252 = 49.1, p < 0.001) and PFTeDA (F2,252 = 12.8, p < 0.001) compared to both fish species. The opposite, with a higher contribution in the fish than in mussel, was true for PFNA (F2,252 = 18.5, p < 0.001), PFDA (F2,252 = 18.9, p < 0.010) and PFOS (F2,252 = 821, p < 0.001). In addition, the PFHxA contribution was significantly higher in mussels than in eel (p < 0.001). The PFUnDA (F2,233 = 1.96, p = 0.143), PFDoDA (F2,252 = 3.03, p = 0.169) and PFTrDA (F2,252 = 2.89, p = 0.057) contributions were similar between mussels and fish. Between the fish species, contributions of PFDA (F2,252 = 18.9, p = 0.022) and PFOS (F2,252 = 821, p < 0.001) were higher in perch compared to eel. The opposite was true for PFDoDA (F2,252 = 3.03, p = 0.039). Contributions of PFBA, PFOA, PFNA, PFUnDA, PFTrDA, PFTeDA and PFDS did not differ between eel and perch (p > 0.179).
One-way ANOVA results showed that only the CI of the quagga mussels exposed at the Dijle (Fig. 1, Loc. 40) was significantly higher than those at the Zenne (Fig. 1, Loc. 31) (F28,144 = 2.44, p = 0.030), while no other differences among locations were observed for the quagga mussels. The Asiatic clams did not differ in CI among the six locations (F6,26 = 2.19, p = 0.077). There was no significant correlation between the CI of the mussels (regardless of species) and concentrations of PFOA (p = 0.991), PFDA (p = 0.950), PFUnDA (p = 0.687), PFDoDA (p = 0.928) and PFTeDA (p = 0.747), nor with ΣPFAS concentrations (p = 0.517). When looking at only the quagga mussels, the PFOA (p = 0.843), PFDA (p = 0.680), PFUnDA (p = 0.590), PFDoDA (p = 0.732), PFTeDA (p = 0.307) and ΣPFAS (p = 0.843) concentrations were not significantly correlated to the CI of the mussels. For all other PFAS, the detection frequencies were below 50% and hence, no correlation with the CI was investigated.
Isotopic niche overlap, trophic levels and associations with PFAS concentrations
The δ13C and δ15N values are reported in Additional file 1: Table S1 for the mussels, and Additional file 1: Table S4 for the fish species. The SIBER analysis revealed that the isotopic niches of perch and eel, as well as those of the quagga mussels and Asian clams, overlapped (Fig. 7, Additional file 1: Table S5). Both mussel species had no, or very limited, overlap in isotopic niche with the fish species (Fig. 7, Additional file 1: Table S5). This difference in isotopic niche between the mussel species and the fish species was mainly the result of significant differences in δ15N values, which were higher in both fish species compared to the mussel species (F3,130 = 120, p < 0.001). The δ13C did only differ between the quagga mussels and the eel, with higher δ13C values in the mussels (F3,130 = 3.18, p = 0.026). The median corrected standard ellipse area (SEAc), representing the isotopic niche width, was larger in eel (23.84‰2), compared to perch (11.00‰2), while the SEAc of the Asian clams (13.46‰2) was higher than those of the quagga mussels (8.39‰2) (Additional file 1: Figure S2).
The trophic levels of the organisms are displayed in Additional file 1: Table S3. The TLs for the mussel species were significantly lower than for the fish species (F3,84 = 100, p < 0.001). Differences in TL between the quagga mussels and the Asian clams, as well as between the perch and eel, were not significant. The TL of all organisms was negatively related to the PFOA (slope (b) = − 0.381, R2 = 0.476, p < 0.001) and PFTeDA (b = − 0.137, R2 = 0.062, p = 0.011) concentrations, and positively related to the concentrations of PFDA (b = 0.101, R2 = 0.212, p < 0.001), PFUnDA (b = 0.097, R2 = 0.116, p = 0.002) and PFOS (b = 0.499, R2 = 0.638, p < 0.001). No relationships between TL and PFDoDA (p = 0.791) and PFTrDA (p = 0.409) were observed. TMFs were only calculated for the compounds that were significantly related with TLs and are displayed in Additional file 1: Table S6.
Significant negative relations have been observed between the PFOA concentrations in mussels and perch (t16 = − 2.31, p = 0.035, R2 = 0.20), whereas concentrations of PFTrDA (t16 = 4.77, p < 0.001, R2 = 0.56) and PFTeDA (t16 = 4.54, p < 0.001, R2 = 0.54) were positively related between both organisms. No relationships between the PFAS concentrations in perch and mussels have been observed for PFDA, PFUnDA, PFDoDA and PFOS (p > 0.05). Between mussels and eels, significant positive relationships were observed for PFDoDA (t19 = 2.16, p = 0.044, R2 = 0.15), PFTrDA (t19 = 6.87, p < 0.001, R2 = 0.70), PFTeDA (t19 = 6.89, p < 0.001, R2 = 0.70), and PFOS (t19 = 4.83, p < 0.001, R2 = 0.53). The concentrations of PFOA, PFDA and PFUnDA (p > 0.05) were not related between eel and mussel. Finally, between both fish species, a positive relationships was observed for PFUnDA (t11 = 9.91, p < 0.001, R2 = 0.89), PFDoDA (t14 = 7.37, p < 0.001, R2 = 0.78), PFTrDA (t14 = 16.8, p < 0.001, R2 = 0.95), PFTeDA (t14 = 5.53, p < 0.001, R2 = 0.66) and PFOS (t14 = 3.18, p = 0.006, R2 = 0.38). The concentrations of PFOA and PFDA (p > 0.05) were not related between eel and perch.
Negative correlations were observed between the δ13C values and concentrations of PFOS (ρ = − 0.405, p < 0.001), PFDA (ρ = − 0.410, p < 0.001), PFUnDA (ρ = − 0.317, p = 0.007), PFDoDA (ρ = − 0.277, p = 0.009) of all organisms, as well as between values of δ15N and concentrations of PFOA (ρ = − 0.659, p < 0.001). The δ13C concentrations were positively correlated with concentrations of PFOA (ρ = 0.275, p = 0.009), whilst the δ15N concentrations were positively correlated with those of PFOS (ρ = 0.753, p < 0.001), PFDA (ρ = 0.439, p < 0.001) and PFUnDA (ρ = 0.363, p = 0.002). No correlations were observed between δ13C and PFTrDA (p = 0.306), δ13C and PFTeDA (p = 0.302), and between δ15N and concentrations of PFDoDA (p = 0.548), PFTrDA (p = 0.644) and PFTeDA (p = 0.101). For all other PFAS, no correlations were examined as detection frequencies were < 50%.
Human health risks
Based on the mean concentrations and the concentration ranges in the eel and perch, the maximum edible amounts of both fish species per day (g) for a person of 70 kg have been calculated and reported in Table 3. This value was determined on the pooled dataset of all sample locations. Calculated using the mean concentrations of the sum of PFOS and PFOA, a person of 70 kg should consume maximally 6.4 g of eel and 5.0 g of perch per day without a potential health risk. A worst-case scenario, using the maximum concentrations detected in the fish species, revealed that humans should not consume more than 0.68 g of eel and 0.82 g of perch per day. For the above results the MRLs by the EFSA Panel on Contaminants in the Food Chain [27] were used, since they are the most strict. For calculations based on ATSDR [2] MRLs for PFOS and PFOA individually, we refer to Table 3.
Discussion
Spatial distribution
Our results confirmed a wide distribution and bioavailability of PFAS in the aquatic environment. The canal Brussel–Schelde (Fig. 1, Loc. 30) showed high accumulated concentrations in all biota. This canal is subject to direct influence from intensive industrial activities. The highest PFAS concentrations in perch were measured in the Zenne River (Fig. 1, Loc. 31), known for its very high background pollution and influences from Brussels [69]. Furthermore, high concentrations were measured in mussels deployed in the canal Bocholt–Herentals (Fig. 1, Loc. 42). This canal is a connection between the Meuse and Scheldt basin, both known for large effects of industrial as well as household waste water as a source of PFAS [69]. The Melsterbeek, however, showing high accumulated concentrations in eel, flows through a more agricultural region. Here, contamination with PFAS might be caused by agriculture, households or undefined point sources. However, our conclusions on this part are mere qualitative and based on personal interpretation and experiences of the general monitoring network of the Flanders Environment Agency. Further investigation using data on population density, area of industrial surfaces and emission indices could be used to investigate the relationship between accumulated concentrations in biota and possible sources with a more quantitative approach.
The use of PFOS is restricted since 2009 [67]. This should eventually lead to a decrease of this substance in the environment. Previous studies on the water bodies used in the present study indeed showed higher concentrations of PFOS. A preliminary monitoring study from 2013 reported PFOS concentrations in muscle tissue of eel of 15, 33, 7.2 and 34 µg/kg ww in the upper-Scheldt, canal Ieper-IJzer, Kleine Nete and Demer, respectively [21]. The present study showed concentrations of 5.6, 14.5, 12 and 11.3, respectively, at the same sampling locations in eel. These results showed a clear decrease and possibly revealed the effects of phasing-out the use of these compounds to the environment, with the exception of the Kleine Nete, which remained stable.
Compared to previous studies on yellow perch (Perca flavescens), the PFOS (118.6 ± 29 ng/g ww) and PFUnDA concentrations (3.8 ± 1.2 ng/g ww) in New Jersey, USA, were considerably higher than those reported in the present study, whilst, on the contrary, concentrations of PFDA (1.1 ± 0.4 ng/g ww) and PFDoDA (0.7 ± 0.2 ng/g ww) were lower in New Jersey [34]. The concentrations of PFOS, PFUnDA, PFTrDA, PFDA, PFDoDA and PFOA were higher in the present study compared to those reported in perch collected in Finnish rivers (3.4 ng/g PFOS, 1.0 ng/g PFUnDA, 0.45 ng/g PFTrDA, 0.5 ng/g PFDA, 0.23 ng/g PFDoDA and 0.03 ng/g PFOA [49]), and to those reported in shad (Alosa agone), European whitefish (Coregonus lavaretus), burbot (Lota lota), rainbow trout (Oncorhynchus mykiss), perch, roach (Rutilus rutilus), brown trout (Salmo trutta) and Arctic char (Salvelinus alpinus) in glacial lakes from the Alps in France, Switzerland and Italy (6.0 ng/g PFOS, 0.3 ng/g PFUnDA, 0.5 ng/g PFDA and 0.3 ng/g PFDoDA [71]). A monitoring study in the Netherlands measured PFOS concentrations in bream (Abramis brama), roach, perch and pike-perch between 4.9 and 120 ng/g ww [31], which were higher than those reported in the present study. In the North Rhine–Westfalen basin in Germany, eel PFOS concentrations ranged between 8.3 and 49 ng/g ww [40]. The PFOS concentrations in the Loire estuary ranged from 17.9 to 39.0 ng/g ww [19]. Kwadijk et al. [50] examined the distribution of multiple PFAS in eel from The Netherlands and reported PFOS concentrations ranging from 7 to 58 ng/g ww. In Lake Möhne, Germany, PFOS concentrations of 37–83 ng/g ww have been reported in eel, whereas PFOA concentrations ranged up to 2.3 ng/g ww [44]. These concentrations are comparable to those reported in eel in the present study. Compared to other studies investigating PFAS concentrations in mussels, the ΣPFAS concentrations were higher in the present study than reported in previous studies in fresh water mussels from Spain [30], and marine mussels from the Netherlands [78], Spain [35, 77], and Denmark [13], but lower than those reported in marine mussels from Portugal [20]. Comparison to literature, revealed a large variation of PFAS concentrations measured in biota, both on a European and global scale. High concentrations might be due to the presence of different sources of PFAS contamination (e.g. point sources, diffusive emission sources). On the other hand, differences between species could be explained by their diet [4].
PFAS bioaccumulation, magnification and dilution
Longer chain PFAS preferentially partition to sediments, as the water solubility of PFAS is inversely proportional to the length of the carbon chain, while short-chain compounds remain dissolved in the water [51, 62]. The carbon chain length, as well as the identity of the anionic functional group of PFAS, is related to their bioaccumulative potential, with PFSAs being more bioaccumulative than PFCAs with the same fluorinated carbon chain length [17]. Furthermore, PFCAs and PFSAs that contain at least eight fluorinated carbons (i.e. PFNA and PFOS and longer compounds) have a greater bioaccumulative potential [17]. Although shorter chain PFAS can also bioaccumulate, they have a much smaller bioaccumulation potential and their bioaccumulation is mainly related to elevated concentrations in the water column [17, 34]. This might also explain the larger contribution of short-chain PFAS in mussels compared to fish, since both isotopic niche analysis and TL confirmed that the mussels occupied a lower position in the trophic food chain.
The differences in PFAS profiles between the mussels and fish species are likely also the result of different ways of exposure, caused by the sampling strategy and experimental design of this study. As the mussel cages were placed in the water column, without contact to the sediment, the mussels have been exposed solely to the water and suspended material, whilst the fish have been exposed to both the water and sediment. Although both species are considered to be spending time in close relation to the sediment compartment [7, 74], eel shows a more bottom-dwelling lifestyle. Consequentially, the dominance of hydrophilic PFCAs and PFSAs, with less than eight fluorinated carbons (i.e. PFOA, PFHxS and shorter PFAS), was expected in the mussels. Similarly, the fish species have also been exposed to sediments and hence to longer chain PFAS with a higher bioaccumulative potential. This also explains why PFOA concentrations were significantly higher in the mussels compared to both fish species, whilst the opposite pattern was often observed for longer chain PFAS. On the other hand, it has been shown that biotransformation (the degradation of PFAS precursors to, for example, PFOS) efficiency increased with increasing trophic level, from invertebrates to fish [4].
Dietary differences between perch and eel could explain the differences in PFAS concentrations and profiles between both fish species. Although on average, the TLs of both species did not differ, eels have a slightly broader range of TLs compared to perch. Despite that both are predatory species, feeding primarily on invertebrates and small fish species [63, 76], there are differences in the feeding ecology between both species in the studied populations. The broader isotopic niche (indicated by SEAc) indicates that, despite the overlap in isotopic niche area between both species, eels have a more diverse and flexible diet, which might consist of different invertebrate or fish species, compared to perch [9]. For example, De Meyer et al. [22] showed that head morphology of eel (broad-headed vs. narrow-headed) could influence diet, trophic level and therefore pollutant accumulations. Furthermore, the diet of both species is known to depend on their size, as size-dependent diet segregation of both species has been reported before [29, 63, 76]. This segregation is also known to vary widely among populations [63], which could also explain dietary differences, and hence differences in exposure, between perch and eel. Additionally, spatial differences in diet may occur depending on local ecological variation in species composition and food availability. Finally, biotransformation of PFAS can be species-specific [4, 32], probably due to specific proteins involved in the process [59], resulting in different contamination profiles.
A positive correlation between accumulated concentrations in mussels and fish was found for PFTrDA, PFTeDA, and PFOS and PFDoDA in eel. This positive relationship reflects the possibility of mussels to predict the pollutant pressure at different locations, since high concentrations at a specific location are found in fish as well as mussels. With a negative correlation, as was the case for PFOA in perch, the relationship between species is contradictory, and mussels will not be able to predict high pollution levels in fish (and therefore a risk of secondary poisoning). Furthermore, all significant relationships between both fish species showed to be positive, which is a logical consequence since they occupied similar trophic levels of the same food web in each location.
The TMFs in the present study were compared to those of other studies on freshwater ecosystems, and were higher than those reported by Loi et al. [54] for PFOS (TMF = 1.3) and lower than those for PFUnDA (TMF = 1.7), although the general trend of biomagnification was comparable to the present study. Furthermore, the TMFPFOS calculated in the present study is in line with other European studies on lake and river food chains as reported by Rüdel et al. [64]. On the other hand, Lescord et al. [53] reported a negative relation between accumulation and trophic level for PFOS, PFNA and PFUnDA in a foodweb from the high Arctic. In a study on alpine lakes in Northern Italy, a TMFPFOS of 3 was found [57]. However, when they analysed a fish-only food web this value became not significant and lower than one. The presence of TMFs greater than 1 for PFOS, PFDA and PFUnDA was expected, as the biomagnification of these PFAS has been reported before [47, 58]. The observed biodilution for PFOA and PFTeDA could, in case of PFOA, be explained by differences in exposure between the mussels and fish species, as was described above. Regarding PFTeDA, its biodilution may be associated with its large molecular size, limiting the penetration of cell membranes [17, 45]. However, since species of different locations were compared, we might need to take into account the possible effect of location (ecological quality, physicochemical parameters) rather than just bare bio-magnification and -dilution effects on bioaccumulated concentrations. This is in agreement with Munoz et al. [58], who stated that the PFAS chemical structure is not be exclusively predictive of TMFs, since they are also influenced by trophic web characteristics.
Suitability of mussels in active biomonitoring of PFAS
The PFAS profiles in the mussels did not differ much among the three species (Additional file 1: Figure S1). Furthermore, isotopic niche determination showed an overlapping niche for both quagga mussels (D. bugensis) and Asian clams (C. fluminea). Therefore, it is appropriate to use both species in monitoring studies on locations with varying salinity and extrapolate the results. Although the PFAS profile of the blue mussels (M. edulis) differs slightly from those of the quagga mussels (D. bugensis) and the Asian clams (C. fluminea), this is likely the result of a smaller dataset for the first species. Therefore, more research, using a larger sample size, is necessary to fully confirm the suitability of using blue mussels simultaneously with the other two species.
Regarding their suitability in active biomonitoring, our results show that mussels can provide an overview of the contaminants present in the environment to which fish are exposed. The PFAS compounds that have been detected in the mussels were similar to those detected in the fish species, although concentrations differed, as was explained above.
Both ABM using mussels and PBM using indigenous fish, have their assets and liabilities. As the mussels provided a short time pollution profile (exposure during 6 weeks), fish allowed the integration of a lifetime exposure. Short time exposure might be influenced by seasonal variations in bioavailability, which is cancelled out using indigenous species. Furthermore, the numerous measurements below LOQ in mussels might give an underestimation of the situation. The bioavailability, however, is made clear through biomagnification in the accumulated concentrations of fish. Measuring in fish also may give additional information towards risk assessment for species of higher trophic levels feeding on fish, such as predatory birds or mammals (including humans). On the other hand, from an ethic perspective, the use of invertebrates might be encouraged. PFAS analysis can be done on a small amount of tissue and therefore on individual mussels, so no large numbers are needed. The results in the present study confirm the possibility of extrapolation between mussels and fish and between both fish species.
Human health risks
The maximum recommended amount of eel that could be consumed without posing health risks, according to the ATSDR guidelines [2], was lower than that of perch concerning PFOA contamination, but for PFOS the opposite was true. Nonetheless, for PFOS the differences between both species (ca. 1.4 times) were smaller than for PFOA (ca. 2 times). On the other hand, when using the EFSA value [27], the maximum recommended amounts of both fish were much lower. This sensitive value was determined with a decreased immunoresponse after consumption as a critical human health response and is to be tested against the sum of PFOS, PFOA, PFNA, PFHxS. However, as stated in the materials section, the sum of PFOS and PFOA was used in the present study. Although, this might have led to an underestimation of the actual risk, we believe it to be a good estimation since PFOS had the largest contribution to the total PFAS sum.
Due to consumption of their catch, in Flanders mainly recreational anglers and their families may be exposed to contaminated fish. A mean consumption of 2.7 g of perch per day and 18 g of eel per day was reported in an interview on anglers (ANB-VF/2015/4). The maximum recommended amounts of fish that could be consumed without posing health risks (Q-values; Table 3), calculated using the mean concentrations in the fish species, were higher than the mean consumption amounts in Flanders for both species using the ATSDR [2] MRL. The Q-values for eel were ca. 50 and 1.2 times higher than the reported consumption amount, for PFOA and PFOS, respectively. For perch, this was ca. 630 and 6 times higher. On the other hand, using the more strict EFSA [27] MRL, Q-values were below the reported consumption amount for eel and only 2 times higher for perch.
However, when using a worst-case scenario, based on the maximum concentrations in both fish species, health risks due to PFAS contamination are expected from the consumption of both fish species. In this worst-case scenario, the maximum edible amount of perch per day was 2.62 g/day and 0.82 g/day (Table 3), calculated using the ATSDR [2] PFOS MRL value and the EFSA [27] MRL values, respectively. For eel, these Q-values in the worst-case scenario are 2.17 g/day and 0.68 g/day, respectively, which are 8 to 26 times lower than the average eel consumption in Flanders.
Therefore, it is likely that the local recreational fishermen have a high chance of experiencing detrimental effects of accumulated PFAS concentrations. Evidentially, calculations were performed on mean consumption rates, indicating individuals exist that consume more. For these people even more locations might pose a health risk, since the Q-values for perch were very close to the mean consumption rate. PFOS accumulation in humans has been associated with multiple hepatotoxic, neurotoxic, reproductive, immunotoxic and thyroid disruptive effects, which could lead to severe diseases and even death (as reviewed by [80]. Even at very low concentrations, PFAS can alter the lipodome, disrupting lipid and weight regulation [36]. The Flemish government, however, already discourages consumption of eel and other predatory fish from Flemish waterbodies due to high concentrations of other pollutants (e.g. PCBs) [55].
Ecological health risk
Under the Water Framework Directive (WFD) the EU [24] defined Biota Quality Standards (EQSbiota) for freshwater, threshold concentrations for protection of the integrity of aquatic ecosystems and specifically for prevention of secondary poisoning and human health risk. For perfluoroalkyl substances, 9.1 µg PFOS/kg ww was set as the (human health based) threshold (EQSbiota, hh). For eel and perch, respectively, 44% and 58% of sampling locations exceeded the EQSbiota, hh, indicating potential health risks to the food web and to top predators (including humans) through fish consumption. In a German monitoring study, PFOS was above the EQSbiota,hh in 33% of the locations in perch muscle [64, 65]. However, the current EQSbiota, hh is based on the previous EFSA tolerable daily intake (TDI) value for PFOS of 150 ng/kg body weight, which can be converted to 10.5 µg per day considering a 70 kg person [26, 56]. This value is more than 200 times higher than the sensitive EFSA group TWI value [27] used in the human health risk determination in the present study. Furthermore, the EQSbiota was calculated considering a mean European daily fish consumption of 115 g [23], while Belgium is known to have a lower fish consumption compared to other European countries [1]. All this leads to the conclusion that the current EQSbiota for PFOS might underestimate the risk for human health consequences through fish consumption, especially for Belgium, and needs to be revised.
On the other hand, the higher EQS of PFOS of 33 µg/kg ww [23] was determined specifically for protection of top predators against secondary poisoning (EQSbiota, secpois). Comparison to this standard resulted in an exceedance for 7% of the sampling locations for eel and 15% for perch. It was, however, stated that when determining the risk for secondary poisoning it is more appropriate to use whole fish measurements instead of fillet [25]. An average conversion factor between both matrices of about 3 was determined for perch [64, 65, 81]. This would increase the exceedance in perch to 45% of all locations.
Furthermore, the setup of our study is in line with general recommendations for biota monitoring under de WFD [25]. All biota used in the present study are considered good biomonitor species. However, in order to estimate the risk for secondary poisoning, taking into account biomagnification effects, the use of top predators (TL of 4 in freshwaters) is recommended. Both fish species included in the present study could be classified as such (TLperch: 4.97 ± 0.15; TLeel: 4.86 ± 0.14). Furthermore, their widespread (European) occurrence and limited home range allow for good monitoring practices [7, 81]. Although, within the present study a limited size range was targeted, differences in ranges and mean fish sizes between locations were detected [69]. This might affect the mean PFAS concentrations per location and comparison between locations, since accumulated concentrations increase with size and age [25]. As stated before, due to the high affinity of PFAS for proteins, liver tissue might have been a better matrix for sole monitoring purpose. However, since human health risk assessment was an important focus of the present study, muscle tissue was considered a more appropriate matrix. Finally, a standardization of hydrophobic compounds was proposed in the Guidance Document [25]. For mercury and perfluorooctane sulfonate (PFOS), which do partition to proteins in contrast to the other lipophilic priority compounds, a standardization to a default dry weight fraction of 26% was recommended. This approach was not included in the present study. However, the standardization had a very limited effect on or even increased the variability of measured concentrations [81, 71]. Furthermore, Valsecchi et al. [71] reported that dry weight standardization, as a proxy for protein content, for PFOS is inappropriate because PFAS bind to specific proteins.
Conclusion
Based on both ABM and PBM, our results show that PFAS are widely bioavailable across Flanders’ aquatic environment. The highest concentrations were measured nearby known densely populated areas, probably with both industry and households being the main sources for PFAS pollution. The PFAS concentrations in the fish species were, in general, comparable to those reported in other industrialized and populated regions in Europe and the USA.
Although biomagnification as well as biodilution have been observed, it should be stated that this was examined on a combination of translocated and indigenous organisms, which have been exposed to different sources throughout the ABM period. Therefore, the outcomes could differ when using indigenous invertebrates, whose concentrations would reflect not only the exposure through the water, but also through the sediment. Nonetheless, translocated mussels have been proven suitable to determine which PFAS are present in indigenous fish, as PFAS profiles were similar among the different species.
Human health risks due to the consumption of PFAS-contaminated fish are expected, especially for eel. Based on the average concentrations, the recommended amounts of fish that could be consumed without posing health risks were lower than the mean consumption amounts in Flanders for eel, but not for perch. However, regarding perch, this difference was very small. Hence, when looking at a worst-case scenario, calculated using the maximum detected concentrations in fish, these recommended consumption amounts were much lower than the mean consumption amounts in Flanders.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its additional information files].
Abbreviations
- ΣPFAS:
-
Sum concentrations of the target analytes
- ABM:
-
Active biomonitoring
- ACN:
-
Acetonitrile
- ATSDR:
-
Agency for Toxic Substances and Disease Registry
- C:
-
Carbon
- CI:
-
Condition index
- EFSA:
-
European Food Safety Authority
- ES:
-
Electrospray
- EQS:
-
Ecological quality standard
- FWO:
-
Research Foundation Flanders
- HPLC:
-
High-performance liquid chromatography
- INBO:
-
Research Institute for Nature and Forest
- IRMS:
-
Infrared mass spectrometry
- ISTD(s):
-
Internal standard(s)
- LOQ:
-
Limit of quantification
- MRL(s):
-
Minimal risk level(s)
- MRM:
-
Multiple reaction monitoring
- MS:
-
Mass spectrometry
- MQ:
-
Milli-Q
- N:
-
Nitrogen
- OCP(s):
-
Organochlorine pesticide(s)
- PAH(s):
-
Polyaromatic hydrocarbon(s)
- PBDE(s):
-
Polybrominated diphenylether(s)
- PBM:
-
Passive biomonitoring
- PCB(s):
-
Polychlorinated biphenyl(s)
- PFAS:
-
Per- and polyfluoroalkyl substances
- PFBA:
-
Perfluorobutanoic acid
- PFBS:
-
Perfluorobutane sulfonate
- PFCA(s):
-
Perfluorocarboxylic acid(s)
- PFDA:
-
Perfluorodecanoic acid
- PFDS:
-
Perfluorodecane sulfonate
- PFDoDA:
-
Perfluorododecanoic acid
- PFHpA:
-
Perfluoroheptanoic acid
- PFHxA:
-
Perfluorohexanoic acid
- PFHxS:
-
Perfluorohexane sulfonate
- PFNA:
-
Perfluorononanoic acid
- PFOA:
-
Perfluorooctanoic acid
- PFOS:
-
Perfluorooctane sulfonate
- PFPeA:
-
Perfluoropentanoic acid
- PFSA(s):
-
Perfluorosulfonic acid(s)
- PFTeDA:
-
Perfluorotetradecanoic acid
- PFTrDA:
-
Perfluorotridecanoic acid
- PFUnDA:
-
Perfluoroundecanoic acid
- POP(s):
-
Persistent organic pollutant(s)
- PP:
-
Polypropylene
- S/N:
-
Signal-to-noise
- SEA:
-
Standard ellipse area
- SIBER:
-
Stable Isotope Bayesian Ellipse in R
- TL:
-
Trophic level
- TMF(s):
-
Trophic magnification factor(s)
- TQD:
-
Triple quadrupole
- TWT:
-
Tissue wet weight
- TWI:
-
Tolerable weekly intake
- TDI:
-
Tolerable daily intake
- UPLC:
-
Ultra-performance liquid chromatography
- VMM:
-
Flanders Environment Agency
- ww:
-
Wet weight
- WFD:
-
Water framework directive
References
Altintzoglou T, Vanhonacker F, Verbeke W, Luten J (2011) Association of health involvement and attitudes towards eating fish on farmed and wild fish consumption in Belgium Norway and Spain. Aquacult Int 19:475–488
Agency for Toxic Substances, Disease Registry (2019) https://www.atsdr.cdc.gov/mrls/pdfs/ATSDR%20MRLs%20-%20June%202019-H.pdf
ANB-VF/2015/4 (2016) Enquête bij hengelaars op openbaar water. ANB-VF/2015/4, 2016. Enquête bij hengelaars op openbaar water. M.A.S. in opdracht van Vlaamse Overheid en Agentschap Natuur en Bos (ANB). Leuven, Belgium
Babut M, Labadie P, Simonnet-Laprade C, Munoz F, Roger MC, Ferrari BJD, Budzinski H, Sivade E (2017) Per- and polyfluoroalkyl compounds in freshwater fish from the Rhône River: influence of fish size, diet, prey contamination and biotransformation. Sci Total Environ 605–606:38–47
Bashnin T, Verhaert V, De Jonge M, Vanhaecke L, Teuchies J, Bervoets L (2019) Relationship between pesticide accumulation in transplanted zebra mussel (Dreissena polymorpha) and community structure of aquatic macroinvertebrates. Environ Pollut 252:591–598
Basack SB, Oneto ML, Guerrero NRV, Kesten EM (1997) Accumulation and elimination of pentachlorophenol in the freshwater bivalve Corbicula fluminea. Bull Environ Contam Toxicol 58:497–503
Belpaire C, Goemans G (2007) Eels: contaminant cocktails pinpointing environmental contamination. ICES J Mar Sci 64:1423–1436
Belpaire C, Smolders R, Vanden Auweele I, Ercken D, Breine J, Can TG, Ollevier F (2000) An index of biotic integrity characterizing fish populations and the ecological quality of Flandrian water bodies. Hydrobiologia 434:17–33
Belpaire C, van Driessche H, Gao FY, Ollevier F (1992) Food and feeding activity of glass eel Anguilla anguilla (L.) stocked in earthen ponds. Irish Fisheries Investigations. Series A (Freshwater) 36:43–54
Bervoets L, Voets J, Chu SG, Covaci A, Schepens P, Blust R (2004) Comparison of accumulation of micropollutants between indigenous and transplanted zebra mussels (Dreissena polymorpha). Environ Toxicol Chem 23:1973–1983
Bervoets L, Voets J, Covaci A, Chu S, Qadah D, Smolders R, Schepens P, Blust R (2005) Use of transplanted zebra mussels (Dreissena polymorpha) to assess the bioavailability of microcontaminants in Flemish surface waters. Environ Sci Technol 39:1492–1505
Borgå K, Kidd KA, Muir DCG, Berglund O, Conder JM, Gobas FAPC, Kucklick J, Malm O, Powell DE (2011) Trophic magnification factors: considerations of ecology, ecosystems, and study design. Integr Environ Assess Manag 8:64–84
Bossi R, Strand J, Sortkjær O, Larsen MM (2008) Perfluoroalkyl compounds in Danish wastewater treatment plants and aquatic environments. Environ Int 34:443–450
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7:513–541
Butt CM, Berger U, Bossi R, Tomy GT (2010) Review: levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 408:2936–2965
Colles A, Bruckers L, Den Hond E, Govarts E, Morrens B, Schettgen T, Buekers J, Coertjens D, Nawrot T, Loots I, Nelen V, De Henauw S, Schoeters G, Baeyens W, van Larebeke N (2020) Perfluorinated substances in the Flemish population (Belgium): levels and determinants of variability in exposure. Chemosphere 242:125250
Conder JM, Hoke RA, de Wolf W, Russell MH, Buck RC (2008) Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol 42:995–1003
Corlett RT (2015) The Anthropocene concept in ecology and conservation. Trends Ecol Evol 30:36–41
Couderc M, Poirier L, Zalouk-Vergnoux A, Kamari A, Blanchet-Letrouvé I, Marchand P, Vénisseau A, Veyrand B, Mouneyrac C, Le Bizec B (2015) Occurrence of POPs and other persistent organic contaminants in the European eel (Anguilla anguilla) from the Loire estuary, France. Sci Total Environ 505:199–215
Cunha I, Hoff P, Van de Vijver K, Guilhermino L, Esmans E, De Coen W (2005) Baseline study of perfluorooctane sulfonate occurrence in mussels, Mytilus galloprovincialis, from north-central Portuguese estuaries. Mar Pollut Bull 50:1128–1132
De Jonge M, Belpaire C, Verhaert V, Dardenne F, Blust R and Bervoets L (2014). Veldstudie naar de monitoring van biota in het kader van de rapportage van de chemische toestand voor de Kaderrichtlijn Water. Universiteit Antwerpen (UA) in samenwerking met het Instituut voor Natuur- en Bosonderzoek (INBO), in opdracht van de Vlaamse Milieumaatschappij (VMM). Antwerpen, België
De Meyer J, Van Wassenbergh S, Bouilliart M, Dhaene J, Adriaens D (2018) Built to bite? Differences in cranial morphology and bite performance between narrow- and broad-headed European glass eels. J Morphol 279:349–360
European Union (EU) (2011) PFOS EQS dossier 2011
European Union (EU) (2013) Directives of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J Eur Union 2013:1–17
European Union (EU) (2014) Common implementation strategy for the water framework directive (2000/60/EC)—guidance document No. 32 on biota monitoring (The implementation of EQSBIOTA) under the Water Framework Directive
European Food Safety Authority (2008) Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts—scientific opinion of the panel on contaminants in the food chain. EFSA J 653:1–131
European Food Safety Authority (2020) Scientific opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):e06223
Environmental Protection Agency (EPA) (2006) 2010/2015 PFOA Stewardship program. www.regulations.gov as docket ID: EPA-HQ-OPPT-2006-0621
Ezzat AE, El-Seraffy SS (1977) Food of Anguilla anguilla in Lake Manzalah. Egypt Marine Biol 41:287–291
Fernández-Sanjuan M, Meyer J, Damásio J, Faria M, Barata C, Lacorte S (2010) Screening of perfluorinated chemicals (PFCs) in various aquatic organisms. Anal Bioanal Chem 398:1447–1456
Foekema EM, Kotterman M and Helder D (2016) Chemische Biotamonitoring conform KRW. Methodeontwikkeling en compliance-check 2014/2015; Wageningen, IMARES Wageningen UR (University & Research Center), IMARES rapport C082/16
Galatius A, Bossi R, Sonne C, Rigét FF, Kinze CC, Lockyer C, Teilmann J, Dietz R (2013) PFAS profiles in three North Sea top predators: metabolic differences among species? Environ Sci Pollut Res 20:8013–8020
Giesy JP, Kannan K (2002) Perfluorochemical surfactants in the environment: these bioaccumulative compounds occur globally, warranting further study. Environ Sci Technol 36:146A-152A
Goodrow SM, Ruppel B, Lippincott RL, Post GB, Procopio NA (2020) Investigation of levels of perfluoroalkyl substances in surface water, sediment and fish tissue in New Jersey, USA. Sci Total Environ 729:138839
Gómez C, Vicente J, Echavarri-Erasun B, Porte C, Lacorte S (2011) Occurrence of perfluorinated compounds in water, sediment and mussels from the Cantabrian Sea (North Spain). Mar Pollut Bull 62:948–955
Gorrochategui E, Pérez-Albaladejo E, Casas J, Lacorte S, Porte C (2014) Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 227:124–130
Groffen T, Lopez-Antia A, D’Hollander W, Prinsen E, Eens M, Bervoets L (2017) Perfluoroalkylated acids in the eggs of great tits (Parus major) near a fluorochemical plant in Flanders, Belgium. Environ Pollut 228:140–148
Groffen T, Eens M, Bervoets L (2019) Do concentrations of perfluoroalkylated acids (PFAAs) in isopods reflect concentrations in soil and songbirds? A study using a distance gradient from a fluorochemical plant. Sci Total Environ 657:111–123
Groffen T, Lasters R, Lemière F, Willems T, Eens M, Bervoets L, Prinsen E (2019) Development and validation of an extraction method for the analysis of perfluoroalkyl substances (PFASs) in environmental and biotic matrices. J Chromatogr B 1116:30–37
Guhl B, Stürenberg F, Santora G (2014) Contamination levels in the European eel (Anguilla anguilla) in North Rhine-Westphalian rivers. Environ Sci Eur 26:1–16
Guo X, Feng C (2018) Biological toxicity response of Asian Clam (Corbicula fluminea) to pollutants in surface water and sediment. Sci Total Environ 631–632:56–70
Hoff PT, Van Campenhout K, Van de Vijver K, Covaci A, Bervoets L, Moens L, Huyskens G, Goemans G, Belpaire C, Blust R, De Coen W (2005) Perfluorooctane sulfonic acid and organohalogen pollutants in liver of three freshwater fish species in Flanders (Belgium): relationships with biochemical and organismal effects. Environ Pollut 137:324–333
Hoff PT, Van de Vijver K, Van Dongen W, Esmans EL, Blust R, De Coen WM (2009) Perfluorooctane sulfonic acid in bib (Trisopterus luscus) and plaice (Pleuronectes platessa) from the Western Scheldt and the Belgian North Sea: Distribution and biochemical effects. Environ Toxicol Chem 22:608–614
Hölzer J, Göen T, Just P, Reupert R, Rauchfuss K, Kraft M, Müller J, Wilhelm M (2011) Perfluorinated compounds in fish and blood of anglers at Lake Möhne, Sauerland Area, Germany. Environ Sci Technol 45:8046–8052
Hong S, Khim JS, Wang T, Naile JE, Park J, Kwon B-O, Song SJ, Ryu J, Codling G, Jones PD, Lu Y, Giesy JP (2015) Bioaccumulation characteristics of perfluoroalkyl acids (PFAAs) in coastal organisms from the west coast of South Korea. Chemosphere 129:157–163
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40:3463–3473
Houde M, Bujas TAD, Small J, Wells RS, Fair PA, Bossart GD, Solomon KR, Muir DCG (2006) Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ Sci Technol 40:4138–4144
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Junttila V, Vähä E, Perkola N, Räike A, Siimes K, Mehtonen J, Kankaanpää H, Mannio J (2019) PFASs in Finnish rivers and fish and the loading of PFASs to the Baltic Sea. Water 11:870
Kwadijk CJAF, Korytár P, Koelmans AA (2010) Distribution of perfluorinated compounds in aquatic systems in the Netherlands. Environ Sci Technol 44:3746–3751
Labadie P, Chevreuil M (2011) Partitioning behavior of perfluorinated alkyl contaminants between water, sediment and fish in the Orge River (nearby Paris, France). Environ Pollut 159:391–397
Land M, de Wit CA, Bignert A, Cousins IT, Herzke D, Johansson JH, Martin JW (2018) What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ Evid 7:4
Lescord GL, Kidd KA, De Silva AO, Williamson M, Spencer C, Wang X, Muir DCG (2015) Perfluorinated and polyfluorinated compounds in lake food webs from the Canadian high arctic. Environ Sci Technol 49:2694–2702
Loi EIH, Yeung LWY, Taniyasu S, Lam PKS, Kannan K, Yamashita N (2011) Trophic magnification of poly- and perfluorinated compounds in a subtropical food web. Environ Sci Technol 45:5506–5513
Maes J, Belpaire C, Goemans G (2008) Spatial variations and temporal trends between 1994 and 2005 in polychlorinated biphenyls, organochlorine pesticides and heavy metals in European eel (Anguilla anguilla L.) in Flanders, Belgium. Environ Pollut 153:223–237
Mazzoni M, Buffo A, Cappelli F, Pascariello S, Polesello S, Valsecchi S, Volta P, Bettinetti R (2019) Perfluoroalakyl acids in fish of Italian deep lakes: Environmental and human risk assessment. Sci Total Environ 653:351–358
Mazzoni M, Ferrario C, Bettinetti R, Piscia R, Cicala D, Volta P, Borgå K, Valsecchi S, Polesello S (2020) Trophic magnification of legacy (PCB, DDT and Hg) and emerging pollutants (PFAS) in the fish community of a small protected Southern Alpine Lake (Lake Mergozzo, Northern Italy). Water 12:1591
Munoz G, Budzinski H, Babut M, Drouinea H, Lauzent M, Le Menach K, Lobry J, Selleslagh J, Simonnet-Laprade C, Labadie P (2017) Evidence for the trophic transfer of perfluoroalkylated substances in a temperate macrotidal estuary. Environ Sci Technol 51:8450–8459
Ng CA, Hungerbühler K (2013) Bioconcentration of perfluorinated alkyl acids: how important is specific binding? Environ Sci Technol 47:7214–7223
Park K-I, Donaghy L, Kang H-S, Hong H-K, Kim Y-O, Choi K-S (2012) Assessment of immune parameters of Manila clam Ruditapes phillipinarum in different physiological conditions using flow cytometry. Ocean Sci J 47:19–26
Powley CR, George SW, Ryan TW, Buck RC (2005) Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrices. Anal Chem 77:6353–6358
Prevedouros KS, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40:32–44
Rask M (1986) The diet and diel feeding activity of perch, Perca fluviatilis L., in a small lake in southern Finland. Ann Zool Fenn 23:49–56
Rüdel H, Kosfeld V, Fliedner A, Radermacher G, Schlechtriem C, Duffek A, Rauert C, Koschorrek J (2020) Selection and application of trophic magnification factors for priority substances to normalize freshwater fish monitoring data under the European Water Framework Directive: a case study. Environ Sci Eur 32:138
Rüdel H, Radermacher G, Fliedner A, Lohmann N, Duffek A (2020) A field study in support of the monitoring of priority substances in German freshwater fish: derivation of fillet-to-whole fish conversion factors. Environ Sci Eur 32:13
Smolders R, Bervoets L, Blust R (2012) Transplanted zebra mussels (Dreissena polymorpha) as active biomonitors in an effluent-dominated river. Environ Toxicol Chem 21:1889–1896
Stockholm Convention (2008) www.pops.int, as document decision SC-4/17
Stockholm Convention (2019) www.pops.int, as document decision SC-9/12
Teunen L, Belpaire C, Dardenne F, Blust R, Covaci A and Bervoets L (2020) Veldstudies naar monitoring van biota in het kader van de rapportage van de chemische toestand voor de Kaderrichtlijn Water 2015–2018 (algemene trends en relaties). Universiteit Antwerpen (UA) in samenwerking met het Instituut voor Natuur- en Bosonderzoek (INBO), in opdracht van de Vlaamse Milieumaatschappij (VMM). Antwerpen, België, 99 blz
US Environmental Protection Agency (USEPA) (2000) EPA and 3M announce phase-out of PFOS. https://archive.epa.gov/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4
Valsecchi S, Babut M, Mazzoni M, Pascariello S, Ferrario C, De Felice B, Bettinetti R, Veyrand B, Marchand P, Polesello S (2020) Per- and polyfluoroalkyl substances (PFAS) in fish from European Lakes: current contamination status, sources, and perspectives for monitoring. Environ Toxicol Chem 40:658–676
Van Leeuwen S, Strub M, Cofino W, Lindström G, van Bavel B (2011) Third interlaboratory study on perfluorinated compounds in environmental and human matrices. IVM Institute for Environmental Studies
Verhaert V, Covaci A, Bouillon S, Abrantes K, Musibono D, Bervoets L, Verheyen E, Blust R (2013) Baseline levels and trophic transfer of persistent organic pollutants in sediments and biota from the Congo River Basin (DR Congo). Environ Int 59:290–302
Westrelin S, Roy R, Tissot-Rey L, Bergès L, Argillier C (2018) Habitat use and preference of adult perch (Perca fluviatilis L.) in a deep reservoir: variation with seasons, water levels and individuals. Hydrobiologia 809:121–139
Wong WH, Gerstenberger S, Baldwin W, Moore B (2012) Settlement and growth of quagga mussels (Dreissena rostriformis bugensis Andrusov, 1897) in Lake Mead, Nevada-Arizona, USA. Aguatic Invasions 7:7–19
Yalçin-Özdilek Ş, Solak K (2007) The feeding of European eel, Anguilla anguilla L. in the river Asi, Turkey. Electr J Ichthyol 1:26–34
Zabaleta I, Bizkarguenaga E, Prieto A, Ortiz-Zarragoitia M, Fernández LA, Zuloaga O (2015) Simultaneous determination of perfluorinated compounds and their potential precursors in mussel tissue and fish muscle tissue and liver samples by liquid chromatography–electrospray-tandem mass spectrometry. J Chromatogr A 1387:13–23
Zafeiraki E, Gebbink WA, Hoogenboom RLAP, Kotterman M, Kwadijk C, Dassenakis E, Van Leeuwen SPJ (2019) Occurrence of perfluoroalkyl substances (PFASs) in a large number of wild and farmed aquatic animals collected in the Netherlands. Chemosphere 232:415–423
Zeng LX, Wu HH, Chen GZ (2006) The bioaccumulation and elimination under low concentration of cadmium from Asian clam (Corbicula fluminea) in the presence of humic acids. Ecol Sci 25:240–242
Zeng Z, Song B, Xiao R, Zeng G, Gong J, Chen M, Xu P, Zhang P, Shen M, Yi H (2019) Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ Int 126:598–610
Fliedner A, Rüdel H, Lohmann N, Buchmeier G, Koschorreck J (2018) Biota monitoring under the Water Framework Directive: On tissue choice and fish species selection. Environ Pollut 235:129–140
Acknowledgements
We thank Tim Willems (University of Antwerp) for the UPLC analysis and Prof. Dr. Steven Bouillon (KULeuven) for the stable isotope analysis. The technical crew of INBO Linkebeek is acknowledged for their help in fish sampling.
Funding
This study was partly funded by the Flanders Environment Agency and the Research Foundation Flanders (FWO, grants G018119N and 12ZZQ21N). TG is a postdoctoral researcher of the Research Foundation of Flanders (FWO).
Author information
Authors and Affiliations
Contributions
LT performed the sample collection and preparation, analysis of fish samples and contributed to the original draft. CB, LB and MDJ contributed to the setup and design of the field study and the review of the original draft. TG analysed mussel samples, performed the statistical analyses and contributed to the original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
At the moment, procedures for ethical approval at INBO are being set up, but are not yet available. INBO collaborators (C. Belpaire and technical crew) have attestations of being experienced with handling animals for experiments (Federale Overheidsdienst Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu- ref.213913/13_23/2013). All procedures were followed in order to minimize suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
PFAS profiles in quagga mussel (Dreissena bugensis; N = 143), Asiatic clam (Corbicula fluminea; N = 30) and blue mussel (Mytilus edulis; N = 5). PFHpA, PFHxS and PFDS were excluded as their concentrations were < LOQ in all the samples. Figure S2. Standard Ellipse Areas (SEA, %o2) of the Bayesian ellipses for each species; perch (Perca fluviatilis, N = 24), eel (Anguilla anguilla, N = 31), quagga mussel (Dreissena bugensis, N = 30) and Asian clam (Corbicula fluminea, N = 5). The red crosses represent the corrected median SEA (SEAc). Table S1. Condition Index, stable isotope data (δ13C and δ15 N), and PFAS concentrations (mean and range (between brackets); ng/g ww) of the mussels (quagga mussel (Dreissena bugensis), Asian clam (Corbicula fluminea) and blue mussel (Mytilus edulis) at each location. Table S2. MRM transitions (precursor and product ions), internal standards (ISTDs), cone voltages (V) and collision energy (eV) for the target perfluoroalkyl substances and their internal standards. Table S3. Mean and range trophic levels (TLs) of the organisms. Table S4. PFAS concentrations (ng/g ww) and stable isotope data (δ13C and δ15N) in fish muscle tissue (eel (Anguilla anguilla) and perch (Perca fluviatilis)) at each location. Table S5. Overlap in the corrected Standard Ellipse Area (SEAc; %o2) of the ellipses of quagga mussel (Dreissena bugensis, N = 30), Asian clam (Corbicula fluminea, N = 5), perch (Perca fluviatilis, N = 43) and eel (Anguilla anguilla, N = 56). Table S6. Slopes and trophic magnification factors (TMFs) for the target analytes that were significantly related with trophic level (TLs) of the organisms. Table S7. Summary of results of PFCs in the environmental study samples (fish, results in ng/g ww) of the interlaboratory study of the reference material [72] and reference concentrations (average values) measured in the present study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teunen, L., Bervoets, L., Belpaire, C. et al. PFAS accumulation in indigenous and translocated aquatic organisms from Belgium, with translation to human and ecological health risk. Environ Sci Eur 33, 39 (2021). https://doi.org/10.1186/s12302-021-00477-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-021-00477-z