Abstract
Background
Large amounts of insecticide-containing dusts produced from abrasion of the seed dressing can be released into the atmosphere during sowing operations. Neonicotinoid pesticides, introduced in the 1990s for several crops, are the leading products for seed-coating treatments in many countries. Neonicotinoid containing dusts can be effectively intercepted by bees in flight over the sowing field, inducing lethal acute effects, so that restrictions in the use of the main neonicotinoids have been adopted in the European Union. This led to the consequent introduction of replacement insecticides for seed-coating, i.e. methiocarb and thiacloprid, despite the lack of information on both the toxicity and the exposure scenarios for honeybees.
Results
In this study, a laboratory apparatus was developed in order to quantify the toxicity of the dusts produced from the abrasion of the seed coating. This quantification is based on (i) an airstream transporting coating particles into an exposure chamber; (ii) exposure of bees to reproducible and measurable concentrations of insecticide, and (iii) direct measurement of the exposure dose on single bees. The method allowed us to perform in vivo experiments of honeybee exposure to provide toxicity data in more realistic exposure conditions. In fact, the formulation rather than the active principle alone can be tested, and the exposure is through dusts rather than a solution so that specific absorption behavior can be studied in representative environmental conditions. The method was used to quantify the acute toxicity (LD50) of dusts obtained from the abrasion of corn seeds coated with clothianidin, thiacloprid and methiocarb.
Conclusions
Our results show that, surprisingly, the replacement insecticide methiocarb has a toxicity (LD50 = 421–693 ng/bee) in the same order of magnitude as clothianidin (LD50 = 113–451 ng/bee) through this specific exposure route, while thiacloprid (LD50 = 16.9·103 ng/bee) has a significantly lower acute toxicity. Moreover, dusts containing methiocarb and clothianidin show a significant increase in toxicity when, after exposure, bees are kept under high humidity conditions. This suggests that the method here presented can be used to obtain complementary toxicity data in the risk assessment procedure for the authorization of new seed-coating insecticides or new formulations.
Similar content being viewed by others
Background
Since their introduction in the 1990s, neonicotinoid insecticides have become one of the most important pesticide classes partly replacing carbamates, organophosphates, and pyrethroids [1]. They are systemic insecticides, because they are able to penetrate through plant tissues, e.g. from leaves or roots, and to spread through the vascular system of the plant. They can be applied to crops in different ways: spray, granular and/or seed coating. The latter is a well-established technique to apply pesticides directly to the seed, before sowing, in order to protect the plant in the early stage of growing. Seed-coating with insecticides grew dramatically between 1990 and 2008, reaching 1 billion dollars in 2008 with neonicotinoids accounting for 93% of the share [2]. Nowadays, the global pesticide-coating market continues to grow and it has approached 1.8 billion dollars in 2019 [3].
The large worldwide use of neonicotinoids is causing a widespread environmental contamination [4,5,6], with dangerous effects on different invertebrate species and the consequent reduction of biodiversity [7,8,9,10]. Particular attention has been posed to honeybees, because they are a highly valued resource used for bee products production and crop pollination [11]. Furthermore, their population is decreasing in many countries of Europe and North America [12,13,14]. Managed honeybee colonies are influenced by many factors: habitat loss, diseases, parasites and pesticides [15,16,17]. Regarding the effect of pesticides on non-target invertebrates, there are several studies investigating and shedding light on sublethal toxic effects of neonicotinoids [18,19,20,21,22,23,24,25,26,27]. Nevertheless, the extent and importance of sublethal effects are still under debate in the scientific community because they are more difficult to quantify in the field due to the presence of co-factors and co-stressors. For example, in a monitoring study in Northern Germany no effects were observed on honeybee colonies exposed to flowering oilseed rape grown from seeds coated with clothianidin + β-cyfluthrin [28, 29]. It is worth noticing that in the case of oilseed rape, sowing occurs in the autumn and flowering only months later in the spring. This means that, by the time of flowering, residues level of seed-coating insecticides in nectar and pollen are low (ppb level) and in fact this large field study did not detect the seed-coating insecticides in nectar and pollen collected by the honeybees [28, 29].
Restrictions on the use of neonicotinoids and fipronil were introduced in several European countries (France, Germany, Swiss, Slovenia and Italy) since 2008, in order to protect bees and other non-target insects. From December 2013, the European Union (EU) banned clothianidin, imidacloprid, thiamethoxam and fipronil as seed-coating product for cereal crops (with the exception for winter ones) and their spray application in bee attractive crops [30, 31]. An extension of this ban was approved by the EU Commission in 2018 [32,33,34].
Despite enforced restrictions [35], seed coating is still used worldwide for the cultivation of many different crops like corn, soybeans, wheat and cotton, also by using supposedly less toxic replacement insecticides like thiacloprid (neonicotinoid) and methiocarb (carbamate). About the active ingredient applied to the seed, only approximately 5% is taken up by the crop while most of it remains in the soil and leaches to ground/surface waters [36,37,38,39,40,41,42,43]. Furthermore, the drilling machine used for sowing releases into the atmosphere particulate matter produced from abrasion of seed coatings, that contains 1–2% of the total amount of pesticide applied in the field [44]. This dust falls on the surrounding vegetation causing the contamination of wild plants growing at the field margins [45, 46], but they can contaminate also larger areas [47, 48]. Moreover, honeybees approaching the drilling machine during sowing collect particulate matter containing high doses of pesticides and lethal acute effects were observed in the exposed honeybees [44, 49,50,51,52]. In Europe, Canada and the US several cases of colony loss related to corn sowing were observed, where beekeepers have reported huge and rapid mortality in their hives due to emission of dust containing insecticides (see Krupke et al. [51], Pistorius et al. [53], the following reviews [54, 55] and references therein). More recently, after the neonicotinoid bans and the introduction of new coatings and sowing technologies aimed to reduce seed abrasion, massive spring colony losses were observed in northeastern Italy. Again, it has been hypothesized that exposure of honeybees to insecticide-containing dusts released during corn sowing may be the cause of mortality [56].
In the literature, there are many studies about the acute toxicity of neonicotinoids to honeybees [57]. Contact lethal dose (LD50) is usually established with topical applications of alcoholic solution containing the pesticides to the dorsal thorax [58] or spraying pesticide on leaves later used for bee exposure [59]. Recently, a method to quantify the toxicity of dusts from coated seeds was developed, but the particulate matter was applied to plants and not directly to honeybees [60]. Furthermore, this method is based on field trials to assess the dust toxicity, which carry limitations concerning the achievement of controlled and reproducible exposure conditions. To the best of our knowledge, there are no laboratory methodologies to apply particulate matter obtained from abrasion of seeds directly to honeybees under “reproducible exposure conditions”. In order to fill this gap, in this study, an easy method to perform tests of particulate matter toxicity to honeybees has been developed. Our purpose was to simulate in controlled conditions in the laboratory the exposure of honeybees to dusts produced from seed-coating fragmentation, in order to reproduce what would happen in the field to flying bees approaching the cloud of particles produced by the drilling machine. Noteworthy, the method includes also the determination of the exposure dose (ng of insecticide/bee) by chemical analysis of single bees.
After method development, the toxicity of dusts containing the insecticides clothianidin, thiacloprid and methiocarb was evaluated. Clothianidin has been chosen as a reference because it is the most used neonicotinoid pesticide [2], whereas thiacloprid (neonicotinoid) and methiocarb (carbamate) were introduced in Europe as replacement insecticides for seed coating after the neonicotinoids ban and they are considered as bee friendly.
Experimental section
A new method was developed to assess the toxicity of insecticide-containing dusts toward honeybees by simulating in the laboratory the exposure of honeybees flying through the cloud of particles emitted by the drilling machine in the field. The method is based on an apparatus to produce dusts from abrasion of seeds coated with insecticides, the exposure of honeybees to insecticides containing dusts in an exposure chamber, and the direct measurement of exposure dose on single bees.
Seeds, insecticides and bees
Corn seeds coated with different pesticides were used: Poncho® (Bayer Cropscience, clothianidin 1.25 mg/seed) supplied in 2009 and 2010 by A.I.S. (Italian seed association) courtesy of MiPAAF (Ministry of Agriculture, Food and Forestry) for the research project APENET; Sonido® (Bayer Cropscience, thiacloprid 1.0 mg/seed) and Mesurol® (Bayer Cropscience, methiocarb, 1.25 mg/seed) purchased in 2014. All seeds were coated also with the fungicide Celest® XL (Syngenta, Fludioxonil and Metalaxyl-M). Apis mellifera L. (hybrid ligustica and carnica) for the toxicity tests were caught every day. Coming from an urban hive, they were attracted with a water/sugar mixture, gently sucked into a tube, and kept in small cages.
Particulate matter production and characterization
The air stream containing seed-coating particles was generated by surface abrasion of 10–60 seeds introduced in a 100-mL flask connected to a rotary evaporator (Fig. 1) working at 300 rpm. A glass tube was introduced into the flask and connected with: (i) the external exposure chamber; (ii) a Ø 37-mm filter holder for the sampling of total suspended particles (TSPs) on glass fiber filters (Omega Specialty Instrument Co.); (iii) a pump (Zambelli ZB1 timer, Milan, Italy; aspiration airflow 10–20 L min−1). The end of the glass tube was placed in the middle of the flask, closed by metallic wire mesh (100 µm) to avoid the introduction of large particles in the airflow line.
The exposure chamber was a plastic tube (Ø 15 mm, 20 cm length) that can host up to 10 bees. Its ends are protected by a plastic gauze to avoid the escape of bees along the airflow. A similar tube may be connected after the exposure chamber to host the sampling line of an optical particle counter (OPC).
In order to optimize the concentration of the insecticides in the airflow for the toxicity tests, many trials using different numbers of seeds, mixing times and flow rates were performed. In these preliminary tests, TSP filters were sampled at regular interval of 5 or 10 min during the seed mixing in order to determine the insecticide concentration in the airflow and its variability.
The size distribution of the particulate matter in the exposure chamber was measured with an OPC (Grimm model 1.108) in the 0.23–32 μm diameter range. Furthermore, TSPs were sampled on polycarbonate filters (Millipore, Ø 37 mm) to evaluate the size distribution using low-vacuum scanning electron microscopy–energy-dispersive spectrometry (SEM–EDS; FEI instrumentation, model Quanta 200).
The abrasion potential (Table 1) was assessed by mixing 12 seeds for 2 h with an airflow of 20 L min−1. TSP sampled at the filter were weighed and then analyzed by ultrahigh performance liquid chromatography with a diode array detection (UHPLC-DAD) to quantify both the total particulate matter and the insecticide concentration. Seeds and filters were kept for 2 days at 20 °C and 50% relative humidity before and after the experiments, as suggested in the Heubach methodology to quantify dust emissions [61].
Toxicity tests
The number of seeds and the mixing time before honeybee exposure were optimized to reach the desired pesticide concentration in the airflow for the toxicity tests (Table 1). Different formulations may have different abrasion potential and therefore may require specific optimization before honeybee exposure tests. During the toxicity tests, eight insects were inserted in the exposure chamber and exposed to insecticide-bearing dusts for 1 or 2 min in an airflow of 10 L min−1. Before and after the honeybee exposure tests, at least one particulate matter sample was collected to quantify the pesticide concentration in the airflow during the exposure tests. After exposure, two honeybees were put at − 20 °C for 1 h and then analyzed to quantify the insecticide dose. The other six honeybees were put in a clean cage to perform the mortality test, using the same procedure described in Girolami et al. [50]. Briefly, honeybees were fed with honey to exclude death from starvation and they were kept at two different humidity conditions: room humidity (40–60% RH) and high humidity (> 90% RH). After 24 h, dead honeybees were counted in every cage. A cage with six non-exposed honeybees was used as control in each experiment, and no mortality was observed. Additional control experiments using non-coated seeds were performed and no mortality was observed. Regarding a possible oral uptake of the insecticide by these caged honeybees, it should be noted that bees are not attracted by seed-coating material and licking activity was never observed.
Statistical analysis
LD50 values were estimated by plotting log dose versus probit [62, 63]. The XLSTAT software was used for the LD50 calculation and the likelihood ratio test was used to evaluate the goodness of the probit model used for data fitting.
Insecticide chemical analysis on dusts and on single bees
For the quantification of the insecticide dose two bees were analyzed for each exposure test. A single bee was introduced in a 10-mL test tube, extracted with 1 mL of methanol in ultrasonic bath for 15 min at room temperature. This treatment was repeated after addition of 1 mL of water. The final solution was analyzed by UHPLC-DAD, after filtration with 0.2-μm syringe filters (Phenomenex, RC). For determination of the insecticide concentration in the airflow, half filter was extracted and analyzed with the same procedure used for bees. A UHPLC-DAD analytical method was optimized for the determination of each single seed-coating insecticide. The method used a Shimadzu Prominence UFLC-XR chromatograph equipped with a Shimadzu SIL 20AC-XR auto sampler, Shimadzu SPDM20A UV–vis diode array detector (DAD), and a Shimadzu XR-ODS II (2.2 μm, 2 mm × 100 mm) analytical column with a Phenomenex (ODS 4 mm × 2 mm) guard column. The following instrumental parameters were used: eluent flow rate of 0.3 mL min−1, with a water–acetonitrile gradient elution, 5 μL of injection volume, 35 °C column temperature. For clothianidin gradient elution was: 0–1.5 min, 27% acetonitrile; 1.5–2.7 min, linear gradient to 100% acetonitrile; 2.7–5.0 min, 100% acetonitrile. For thiacloprid gradient elution was: 0–2.0 min, 32.5% acetonitrile; 2.0–2.5 min linear gradient to 100% acetonitrile; 2.5–4.5 min, 100% acetonitrile. For methiocarb gradient elution was: 0–3.0 min, linear gradient from 10% to 20% acetonitrile; 3.0–3.5 min, linear gradient to 50% acetonitrile; 3.5–5.0 min, linear gradient to 100% acetonitrile; 5.5–8.5 min, 100% acetonitrile. Detector signals at λ = 269 nm for clothianidin, at λ = 244 nm for thiacloprid and at λ = 202 nm for methiocarb were used for analytes quantification. Instrumental calibration (external) was performed by analysis of 0.05–10 mg L−1 standard solutions of each analyte in 50% water–methanol.
Chemicals for the preparation of the standard solutions of clothianidin, thiacloprid and methiocarb were purchased from Fluka (Pestanal, purity > 99.7%). Methanol (VWR) and acetonitrile (Riedel-de-Haen) were of HPLC grade. Water was purified using a Millipore Milli-Q equipment.
Result and discussion
Particulate matter size distribution
The size distribution of the dusts produced in the experimental apparatus was mainly characterized by coarse particles with a mode centered around 5–7.5 µm in diameter (Fig. 2). Besides large particles, there was also a significant production of fine particles with diameter of 1–2 µm. The observed size distribution is comparable with the one previously measured in field experiments of sowing of corn seeds from the same batch [44]. Therefore, this setup can be used to obtain in laboratory a particulate matter comparable to that released by the drilling machine.
Production of particulate matter from coated seeds
As expected, a different behavior in the particulate matter production was observed for seeds coated with different insecticides. In particular, corn seeds coated with clothianidin produced more dusts compared with seeds coated with methiocarb and thiacloprid (Table 1). This is related to the different coating technologies of seeds that have changed during the past years. In fact, new coatings have a better resistance to abrasion and this improvement has an important ecological impact [61, 64, 65]. Anyway, the assessment of seed-coating abrasion potential is not the goal of this study, but it had important implication in the optimization of the experimental settings to reach the desired concentrations when seeds coated with different pesticides were used.
For all the tested seeds, the insecticides concentration in the airflow reached a stable value, after an initial rising period. Therefore, it was possible to modulate the concentration in the airflow of a particular insecticide by changing the number of mixed seeds. However, due to the different toxicities of each insecticide the best number of seeds had to be optimized for each of them (Table 1). Different number of seeds required also different mixing time to reach a stable concentration. By changing the number of seeds and mixing time it is possible to obtain a wide range of insecticide concentration in the airflow. Toxicity tests started only when an appropriate and stable concentration of dusts was reached. The concentration and exposure time of the honeybees could be used to modify the insecticide doses, because a clear correlation between the exposure to dusts and their collection by honeybees was observed for each insecticide tested (Fig. 3). Applied doses ranged between few ng to hundreds of µg of insecticide in the form of suspended particulate matter. This wide range allowed us to quantify the effect of pesticides with different toxicity (e.g. clothianidin and thiacloprid). The reproducibility of the method was good enough to modulate the doses applied to honeybees by acting on the experimental parameters. However, chemical analysis remains necessary for the accurate quantification of the applied dose (Additional file 1: Table S1–S3).
Amount of insecticide (i.e. the exposure dose) measured on honeybees exposed to different streams of seed-coating dusts, characterized by different concentration of particles. The emitted mass represents the total amount of insecticide produced in each exposure experiment and collected on the filter placed at the end of the exposure chamber. It was calculated as insecticide concentration (µg/m3) · airflow (m3/min) · exposure time of the honeybees (min). Significant linear relationships (dose vs emitted amount) were obtained for all insecticides (F test, p < 0.0001)
It is appropriate to underline that the large exposure doses used in the tests with thiacloprid induce the concomitant exposure to very high doses of all other seed-coating components, including the fungicide. In view of the negligible acute toxicity of these components (with respect to the active principle) and the no effect observed up to 11 µg/bee of thiacloprid administered (see next section), these exposure condition represent an “experimental control” for the exposure tests with clothianidin and methiocarb in which the observed lethal effect must be associated to the dose of insecticide with a non-significant contribution of the fungicide or other components of the seed coating.
Particulate matter toxicity
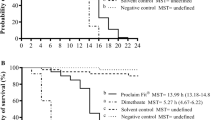
The developed method has been applied to the quantification of the contact toxicity to honeybees of dusts produced from corn seeds coated with insecticides (Table 2). Our results show that, surprisingly, the replacement insecticide methiocarb has a toxicity (LD50 = 421–693 ng/bee) in the same order of magnitude as clothianidin (LD50 = 113–451 ng/bee) through this specific exposure route, while thiacloprid (LD50 = 16.9·103 ng/bee) has a significantly lower acute toxicity. Furthermore, for clothianidin and methiocarb an effect of the air relative humidity to dust toxicity was observed. Honeybees kept at high relative humidity (> 90%) after exposure to particulate matter showed toxic effects at lower doses, compared with honeybees kept at room air humidity (40–60%). This is in agreement with previous results obtained during field experiments [50]. This effect was not observed with thiacloprid, therefore, for this particular insecticide, data collected at high and room humidity were in agreement. Moreover, dusts containing thiacloprid resulted to be only slightly toxic to honeybees and high doses were necessary to observe acute toxic effects. Because of this lower toxicity, data collected with thiacloprid were less precise and it may be possible that humidity effect cannot be correctly observed. Furthermore, when such high amounts of thiacloprid were applied to honeybees, their ability to clean themselves from the dusts was clearly observed, but deeper studies are needed to quantify the phenomenon. In addition, further experiments are necessary to understand why air relative humidity increases dust toxicity. Our hypothesis is that high air relative humidity negatively affects the honeybee cleaning ability from dusts or increases the absorption of the active principle through the cuticle. Thanks to the method presented here, it will be possible to better investigate this phenomenon.
As for the possible oral uptake of the insecticide by honeybees during the 24 h of the toxicity test (from particles on the cage mate or on the cage walls), we want to emphasize that bees are not attracted by seed-coating material and licking activity was never observed. Furthermore, the much higher oral toxicity (with respect the contact one) manifested by these insecticides represents a good indication of the scarce significance of the oral uptake.
Concerning the toxicity of different active principles, our results show that powders produced from seeds coated with clothianidin are highly toxic to honeybees, especially in the worst (high relative humidity) conditions. Instead, dusts containing thiacloprid are much less toxic and unrealistically high doses were required to observe acute toxic effects. This result is in agreement with the contact toxicity observed with topical application of neonicotinoid alcohol solutions [58] and they confirm that the toxicity of the insecticide thiacloprid to honeybees is relatively low in comparison with the other two active ingredients. It is worth noticing that LD50 values here reported are different (e.g. significantly higher for clothianidin) from those obtained by topical application of alcoholic solutions [58, 66] due to the different exposure route and absorption efficiency.
Surprisingly, the toxicity of dusts containing methiocarb is only slightly lower of that containing clothianidin, but significantly higher than that of dusts containing thiacloprid. It is worth noticing that, during field experiments, honeybees flying close to the drilling machine collected approximately 500 ng/bee of insecticide [50]. Our results show that these doses of clothianidin and methiocarb can have severe toxic effects and therefore they support the hypothesis that massive colony losses recently observed in northeastern Italy may be associated to exposures of honeybees to methiocarb-containing dusts released during corn sowing.
This method could be used to quantify powder toxicity also for other pollinators like bumblebees and solitary bees. This is an important factor because new data have pointed out that pesticide effects could be very different for different pollinators. As an example, the study from Rudlöf et al. [67] showed no effects on honeybees of seed-coating with the neonicotinoid clothianidin and the non-systemic pyrethroid β-cyfluthrin applied to oilseed rape seeds, but, conversely, they observed reduced wild bee density, solitary bee nesting, and bumblebee colony growth. Furthermore, this method is comparable with realistic field conditions and allows quantification of the toxicity of each pesticide used for seed coating without the concern of expensive field experiments.
Conclusions
A new method was presented here for the quantification of the toxicity to honeybees of particulate matter produced from abrasion of corn seeds coated with insecticides. A wide range of doses could be applied, therefore is possible to evaluate the toxicity of pesticides with different characteristics. Mortality tests confirmed that clothianidin is highly toxic to honeybees, especially in the worst conditions (high humidity). The obtained LD50 explains how relatively low doses of the insecticide, easily reached during sowing in the field [50, 68], may cause high mortality in honeybees. Instead, particulate matter containing thiacloprid are less toxic and so they probably do not represent a threat for honeybees. Surprisingly, the toxicity of the replacement insecticide methiocarb as dusts is much more similar to that of clothianidin and it could be harmful for honeybees through exposure of particulate matter emitted by the drilling machine during normal sowing operations. This result suggests that recent colony losses observed in northeastern Italy may be caused by methiocarb-containing dusts emitted during corn sowing. In view of a better risk assessment procedure [35, 69] for the authorization of new seed-coating insecticides or new formulations, together with the quantification of the abrasion capability of the seeds complementary toxicity data can be obtained by the method here presented.
Availability of data and materials
All relevant scientific data are reported in the manuscript. Raw data regarding toxicity experiments are in Additional file 1. More details can be provided upon request.
Abbreviations
- LD50 :
-
Lethal dose, 50%
- TSP:
-
Total suspended particulate
- OPC:
-
Optical particle counter
- SEM–EDS:
-
Scanning electron microscopy with energy-dispersive spectrometry
- UHPLC-DAD:
-
Ultra-high performance liquid chromatography with a diode array detection
References
Jeschke P, Nauen R (2008) Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag Sci 64:1084–1098. https://doi.org/10.1002/ps.1631
Simon-Delso N, Amaral-Rogers V, Belzunces LP et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Seed Coating Market by Additive (Polymers, Colorants, Pellets, Minerals/Pumice, Active Ingredients), Process (Film Coating, Encrusting, Pelleting), Active Ingredient (Protectants and Phytoactive Promoters), Crop Type, Region - Global Forecast to 2025, https://www.marketsandmarkets.com/Market-Reports/seed-coating-materials-market-149045530.html, Accessed on 17 Apr 2020
Bonmatin J-M, Giorio C, Girolami V et al (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67. https://doi.org/10.1007/s11356-014-3332-7
Giorio C, Safer A, Sánchez-Bayo F et al (2017) An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: new molecules, metabolism, fate, and transport. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0394-3
Becker JM, Ganatra AA, Kandie F et al (2020) Pesticide pollution in freshwater paves the way for schistosomiasis transmission. Sci Rep 10:3650. https://doi.org/10.1038/s41598-020-60654-7
van Lexmond MB, Bonmatin J-M, Goulson D, Noome DA (2015) Worldwide integrated assessment on systemic pesticides: global collapse of the entomofauna: exploring the role of systemic insecticides. Environ Sci Pollut Res 22:1–4. https://doi.org/10.1007/s11356-014-3220-1
Beketov MA, Kefford BJ, Schafer RB, Liess M (2013) Pesticides reduce regional biodiversity of stream invertebrates. Proc Natl Acad Sci 110:11039–11043. https://doi.org/10.1073/pnas.1305618110
Forfert N, Troxler A, Retschnig G et al (2017) Neonicotinoid pesticides can reduce honeybee colony genetic diversity. PLoS ONE 12:e0186109. https://doi.org/10.1371/journal.pone.0186109
Hallmann CA, Sorg M, Jongejans E et al (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Breeze TD, Bailey AP, Balcombe KG, Potts SG (2011) Pollination services in the UK: how important are honeybees? Agric Ecosyst Environ 142:137–143
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apic Res 49:1–6
2018/19 Average Annual Colony Loss. https://research.beeinformed.org/loss-map/. Accessed 17 Apr 2020
Jacques A, Laurent M, EPILOBEE Consortium et al (2017) A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 12:e0172591. https://doi.org/10.1371/journal.pone.0172591
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. https://doi.org/10.1126/science.1255957
Martinello M, Baratto C, Manzinello C et al (2017) Spring mortality in honey bees in northeastern Italy: detection of pesticides and viruses in dead honey bees and other matrices. J Apic Res 56:239–254. https://doi.org/10.1080/00218839.2017.1304878
vanEngelsdorp D, Hayes JJ, Underwood RM, Pettis JS (2010) survey of honey bee colony losses in the United States, fall 2008 to spring 2009. J Apic Res 49(1):7–14
Desneux N, Decourtye A, Delpuech J-M (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Feltham H, Park K, Goulson D (2014) Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23:317–323. https://doi.org/10.1007/s10646-014-1189-7
Henry M, Béguin M, Requier F et al (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350. https://doi.org/10.1126/science.1215039
Mommaerts V, Reynders S, Boulet J et al (2009) Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 19:207–215. https://doi.org/10.1007/s10646-009-0406-2
Pisa L, Goulson D, Yang E-C et al (2017) An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ Sci Pollut Res 9:1–49
Pisa LW, Amaral-Rogers V, Belzunces LP et al (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68–102
Rondeau G, Sánchez-Bayo F, Tennekes HA et al (2014) Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci Rep. https://doi.org/10.1038/srep05566
Whitehorn PR, O’Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352. https://doi.org/10.1126/science.1215025
Yang E-C, Chang H-C, Wu W-Y, Chen Y-W (2012) Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 7:e49472. https://doi.org/10.1371/journal.pone.0049472
Yang EC, Chuang YC, Chen YL, Chang LH (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101:1743–1748. https://doi.org/10.1603/0022-0493-101.6.1743
Rolke D, Persigehl M, Peters B et al (2016) Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in northern Germany: residues of clothianidin in pollen, nectar and honey. Ecotoxicology 25:1691–1701. https://doi.org/10.1007/s10646-016-1723-x
Rolke D, Fuchs S, Grünewald B et al (2016) Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: effects on honey bees (Apis mellifera). Ecotoxicology 25:1648–1665. https://doi.org/10.1007/s10646-016-1725-8
(2013) Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off J Eur Union L. 2013;139:12-26
Commission Implementing Regulation (EU) (2013) No 781/2013 of 14 August 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substance fipronil, and prohibiting the use and sale of seeds treated with plant protection products containing this active substance
Commission Implementing Regulation (EU) (2018) No 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid
Commission Implementing Regulation (EU) (2018) No 2018/784 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance clothianidin
Commission Implementing Regulation (EU) (2018) No 2018/785 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam
Sgolastra F, Medrzycki P, Bortolotti L et al (2020) Bees and pesticide regulation: lessons from the neonicotinoid experience. Biol Conserv 241:108356. https://doi.org/10.1016/j.biocon.2019.108356
Goulson D (2014) Ecology: pesticides linked to bird declines. Nat Adv Online Publ. https://doi.org/10.1038/nature13642
Hallmann CA, Foppen RPB, van Turnhout CAM et al (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:341–343. https://doi.org/10.1038/nature13531
Zhang P, Ren C, Sun H, Min L (2018) Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci Total Environ 615:59–69. https://doi.org/10.1016/j.scitotenv.2017.09.097
Bradford BZ, Huseth AS, Groves RL (2018) Widespread detections of neonicotinoid contaminants in central Wisconsin groundwater. PLoS ONE 13:e0201753. https://doi.org/10.1371/journal.pone.0201753
Schaafsma A, Limay-Rios V, Baute T et al (2015) Neonicotinoid Insecticide Residues in Surface Water and Soil Associated with Commercial Maize (Corn) Fields in Southwestern Ontario. PLoS ONE 10:e0118139. https://doi.org/10.1371/journal.pone.0118139
Schaafsma AW, Limay-Rios V, Baute TS, Smith JL (2019) Neonicotinoid insecticide residues in subsurface drainage and open ditch water around maize fields in southwestern Ontario. PLoS ONE 14:e0214787. https://doi.org/10.1371/journal.pone.0214787
Morrissey CA, Mineau P, Devries JH et al (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303. https://doi.org/10.1016/j.envint.2014.10.024
Douglas MR, Rohr JR, Tooker JF (2015) EDITOR’S CHOICE: neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. J Appl Ecol 52:250–260. https://doi.org/10.1111/1365-2664.12372
Tapparo A, Marton D, Giorio C et al (2012) Assessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds. Environ Sci Technol 46:2592–2599. https://doi.org/10.1021/es2035152
Greatti M, Barbattini R, Stravisi A et al (2006) Presence of the a.i. imidacloprid on vegetation near corn fields sown with Gaucho® dressed seeds. Bull Insectology 59:99–103
Greatti M, Sabatini AG, Barbattini R et al (2003) Risk of environmental contamination by the active ingredient imidacloprid used for corn seed dressing. Preliminary results. Bull Insectology 56:69–72
Forero LG, Limay-Rios V, Xue Y, Schaafsma A (2017) Concentration and movement of neonicotinoids as particulate matter downwind during agricultural practices using air samplers in southwestern Ontario, Canada. Chemosphere 188:130–138. https://doi.org/10.1016/j.chemosphere.2017.08.126
Krupke CH, Holland JD, Long EY, Eitzer BD (2017) Planting of neonicotinoid-treated maize poses risks for honey bees and other non-target organisms over a wide area without consistent crop yield benefit. J Appl Ecol. 54(5):1449–1458
Girolami V, Marzaro M, Vivan L et al (2013) Aerial powdering of bees inside mobile cages and the extent of neonicotinoid cloud surrounding corn drillers. J Appl Entomol 137:35–44. https://doi.org/10.1111/j.1439-0418.2012.01718.x
Girolami V, Marzaro M, Vivan L et al (2012) Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J Appl Entomol 136:17–26. https://doi.org/10.1111/j.1439-0418.2011.01648.x
Krupke CH, Hunt GJ, Eitzer BD et al (2012) Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 7:e29268. https://doi.org/10.1371/journal.pone.0029268
Pochi D, Biocca M, Fanigliulo R et al (2012) Potential Exposure of Bees, Apis mellifera L., to Particulate Matter and Pesticides Derived from Seed Dressing During Maize Sowing. Bull Environ Contam Toxicol 89:354–361. https://doi.org/10.1007/s00128-012-0664-1
Pistorius J, Bischoff G, Heimbach U, Stähler M (2010) Bee poisoning incidents in Germany in spring 2008 caused by abrasion of active substance from treated seeds during sowing of maize. Julius-Kühn-Arch 118
Cutler GC, Scott-Dupree CD, Drexler DM (2014) Honey bees, neonicotinoids and bee incident reports: the Canadian situation. Pest Manag Sci 70:779–783
Mutinelli F, Costa C, Lodesani M et al (2010) Honey bee colony losses in Italy. J Apic Res 49:119–120. https://doi.org/10.3896/IBRA.1.49.1.24
Sgolastra F, Porrini C, Maini S et al (2017) Healthy honey bees and sustainable maize production: why not? Bull Insectol 70:156–160
Decourtye A, Devillers J (2010) Ecotoxicity of Neonicotinoid Insecticides to Bees. In: Thany SH (ed) Insect nicotinic acetylcholine receptors. Springer, New York, pp 85–95
Iwasa T, Motoyama N, Ambrose JT, Roe RM (2004) Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot 23:371–378. https://doi.org/10.1016/j.cropro.2003.08.018
Laurino D, Porporato M, Patetta A, Manino A (2011) Toxicity of neonicotinoid insecticides to honey bees: laboratory tests. Bull Insectol 64:107–113
Pistorius J, Wehner A, Kriszan M et al (2015) Application of predefined doses of neonicotinoid containing dusts in field trials and acute effects on honey bees. Bull Insectol 68:161–172
Zwertvaegher IKA, Foqué D, Devarrewaere W et al (2016) Assessment of the abrasion potential of pesticide-treated seeds using the Heubach test. Int J Pest Manag. https://doi.org/10.1080/09670874.2016.1206993
Finney DG (1971) Probit Analysis, 3rd edn. Cambridge University Press, Cambridge
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. New York, W.H, Freeman and Co
Biocca M, Pochi D, Fanigliulo R et al (2017) Evaluating a filtering and recirculating system to reduce dust drift in simulated sowing of dressed seed and abraded dust particle characteristics. Pest Manag Sci 73:1134–1142. https://doi.org/10.1002/ps.4428
Foque D, Beck B, Devarrewaere W et al (2017) Comparing different techniques to assess the risk for dust drift from pesticide-coated seeds. Pest Manag Sci n/a-n/a. https://doi.org/10.1002/ps.4557
Sanchez-Bayo F, Goka K (2014) Pesticide residues and bees—a risk assessment. PLoS ONE 9:e94482. https://doi.org/10.1371/journal.pone.0094482
Rundlöf M, Andersson GKS, Bommarco R et al (2015) Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80. https://doi.org/10.1038/nature14420
Marzaro M, Vivan L, Targa A et al (2011) Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bull Insectol 64:119–126
Hitaj C, Smith DJ, Code A et al (2020) Sowing uncertainty: what we do and don’t know about the planting of pesticide-treated seed. BioScience 70:390–403. https://doi.org/10.1093/biosci/biaa019
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Authors contributed equally to the realization of present study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lentola, A., Giorio, C., Petrucco Toffolo, E. et al. A new method to assess the acute toxicity toward honeybees of the abrasion particles generated from seeds coated with insecticides. Environ Sci Eur 32, 93 (2020). https://doi.org/10.1186/s12302-020-00372-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00372-z