Abstract
Background
Pesticide residue contamination of surface water in agricultural areas can have adverse effects on the ecosystem. We have performed an integrated chemical and bioanalytical profiling of surface water samples from Swedish agricultural areas, aiming to assess toxic activity due to presence of pesticides. A total of 157 water samples were collected from six geographical sites with extensive agricultural activity. The samples were chemically analyzed for 129 commonly used pesticides and transformation products. Furthermore, the toxicity was investigated using in vitro bioassays in the water samples following liquid–liquid extraction. Endpoints included oxidative stress response (Nrf2 activity), estrogen receptor (ER) activity, and aryl hydrocarbon receptor (AhR) activity. The bioassays were performed with a final enrichment factor of 5 for the water samples. All bioassays were conducted at non-cytotoxic conditions.
Results
A total of 51 pesticides and transformation products were detected in the water samples. Most of the compounds were herbicides, followed by fungicides, insecticides and transformation products. The highest total pesticide concentration in an individual sample was 39 µg/L, and the highest median total concentration at a sample site was 1.1 µg/L. The largest number of pesticides was 31 in a single sample. We found that 3% of the water samples induced oxidative stress response, 23% of the samples activated the estrogen receptor, and 77% of the samples activated the aryl hydrocarbon receptor. Using Spearman correlation coefficients, a statistically significant correlation was observed between AhR and ER activities, and AhR activity was strongly correlated with oxidative stress in samples with a high AhR activity. Statistically significant relationships were observed between bioactivities and individual pesticides, although the relationships are probably not causal, due to the low concentrations of pesticides. Co-occurrence of non-identified chemical pollutants and naturally occurring toxic compounds may be responsible for the induced bioactivities.
Conclusions
This study demonstrated that integrated chemical analysis and bioanalysis can be performed in water samples following liquid/liquid extraction with a final enrichment factor of 5. AhR and ER activities were induced in water samples from agricultural areas. The activities were presumably not caused by the occurrence of pesticides, but induced by other anthropogenic and natural chemicals.
Similar content being viewed by others
Background
Surface water in agricultural areas can be contaminated by pesticide residues [1] with possible adverse effects on the ecosystem [2,3,4]. Environmental monitoring of pesticide residues in water is generally based on chemical analysis of pesticides and known metabolites or degradation products thereof. In Sweden, pesticide levels in surface waters from four agricultural areas have been monitored since 2002 within the Swedish monitoring program for pesticides in surface water [5]. The program is unique considering the long-term and comprehensive data set. However, targeted chemical analysis alone has limitations. It is not possible to detect unknown substances (e.g, unknown pesticide metabolites or degradation products), it does not assess effects of individual chemicals or mixture effects, and there is often not a clear link to adverse outcome. Therefore, it has repeatedly been suggested that an effect-based approach by a combination of chemical characterization and toxicological profiling would be a more suitable way of performing environmental monitoring for a better understanding of the environmental pollutants effects on the ecosystem [6,7,8,9,10,11,12,13]. Recently, Könemann et al. [14] identified effect-based screening methods as sensitive and valuable complements to chemical analysis for monitoring estrogens under the European Water Framework Directive.
The use of in vitro bioassays for environmental monitoring is a rapidly expanding field of research [15,16,17,18,19,20,21,22,23,24,25]. While the bioassays cannot discriminate between the toxic effects of two or more compounds that are present in the same sample, the great strength with bioassays is that they show the total toxicity exerted by a sample—regardless whether the toxicity is caused by a known anthropogenic compound, an unknown anthropogenic compound, a naturally occurring compound, or a combination of these [6, 26, 27]. The contribution of micropollutants to the bioactivity in surface water affected by wastewater has been evaluated in recent studies, showing that the major part of the bioactivities cannot be explained by target compounds [16, 22, 28,29,30,31,32]. Bioanalytical studies focusing on pesticides in water from agricultural areas are scarce. However, acute toxic effects in wastewater-impacted streams in Switzerland were found to be driven by pesticides with diazinon and clothianidine as main drivers [30]. Furthermore, positive responses in cellular bioassays have been demonstrated for a few pesticides [31].
Sample preparation and extraction are crucial steps in studies with integrated bioanalysis and chemical analysis. Recovery of both toxic activities and chemicals should be considered, as well as cost and time efficiency especially for large sample sizes. When using toxicity bioassays for environmental monitoring purposes, it is often necessary to concentrate the water samples before applying the sample to the in vitro assay—because the levels of environmental pollutants are low (in the nanogram or microgram per liter range) and the sample needs to be diluted approximately 100 times in cell-culture media in the assay. A final enrichment factor in the cell-culture media of 10–50 as compared to the original water sample is generally needed [15, 16]. Solid-phase extraction (SPE) has the ability to eliminate matrix effects and concentrate dissolved organic chemicals and is a technique often used for enrichment of water samples. However, the SPE methods are optimized to give maximum recovery for the chemical analysis, while the effects on the bioassays are less investigated [33,34,35]. In the present study, the chemical analyses of pesticides were performed by GC–MS following liquid–liquid extraction (additional extract available) and by LC–MS/MS following online SPE extraction (no additional extract available). The remaining extracts from liquid/liquid extraction were used for the bioanalysis and one aim of the study was to investigate the possibility to use these extracts for bioanalysis.
In this study, we have performed an integrated chemical and toxicological profiling of surface water samples to investigate the association between bioactivity and occurrence of pesticides in field water. The water samples were collected within the long-term Swedish environmental monitoring program from streams and rivers located in areas with extensive agricultural activity and analyzed for a broad range of commonly used pesticides [36]. The liquid/liquid extracts used for the GC–MS analysis were also used for toxicological profiling, including measurements of oxidative stress response, estrogen receptor (ER) activity, and aryl hydrocarbon receptor (AhR) activity. The associations between the toxicity parameters and between toxic activity and pesticide concentrations were evaluated by calculation of Spearman correlation coefficients. In this investigation, we show a methodology to integrate chemical and toxicological profiling of water samples, to investigate the association between toxic activity and the occurrence of pesticides in surface water.
Materials and methods
Collection of water samples
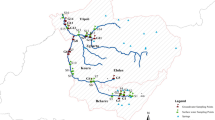
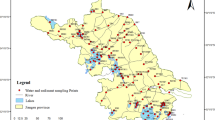
Time-integrated water samples were collected from four streams located in agricultural areas in southern Sweden (Fig. 1). The main sampling period was May to November 2013 with samples collected from sites O18, E21, N34, and M42, with occasional intermission during low-flow periods. For the southernmost sampling sites (N34 and M42), the sampling continued until the end of April the following year, since the agricultural activities are spanning over a greater part of the year in these areas, compared to the two sampling sites located further north (O18 and E21).
Water samples were collected using an automatic ISCO sampler (Teledyne Isco, Lincoln, NE, USA). Small subsamples were taken every 80 min and pooled into a weekly time-integrated sample that was stored in a refrigerator during the sampling period. For the December–April periods, the samples were pooled into biweekly samples. For sampling site M42, flow-proportional sampling at high water flow rates following intensive rain was also performed for 24 samples. Additional sampling was done in the two rivers Skivarpsån and Vege å (Fig. 1), where grab samples were collected twice a month in May and June and then monthly from July to November. Sampling periods and number of water samples collected at each site are presented in Table 1.
Chemical analysis and water sample preparation
The concentrations of 129 pesticides and pesticide transformation products were determined using GC–MS or LC–MS/MS [37] in a SWEDAC accredited laboratory at the Swedish University of Agricultural Sciences. The analyzed compounds and information about the analytical methods for each compound are presented as Additional file 1: Table S1.
Each water sample (500 mL) with internal standard compounds added (30 ng ethion, 77 ng terbuthylazine D5, 100 ng pendimethalin D5, and 100 ng trans-cypermethrin D6) was extracted three times with 35 mL dichloromethane for GC–MS analysis. The three extracts were pooled and concentrated to 1 mL in cyclohexane/acetone (9:1). For the toxicological profiling, the cyclohexane/acetone extracts were evaporated to dryness under nitrogen gas at room temperature and dissolved in 1 mL of ethanol. For LC–MS/MS analysis, an online solid-phase extraction of water sample was used (Additional file 1: Table S1).
Since the toxicological screening was performed on extracts prepared for GC–MS analysis, we performed an extraction recovery experiment to investigate to which extent the compounds determined with LC–MS/MS were also extracted with the dichloromethane extraction method, and thereby potentially present in the extracts used for toxicity bioassays. Pooled surface water was spiked in duplicate with a mixture of all the LC–MS/MS compounds and extracted according to the GC–MS method as described above, followed by evaporation and solvent change to 1 mM ammonium acetate at pH 5. An extract of the same (non-spiked) water was spiked with an equal amount of the compounds and used as reference. The extracts were then analyzed with LC–MS/MS and recoveries calculated.
In vitro bioassays
The water sample extracts used for the GC–MS analysis were tested for effects on cell viability, oxidative stress, estrogen receptor (ER) activity, and aryl hydrocarbon receptor (AhR) activity. The vehicle control for the bioassays consisted of a sample, where pure cyclohexane/acetone, containing the same amount of internal standard compounds as the water samples, had been evaporated under nitrogen gas and then dissolved in ethanol. This control was compared to a vehicle control consisting of ethanol only, to investigate if the internal standard compounds induced any activity. The internal standards were found to not induce any activities above the thresholds for bioactivity. The vehicle control including internal standard showed an Nrf2 activity of 0.93 ± 0.32 as compared to 1.00 ± 0.27 for the vehicle control without internal standards. For the ER assay, the vehicle control including internal standard showed an activity of 0.84 ± 0.05 compared to 1.00 ± 0.16 and for the AhR 1.42 ± 0.22 as compared to 1.00 ± 0.11 for the vehicle control without internal standard.
The relative enrichment factor (REF) of the samples was calculated as described by Escher et al. [27]. The concentrated water sample extracts and vehicle controls were added to the cell-culture media in a concentration of 1% leading to an REF of 5 in the cell culture. The oxidative stress response was measured using a nuclear factor erythroid 2-related factor 2 (Nrf2) bioassay in human adrenocortical H295R cells, as previously described [38]. Shortly, human adrenocortical H295R cells (ATCC number CRL-2128) were grown in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 Ham without phenol red (Sigma-Aldrich) supplemented with 1% ITS Plus premix (BD Biosciences), 2.5% NuSerum (VWR), 2 mM l-glutamine (Gibco Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco Life Technologies). The cells were cultured as monolayers in a humidified environment at 37 °C with 5% CO2.
The cells were transiently transfected with a pGL4 based luciferase reporter plasmid, where the gene expression of luciferase is under the control of an antioxidant responsive element (pGL4.37[luc2P/ARE/Hygro] Vector, E3641) purchased from Promega. The transfection efficiency was controlled for by co-transfection of a renilla luciferase plasmid. The transient transfection was performed using Lipofectamine (Life Technologies) in accordance with the manufacturer’s recommendations. Cells were seeded in a 96 well plate with a density of 17,000 cells per well. After 24 h, the cells in each well were transfected with 90 ng pGL4.37[luc2P/ARE/Hygro] vector and 30 ng renilla luciferase plasmid using 0.3 µL lipofectamine reagent. The total volume in each well was 100 µL. Following transfection, the cells were incubated for 24 h before exposure to the water sample extracts and controls.
After 24 h exposure, the luciferase activity was assayed using the Dual-Luciferase Reporter Assay System (Promega). Luminescence was measured using a Wallac Victor2 1420 microplate reader (PerkinElmer, Massachusetts, USA). The luciferase activity is expressed as fold change compared to the control (vehicle)-treated cells. A vehicle control was prepared, as described in “Chemical analysis and water sample preparation” and tert-butylhydroquinone (tBHQ) was used as a positive control.
Estrogen receptor activity was assayed using the VM7Luc4E2 cell line, generally in accordance with the OECD guideline 457 [39]. The cell line VM7Luc4E2, based on human breast cancer MCF-7 cells, contains a stably transfected firefly luc reporter construct, controlled by estrogen response elements, which will detect substances with in vitro ER agonist activity. This cell line was a generous gift from Michael Denison, University of California at Davis [40, 41]. This cell line was previously named BG1Luc4E2, but due to a misidentification of the cell line, the name has been changed into VM7Luc4E2 (rational for this name change can be found in the corrigendum to Ref. [41]). The cells were cultured as a monolayer in a humidified environment at 37 °C and 5% CO2.
The cells were routinely cultured in RPMI 1640 (Lonza) supplemented with 8% fetal bovine serum (FBS) (Gibco) and 45 U/mL penicillin, 45 µg/mL streptomycin, and 550 µg/mL gentamycin (Lonza). Two-to-three days before experiments, cells were transferred to a medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) (Lonza) with 4.5 g/L glucose, sodium pyruvate, but without phenol red and l-glutamine. This experimental medium was supplemented with 4.5% charcoal/dextran-treated FBS (Thermo Scientific), together with 4 mM l-glutamine (Lonza), 45 U/mL penicillin, 45 µg/mL streptomycin, and 375 µg/mL gentamycin (Lonza). For experiments, cells were seeded in a 96 well plate with a density of 40,000 cells per well in the above-mentioned experimental medium but without addition of gentamycin. A vehicle control was prepared as described above. 17β-Estradiol (Sigma) and methoxychlor (Fluka) were used as positive controls. On the day of exposure, medium was removed and replaced with experimental medium containing solvent control, positive control, or concentrated water sample. After 24 h exposure, the luciferase activity was assayed using the Luciferase Assay System (Promega). The luciferase activity is expressed as fold change compared to the control (vehicle) treated cells.
To assay the AhR activity, human hepatoma HepG2 cells were used. HepG2 cells (ECACC number 85011430) were grown in Dulbecco’s Modified Eagle Medium (DMEM) with GlutaMAX (Gibco Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco Life Technologies). The cells were cultured as monolayers in a humidified environment at 37 °C with 5% CO2.
The cells were transiently transfected with a pGL3-based luciferase reporter plasmid, where the gene expression of luciferase is under the control of a promoter sequence from the human CYP1A1 promoter, including 20 copies of dioxin responsive elements (pGudLuc7.5) [42]. The plasmid was a generous gift from Prof. Michael Denison, University of California at Davis. The transfection efficiency was controlled for by co-transfection of a renilla luciferase plasmid. The transient transfection was performed using Lipofectamine (Life Technologies) in accordance with the manufacturer’s recommendations. Cells were seeded in a 96 well plate with a density of 15,000 cells per well. After 24 h, the cells in each well were transfected with 90 ng pGudLuc7.5 plasmid and 30 ng renilla luciferase plasmid using 0.3 µL Lipofectamine reagent. The total volume in each well was 100 µL. Following transfection, the cells were incubated for 24 h before exposure to the water sample extracts and controls.
After 24 h exposure, the luciferase activity was assayed using the Dual-Luciferase Reporter Assay System (Promega). The luciferase activity is expressed as fold change compared to the control (vehicle)-treated group. A vehicle control was prepared as described in “Chemical analysis and water sample preparation” and 2,3,7,8-tetrachlorodibenzo-dioxin (TCDD) was used as a positive control for AhR activity.
For HepG2 cells and H295R cells, the cell viability was analyzed with an MTS test using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit (Promega) in accordance with the manufacturer’s recommendations. Cells were cultured and exposed to concentrated water samples as described above. After the exposure, the cell-viability assay substrate was added and the absorbance was measured using a Wallac Victor2 1420 microplate reader (PerkinElmer, Massachusetts, USA). Relative effects on cell viability compared to vehicle control were determined from the mean absorbance value.
For VM7Luc4E2, the cell viability was analyzed using the CellTiter-Glo® Luminescent Cell-Viability Assay (Promega). Cells were cultured and exposed to concentrated water samples as described above. After the exposure, the cell-viability assay substrate was added and the luminescence was measured in a Wallac Victor2 1420 microplate reader (PerkinElmer) and relative effects on cell viability were calculated in relation to the vehicle control.
Statistical analysis
Pesticides that were detected in less than four of the 157 water samples were excluded from the statistical analysis to avoid results that are not generalizable (Additional file 2: Table S2). Pesticides analyzed by LC–MS/MS were analyzed for recovery when using the dichloromethane extraction procedure used to generate extracts for the toxicity bioassays (Additional file 3: Table S3). Pesticides with a recovery of < 15% were excluded from the statistical analysis. Pesticides with a recovery in the range 15–63% were included in the statistical analysis, and the pesticide level was adjusted according to the recovery. Pesticides with a recovery of > 63%, the median recovery was 85%, were included in the statistical analysis without adjustment for recovery. After these changes, the data set consisted of 51 pesticides, used for the statistical analysis. Samples with a fold induction below 1 in the bioassays, as compared to the vehicle control, were excluded from the analysis.
We investigated if there were any statistical correlations between the biological parameters analyzed, using Spearman correlation coefficients, computed with the SAS software, Version 9.4 for Windows. First, the entire data set was analyzed. Second, all data were analyzed excluding the 20 samples collected using flow-proportional sampling at site M42. Third, the data for the 20 samples collected using flow-proportional sampling at site M42 were analyzed separately. Since several samples were collected at the same geographical location, the observations are not independent in the statistical sense. Hence, p values will most probably be underestimated (and statistical significance overestimated) and should, therefore, be interpreted with care. Still, here, we use p values below 0.05 to indicate interesting associations. For samples that were collected proportional to flow, data are available only for one location, M42.
For the individual pesticides, we calculated the Spearman correlation coefficient between the pesticide concentration and the responses in the toxicity bioassays and present correlations with a p value below 0.005 to account for multiple comparisons, so-called multiple hypothesis testing.
Results
Pesticide data set
Recovery of pesticides analyzed by LC–MS/MS was determined after extraction by dichloromethane to estimate the concentration present of these pesticides in the bioassays (Additional file 3: Table S3). Of the 102 pesticides, 69 had a recovery of > 63%, the median recovery was 85%, and the concentrations from the LC–MS/MS were used in the statistical analysis. Three pesticides had a recovery of 15–63% and the concentrations were adjusted according to the recovery. The remaining 30 pesticides were recovered by < 15% and not used in the statistical analysis.
The water samples collected within the monitoring program included a total of 51 different pesticides and transformation products that were detected and relevant for the toxicity testing evaluation. The 30 pesticides mentioned above with recovery < 15% were considered not relevant for the statistical analysis, as were 12 pesticides due to less than 4 observations (Additional file 2: Table S2). Twenty of the 51 compounds were herbicides, followed by 17 fungicides, 8 insecticides, 5 transformation products, and 1 growth regulator. The data set included a total of 2533 single detections, with 45% being herbicides, 24% fungicides, 18% transformation products, 14% insecticides, and < 1% growth regulators.
Concentrations of the 51 pesticides measured in each of the 157 water samples are presented in Additional file 4: Table S4. Ten of the pesticides were detected in > 50% of the samples and three of the pesticides [2,6-dichlorobenzamide (BAM), isoproturon, and metazachlor] in > 80% of the samples. The highest concentration in an individual sample was found for isoproturon, 38 µg/L and methiocarb, 16 µg/L, while 41 of the 51 pesticides (80%) had maximum concentrations < 0.5 µg/L. In general, pesticide concentrations were lower during the winter period (median total concentration 0.06–0.09 µg/L per sample with detected concentrations) and at the O18 location (0.04 µg/L). Median total concentrations were relatively higher at the locations in the very south (M42, Skivarpsån and Vege å; 0.25–0.38 µg/L) and also with a larger number of pesticides detected in each sample (median number 15–24). The highest median total concentrations and the largest number of pesticides were detected in samples collected flow proportionally at location M42 (1.05 µg/L and 31 pesticides).
Cell viability
To ensure that the studies were performed at non-cytotoxic concentrations, the cell viability was assayed after exposure to the concentrated water samples. In H295R cells and HepG2 cells, an MTS test was used to assay cell viability and < 75% cell viability as compared to the vehicle control was defined as a threshold for toxicity. In the estrogen receptor activation assay, a luminescence-based cell-viability assay was used, and the same threshold for toxicity was used. We found that none of the concentrated water samples caused cytotoxicity above the threshold in H295R, HepG2, or VM7Luc4E2 cells (Additional file 4: Table S4 and Additional file 5: Figure S1).
Oxidative stress response
The oxidative stress response was measured by an Nrf2 activity assay in transiently transfected human adrenocortical H295R cells. The cut-off value for activation was based on the limit of detection (LOD), calculated as 1 plus 3 times the standard deviation (SD) of the normalized vehicle control. The LOD was 1.75-fold induction and the cut-off values for Nrf2 activation were set at a fold induction ≥ 2 of the vehicle control. When exposed to the concentrated water samples, Nrf2 activity was triggered by five samples, equivalent to 3% of the total samples (Fig. 2 and Additional file 4: Table S4). Nrf2 activity was observed only in the flow-proportional samples from M42, collected on the same day (2013-10-21). The highest induction of oxidative stress response was 2.8-fold as compared to the vehicle control.
Oxidative stress response measured in human adrenocortical H295R cells transiently transfected with an Nrf2-sensitive luciferase reporter. Cells were exposed to concentrated water samples for 24 h prior to measurement of luciferase activity (mean, n = 4). Bioactivity was normalized to vehicle control, set to 1. Solid line indicates the cut-off value of ≥ 2 fold induction. For standard deviation, refer to Additional file 4: Table S4
Estrogen receptor activity
The estrogen receptor activity was assayed using the cell line VM7Luc4E2. The cut-off value for activation was based on the limit of detection (LOD), calculated as above as 1 plus 3 times the standard deviation (SD) of the normalized vehicle control. The LOD was 1.3-fold induction and the cut-off values for ER activation were set at a fold induction ≥ 1.5 of the vehicle control. Of the 157 concentrated water samples, 36 activated the estrogen receptor equivalent to 23% of the total samples (Fig. 3 and Additional file 4: Table S4), of which 17 were sampled at the M42 sampling site. The highest induction of ER activity was fivefold as compared to the vehicle control induced by a sample at the O18 site.
Estrogen receptor activity measured in human breast carcinoma VM7Luc4E2, stably transfected with an estrogen receptor sensitive luciferase reporter. Cells were exposed to concentrated water samples for 24 h prior to measurement of luciferase activity (mean, n = 4). Bioactivity was normalized to vehicle control, set to 1. Solid line indicates the cut-off value of ≥ 1.5-fold induction. For standard deviation, refer to Additional file 4: Table S4
Aryl hydrocarbon receptor activation
The induction of aryl hydrocarbon receptor (AhR) was measured using human hepatoma HepG2 cells, transiently transfected with an AhR sensitive luciferase reporter. Based on the LOD, which was 1.6-fold induction, the cut-off value for AhR activity was set at a fold induction ≥ 2 of the vehicle control. We found that 121 concentrated water samples (77%) activated the AhR (Fig. 4 and Additional file 4: Table S4). The highest AhR activities and the highest frequency of AhR activation were observed in samples from M42, where 42 of 44 samples induced AhR activity. The highest induction of AhR activity was 31.7-fold induction as compared to the vehicle control.
Aryl hydrocarbon receptor activity measured in human hepatoma HepG2 cells, transiently transfected with an aryl hydrocarbon receptor sensitive luciferase reporter. Cells were exposed to concentrated water samples for 24 h prior to the measurement of luciferase activity (mean, n = 4). Bioactivity was normalized to vehicle control, set to 1. Solid line indicates the cut-off value of ≥ 2-fold induction. For standard deviation, refer to Additional file 4: Table S4
Correlations between the biological parameters—effect of high flow rate
The data set, including all samples with a positive fold change in the bioassays, was analyzed to identify correlations between the fold change in biological parameters by calculating Spearman correlation coefficients. Initially, we analyzed the entire data set, including water samples collected both with time-integrated and flow-proportional sampling techniques. We found (Table 2) that the AhR activity was correlated with both Nrf2 response (r = 0.22, p = 0.0159) and ER activity (r = 0.43, p < 0.0001).
To investigate the impact of water flow rate on toxicological activity, we analyzed separately first the data from the water samples collected with time-integrated weekly sampling, and then, the data from the flow-proportional water samples collected at high peak flows. For the samples collected with time-integrated sampling, we found a correlation between AhR activity and ER activity (r = 0.43, p < 0.0001) (Table 2). For the high flow samples, we found a strong correlation between the AhR activity and the Nrf2 response (r = 0.94, p < 0.0001) (Table 2).
When comparing the observed toxic responses for the water samples collected according to the two different sampling principles (Table 3), we found that samples collected at high water flow exerted a higher mean AhR activity than samples collected with a time-integrated sampling technique (median of 6.2-fold induction for sampling at high flows compared to median of 3.5-fold induction for time-integrated).
Correlations between biological parameters and pesticide concentrations
To investigate the association between biological response and pesticide concentrations, we calculated Spearman correlation coefficients for each pesticide and biological parameter. The statistical analysis was based on the occurrence of 51 pesticides, since pesticides detected in less than four water samples and pesticides with an extraction recovery of less than 15% were excluded from the analysis (refer to section on “Statistical analysis” for further details). We found correlations (with p < 0.005) between AhR activity and 27 pesticides, between ER activity and 12 pesticides and between Nrf2 response and two pesticides (Table 4). Among the significant correlations between AhR activity and pesticides, three were negative. The occurrence of BAM, prothioconazole-desthio, azoxystrobin, diuron, carbendazim, methabenzthiazuron, methiocarb, pirimicarb, propiconazole, pyraclostrobin, terbuthylazine, and terbuthylazine-desethyl was correlated with both AhR activity and ER activity. Bitertanol was significantly correlated with both AhR activity and Nrf2 response.
Discussion
In this study, we have performed an integrated chemical and toxicological profiling of 157 water samples, collected as part of a pesticide-monitoring program in Sweden. In the program, water samples are routinely analyzed for the presence of 129 pesticides and relevant transformation products, and one aim of the present project was to investigate if it was possible to use the liquid/liquid extracts, available from the GC–MS analysis of pesticides, to complement the chemical analysis with a toxicological profiling of the samples using toxicity bioassays.
One challenge in this project was the relatively small water volumes extracted, which resulted in a final enrichment factor in the bioassays of only 5 as compared to the original water sample. Despite that, we were able to detect induced bioactivity in all three bioassays investigated. The incidence and potency of the effects were particularly high in the AhR assay. This shows that our bioassays are sensitive enough to detect toxic responses in field-collected water samples from agricultural areas, following liquid/liquid extraction and with a final enrichment factor as low as 5. The biological implications of the high AhR activity in some samples are not known, but AhR is involved in a wide variety of species- and tissue-specific biologic and toxic effects, involving developmental, immunological, and reproductive functions [43].
We investigated the potential correlation between the three toxicity parameters, using a Spearman correlation test. We found that AhR was positively correlated with ER and also to Nrf2 when assessing all samples, with correlations between AhR and ER in the time-integrated samples and AhR and Nrf2 in the flow-proportional samples. Such a correlation could be due to (1) linked biological effects (e.g., interactions between AhR and ER signaling [44, 45] and by Nrf2 activation of the AhR pathway [46]), (2) co-presence in the sample of chemicals that are regulating the different biological responses, or (3) that the same chemical affects both pathways. The strong correlation between AhR and Nrf2 in the flow-proportional samples may indicate concomitant release, due to the high flow, of chemicals regulating the two pathways.
The observed differences in AhR bioactivity in the time-integrated samples collected over 1 week compared to those collected at high flow events during the same time period may be explained by differences in the concentrations of the analyzed pesticides. However, the samples with the total highest pesticide concentrations did not induce the highest AhR activities in the flow-proportional samples from the M42 area (Additional file 4: Table S4; Fig. 4). More likely, the higher activities in flow-proportional samples are explained by differences in occurrence of other bioactive compounds, e.g., naturally occurring compounds or environmental pollutants in rain or from surface run-off. Many persistent organic pollutants have the capacity to activate AhR, such as PAHs and halogenated aromatic hydrocarbons as well as chemicals of natural origin [47, 48]. Samples for bioassay analysis were extracted by dichloromethane and PAHs and halogenated aromatic hydrocarbons, if present, would have been co-extracted. Flow-proportional sampling was performed at high water flow rates following intensive rain and the pesticide occurrence was considerably higher in these samples compared to the samples collected with time-integrated sampling at the same location. It can be suggested that the flow-proportional samples, apart from pesticides, also contained higher concentrations of other chemicals of natural or anthropogenic origin, inducing AhR activity.
A total of 51 pesticides were detected in 157 water samples, and each pesticide was detected in at least four samples in the extracts for GC–MS analysis, enabling an evaluation of associations between bioactivity and pesticide concentrations. Using Spearman correlation coefficients, two pesticides showed a statistically significant correlation to the Nrf2 response, 12 pesticides significantly correlated with ER activity, and 27 pesticides significantly correlated with AhR activity. Some pesticides were statistically linked to two biological responses, either both AhR activity and ER activity or both AhR activity and Nrf2 response. However, no pesticide showed correlation to both ER activity and Nrf2 response, which is also in line with the correlation analysis between the biological parameters, where we observed statistically significant correlations between AhR activity and Nrf2 response and between AhR activity and ER activity, but not between ER activity and Nrf2 response.
However, the correlation coefficients were low, 0.25–0.5, and it is doubtful whether the associations really are causal. Pesticides were present in low concentrations in most samples, although in a large number in many of the samples. The highest total concentration of pesticides in a sample in the present study was 39 µg/L, approximately corresponding to 0.2 µM (at a molecular weight of 200). With the enrichment factor of 5, the maximum total concentration of pesticides in the bioassays is approximately 1 µM, while the median concentrations of the samples are more than two order of magnitude lower. Neale et al. [31] reported EC10 (concentration causing 10% effect) or lowest observed effect concentrations (LOECs) of seven pesticides (carbendazim, chlorpyrifos, cyprodinil, diazinon, fipronil, and propiconazole) in bioassays: for AhR from 1.25 µM, for ER activity from 9 µM, and for Nrf2 from 27 µM. Two of the tested pesticides, carbendazim, and propiconazole were found in our study to be statistically significantly correlated with both AhR and ER activity, according to the Spearman correlation coefficients. However, the maximum concentrations of carbendazim and propiconazole in the concentrated water samples, tested for bioactivity in our study, were approximately 0.006 µM, which is more than 2–4 order of magnitude lower than the lowest effect concentrations, reported by Neale et al. [31]. Much higher toxicity was demonstrated by Neale et al. [31] for polycyclic aromatic hydrocarbons (PAHs) with effects on AhR from 0.0008 µM, on ER activity from 0.5 µM, and on Nrf2 from 0.2 µM. Takeuchi et al. [49] tested 200 pesticides for AhR activity in a bioassay and reported activity for 11 of the pesticides, of which diuron and prochloraz were detected in our study. Diuron had an EC10 of approximately 0.5 µM, which can be compared with the maximum concentration in the concentrated water samples, tested for bioactivity in our study, which was 0.0003 µM. Thus, from literature data, it seems not probable that the AhR activities observed in our study are due to the occurrence of the pesticides. It is rather probable that the correlation between pesticides and bioactivities is a result of co-occurrence of the pesticides with other anthropogenic and/or natural chemicals, which are bioactive in the water samples.
Induction of ER activity has been correlated with presence of natural estrogens and ethinylestradiol from contraceptives and also to bisphenol A and genistein [18, 50]. Kojima et al. [51] tested 200 pesticides for ER activity, of which 47 activated human estrogen receptor α. Two of these, HCH-gamma (lindane) and methiocarb, were detected in our study, however, at much lower concentrations than those active in the study by Kojima et al. [51]. We detected ER activity in 23% of the samples and the activity may partly originate from nearby livestock farming.
Oxidative stress can be induced in fish by pesticides [52]. Lungu-Mitea et al. [53] reported Nrf2 activation in zebrafish cell lines after incubation by the pesticides diazinon, deltametrin, diuron, and metazachlor at concentrations of 50 µM or higher, which are far higher than the concentrations of pesticides found in the present study.
Conclusions
The present study demonstrates that it is possible to use liquid/liquid extracts of water samples, prepared for GC–MS analysis, with a final enrichment factor of 5 in the bioassay, to complement the chemical analysis with a toxicological profiling using bioassays. Our results show that AhR and ER are sensitive endpoints for the assessment of bioactivity in surface water. Based on literature data on bioactivities of pesticides, the detected levels of pesticides in the present study were too low to induce the observed bioactivities. Thus, other environmental factors than pesticides are likely to influence the response of the bioassays, such as other chemical pollutants as well as naturally occurring compounds. Further studies are needed to identify chemicals that are responsible for toxic activities in surface water samples, which may have an impact on aquatic organisms.
Availability of data and materials
The data sheets are available in the additional file and from the corresponding author.
Abbreviations
- AhR:
-
aryl hydrocarbon receptor
- ER:
-
estrogen receptor
- LOD:
-
limit of detection
- Nrf2:
-
nuclear factor erythroid 2-related factor 2
- PAHs:
-
polycyclic aromatic hydrocarbons
- REF:
-
relative enrichment factor
- SD:
-
standard deviation
- TCDD:
-
2,3,7,8-tetrachlorodibenzo-dioxin
- tBHQ:
-
tert-butylhydroquinone
References
Loos R et al (2009) EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157(2):561–568
Staley ZR et al (2015) A synthesis of the effects of pesticides on microbial persistence in aquatic ecosystems. Crit Rev Toxicol 45(10):813–836
Kohler HR et al (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341(6147):759–765
Cruzeiro C et al (2017) Determination of 54 pesticides in waters of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemia salina and Daphnia magna bioassays. Ecotoxicol Environ Saf 145:126–134
Boye K et al (2019) Long-term data from the Swedish National environmental monitoring program of pesticides in surface waters. J Environ Qual. https://doi.org/10.2134/jeq2019.02.0056
Brack W et al (2016) Effect-directed analysis supporting monitoring of aquatic environments—an in-depth overview. Sci Total Environ 544:1073–1118
Ankley G et al (2016) Pathway-based approaches for environmental monitoring and risk assessment. Environ Sci Technol 50(19):10295–10296
Escher BI et al (2017) From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 99:97–106
Escher BI et al (2013) Most oxidative stress response in water samples comes from unknown chemicals: the need for effect-based water quality trigger values. Environ Sci Technol 47(13):7002–7011
Brack W et al (2019) Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality. Environ Sci Eur 31:10
Brack W et al (2018) Towards a holistic and solution-oriented monitoring of chemical status of European water bodies: how to support the EU strategy for a non-toxic environment? Environ Sci Eur 30(1):33
Altenburger R et al (2019) Future water quality monitoring: improving the balance between exposure and toxicity assessments of real-world pollutant mixtures. Environ Sci Eur 31(1):12
Dopp E et al (2019) Effect-based methods in combination with state-of-the-art chemical analysis for assessment of water quality as integrated approach. Int J Hyg Environ Health 222(4):607–614
Könemann S et al (2018) Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. Trends Anal Chem 102:225–235
König M et al (2017) Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ Pollut 220(Part B):1220–1230
Neale PA et al (2017) Integrating chemical analysis and bioanalysis to evaluate the contribution of wastewater effluent on the micropollutant burden in small streams. Sci Total Environ 576:785–795
Tan L et al (2017) Cell culture-based biosensing techniques for detecting toxicity in water. Curr Opin Biotechnol 45:59–68
Conley JM et al (2017) Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ Sci Technol 51(9):4781–4791
Niss F et al (2018) Toxicity bioassays with concentrated cell culture media—a methodology to overcome the chemical loss by conventional preparation of water samples. Environ Sci Pollut Res 25(12):12183–12188
Rosenmai AK et al (2018) Effect-based assessment of recipient waters impacted by on-site, small scale, and large scale waste water treatment facilities-combining passive sampling with in vitro bioassays and chemical analysis. Sci Rep 8:17200
Pieterse B et al (2015) Effect-based assessment of persistent organic pollutant and pesticide dumpsite using mammalian CALUX reporter cell lines. Environ Sci Pollut Res 22(19):14442–14454
blackwell br et al (2019) potential toxicity of complex mixtures in surface waters from a nationwide survey of United States streams: identifying in vitro bioactivities and causative chemicals. Environ Sci Technol 53(2):973–983
Dingemans MM et al (2019) Risk-based approach in the revised European Union drinking water legislation: opportunities for bioanalytical tools. Integr Environ Assess Manag 15(1):126–134
Konig M et al (2017) Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ Pollut 220:1220–1230
Tousova Z et al (2017) European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci Total Environ 601:1849–1868
Escher B et al (2012) Bioanalytical tools in water quality assessment. IWA Publishing, London
Escher BI et al (2014) Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ Sci Technol 48(3):1940–1956
Tang JY et al (2014) Which chemicals drive biological effects in wastewater and recycled water? Water Res 60:289–299
Tousova Z et al (2017) European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci Total Environ 601–602:1849–1868
Munz NA et al (2017) Pesticides drive risk of micropollutants in wastewater-impacted streams during low flow conditions. Water Res 110:366–377
Neale PA et al (2017) Development of a bioanalytical test battery for water quality monitoring: fingerprinting identified micropollutants and their contribution to effects in surface water. Water Res 123:734–750
Müller ME et al (2018) Combining in vitro reporter gene bioassays with chemical analysis to assess changes in the water quality along the Ammer River, Southwestern Germany. Environ Sci Eur 30(1):20
Niss F et al (2018) Toxicity bioassays with concentrated cell culture media-a methodology to overcome the chemical loss by conventional preparation of water samples. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-018-1656-4
Neale PA et al (2018) Solid-phase extraction as sample preparation of water samples for cell-based and other in vitro bioassays. Environ Sci Process Impacts 20(3):493–504
Abbas A et al (2019) What you extract is what you see: optimising the preparation of water and wastewater samples for in vitro bioassays. Water Res 152:47–60
Bundschuh M et al (2014) Evaluation of pesticide monitoring strategies in agricultural streams based on the toxic-unit concept—experiences from long-term measurements. Sci Total Environ 484:84–91
Jansson C et al (2010) Multiresidue analysis of 95 pesticides at low nanogram/liter levels in surface waters using online preconcentration and high performance liquid chromatography/tandem mass spectrometry. J AOAC Int 93(6):1732–1747
Lundqvist J et al (2016) Fungicide prochloraz induces oxidative stress and DNA damage in vitro. Food Chem Toxicol 91:36–41
OECD (2012) Test No. 457: BG1Luc estrogen receptor transactivation test method for identifying estrogen receptor agonists and antagonists. OECD Publishing, Paris
Rogers JM et al (2000) Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In Vitro Mol Toxicol 13(1):67–82
Brennan JC et al (2016) Development of a recombinant human ovarian (BG1) cell line containing estrogen receptor α and β for improved detection of estrogenic/antiestrogenic chemicals. Environ Toxicol Chem 35(1):91–100
He G et al (2011) Third-generation Ah receptor-responsive luciferase reporter plasmids: amplification of dioxin-responsive elements dramatically increases CALUX bioassay sensitivity and responsiveness. Toxicol Sci 123(2):511–522
Bock KW (2019) Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol 168:65–70
Chang Z et al (2014) Potential role of HSP90 in mediating the interactions between estrogen receptor (ER) and aryl hydrocarbon receptor (AhR) signaling pathways. Toxicol Lett 226(1):6–13
Helle J et al (2016) Cross-talk in the female rat mammary gland: influence of aryl hydrocarbon receptor on estrogen receptor signaling. Environ Health Perspect 124(5):601–610
Wakabayashi N et al (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13(11):1649–1663
Denison MS et al (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43(1):309–334
Stejskalova L et al (2011) Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab 12(2):198–212
Takeuchi S et al (2008) In vitro screening for aryl hydrocarbon receptor agonistic activity in 200 pesticides using a highly sensitive reporter cell line, DR-EcoScreen cells, and in vivo mouse liver cytochrome P450-1A induction by propanil, diuron and linuron. Chemosphere 74(1):155–165
Gutendorf B et al (2001) Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 166(1–2):79–89
Kojima H et al (2004) Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112(5):524–531
Slaninova A et al (2009) A review: oxidative stress in fish induced by pesticides. Neuro Endocrinol Lett 30(Suppl 1):2–12
Lungu-Mitea S et al (2018) Development of an oxidative stress in vitro assay in zebrafish (Danio rerio) cell lines. Sci Rep 8(1):12380
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Research Council Formas (Grants no 2012-2124 and 2014-1435), the SLU Programme on a Non-toxic Environment (Foma), and the Royal Swedish Academy of Agriculture and Forestry (Grant no H14-0162-CHF).
Author information
Authors and Affiliations
Contributions
JL and AO have been responsible for the concept of the manuscript, analysis, and interpretation of data and drafted the manuscript. JL made substantial contributions to the bioassay measurements and the design of figures. JK and OJ made all contributions to the sampling and chemical analysis, contributed to the interpretation of data, and were involved in revising the manuscript. CvB made all contributions to the statistical analysis of the data. AKR, ÅO, and TLG made substantial contributions to the bioassay measurements. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Analyzed pesticides, CAS numbers and method of analysis.
Additional file 2: Table S2.
Pesticides excluded from the statistical analysis, based on the fact that they were detected in less than four samples.
Additional file 3: Table S3
. Recovery for pesticides normally analyzed with an LC–MS/MS method, when extracted according to the GC–MS method based on dichloromethane extraction and subsequently used for toxicity bioassays.
Additional file 4: Table S4
. Bioactivity and cell viability (fold change; mean ± standard deviation, n = 4) and pesticide concentrations (µg/L) in 157 water samples.
Additional file 5: Figure S1.
Cell viability of H295R, HepG2, and VM7Luc4E2 cells, after exposure to concentrated water samples for 24 h.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lundqvist, J., von Brömssen, C., Rosenmai, A.K. et al. Assessment of pesticides in surface water samples from Swedish agricultural areas by integrated bioanalysis and chemical analysis. Environ Sci Eur 31, 53 (2019). https://doi.org/10.1186/s12302-019-0241-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-019-0241-x