Abstract
Background
Bladder cancer surgery is critical for treatment, and systemic treatment before or after cystectomy may be necessary. We aimed to investigate the efficacy and response to neoadjuvant and adjuvant treatments.
Methods
Data on 93 patients with resectable muscle-invasive bladder cancer were analyzed retrospectively. Patients who received neoadjuvant and adjuvant chemotherapies were included. The neoadjuvant treatment group was divided into pathological responders and non-responders. Overall survival and disease-free survival were calculated.
Results
The median age was 61.5 years; there were 6 female and 87 male patients. Baseline characteristics were similar between the groups. While there was no difference in OS between the neoadjuvant and adjuvant treatment groups (20 months vs. not reached), DFS was significantly higher in the adjuvant group (20.6 vs. 25.3 months). While there was no significant difference in DFS between the responders and non-responders to neoadjuvant treatment (20.6 vs. 19.1 months), OS was significantly longer in the responders (Not reached vs. 12.3 months).
Conclusions
Our results concluded that neoadjuvant and adjuvant chemotherapies have similar survival rates, but no response was associated with poor outcomes. Determining the group for patient selection may be helpful for optimal management.
Similar content being viewed by others
1 Background
Bladder cancer is the sixth most frequent cancer in the USA and is more common in older individuals with medical comorbidities [1]. Non-muscle-invasive bladder cancer is treated with urological interventions, while locally advanced muscle-invasive bladder cancer (MIBC) requires systemic treatment. The goals are cure, resection of the disease, relapse prevention, and survival prolongation [2].
Radical cystectomy is the cornerstone of treatment for MIBC. Neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) after cystectomy showed superiority to surgery alone [3]. For locally advanced diseases, gemcitabine plus cisplatin provided promising positive effects similar to MVAC with less toxicity. Thus, gemcitabine plus cisplatin before surgery was accepted by prominent authorities, but adjuvant approaches were still used for eligible patients [4]. Neoadjuvant chemotherapy was a safe modality before surgery, even for malignant obstructive disease [5,6,7]. However, many patients are ineligible for platinum-based chemotherapy and should be offered surgery and adjuvant regimens, but these regimens have less clear data [8].
There is a lack of knowledge about predicting factors of responses to neoadjuvant and adjuvant treatments. Head-to-head comparisons of neoadjuvant and adjuvant regimens with randomized clinical trials were limited.
We aimed to investigate the outcomes of patients with resectable MIBC after surgery with neoadjuvant and adjuvant chemotherapies. Also, we purposed to define predictive factors for treatment response.
2 Methods
2.1 Patients and measures
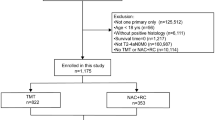
Patients over 18 years of age diagnosed with muscle-invasive bladder cancer receiving neoadjuvant or adjuvant treatment at the Department of Medical Oncology, xxxxxx Hospital, between 2010 and 2022 were included in the study. Postoperative pathological evaluation was taken as the basis for evaluating the response. Patients who could not undergo surgery were excluded. Patient characteristics, age at diagnosis, clinical stage, Eastern Cooperative Oncology Group performance status (ECOG-PS), survival at follow-up, and baseline laboratory values were noted. However, there does not exist any pathological assessment for response to neoadjuvant treatment. Patients with downstaging after neoadjuvant treatment were evaluated as pathologically responded, and patients whose stage did not decrease were evaluated as unresponsive. The same parameters were assessed for patients receiving adjuvant treatment. Factors affecting survival and progression were considered for all participants.
2.2 Statistical analysis
IBM SPSS version 25 was used for all statistical analyses. Categorical variables are given as n (%). Histogram and Shapiro–Wilk test were used to determine the normal distribution. Non-normally distributed numerical variables were presented as median (min–max). Fisher-exact or Chi-square test was used to compare categorical variables, and Mann–Whitney U test was used to compare continuous variables. Log-rank test, Cox regression analysis, and Kaplan–Meier survival curves were used for survival analysis. P < 0.05 was considered significant.
3 Results
Records of 63 patients were analyzed retrospectively. Thirty-four patients received neoadjuvant treatment before surgery, and twenty-nine patients received adjuvant chemotherapy after cystectomy. The median age was 61.5 years, and 90.5% of the patients were male. Nineteen of the patients who received neoadjuvant treatment were pathological responders. All patients received gemcitabine and cisplatin as a neoadjuvant regimen. Baseline patient and disease characteristics are given in Table 1.
Neoadjuvant and adjuvant treatment groups had similar disease characteristics and laboratory values except for stage distribution (Table 2). During follow-up, 26 patients died. There was no significant difference in the baseline characteristics of the surviving and deceased patients (Table 3).
While there was no difference in overall survival (OS) between the neoadjuvant and adjuvant treatment groups (20 months vs. not reached), disease-free survival (DFS) was significantly higher in the adjuvant group (20.6 vs. 25.3 months) (Figs. 1 and 2).
There was no significant difference in laboratory values between the responders and non-responders to neoadjuvant treatment (Table 4). However, the subtypes are small groups, and the distribution was not different in responsive and unresponsive groups (P = 0.562).
While there was no significant difference in DFS between the responders and non-responders to neoadjuvant treatment (20.6 vs. 19.1 months), OS was significantly longer in the responders (Not reached vs. 12.3 months) (Figs. 3 and 4).
4 Dıscussıon
Curing muscle-invasive bladder cancer is a goal for oncologists. Providing maximum benefit to patients is crucial, and the best treatment should be administered. Our results showed that patients with an unsatisfactory response to neoadjuvant chemotherapy had a poor prognosis.
Several trials have compared the effectiveness of neoadjuvant and adjuvant chemotherapies with different designs and results. Jue et al. [9] revealed that neoadjuvant chemotherapy was superior to adjuvant chemotherapy and had better survival rates (46.2% vs. 37.6%).
The Retrospective International Study of Cancers of the Urothelial Tract (RISC) showed that DFS was better in the neoadjuvant chemotherapy group than in the adjuvant chemotherapy group (34.6 vs. 24.9 months), but cancer-specific survival was similar [10]. A recent study with a larger cohort found no difference in overall survival, but some bias was noted. After propensity correction, neoadjuvant chemotherapy was found to be a predictive factor for increased OS [11]. Another retrospective analysis found that 5-year survival was 55.7% in the neoadjuvant chemotherapy group and 30.4% in the adjuvant chemotherapy group; median survival was also better in the neoadjuvant chemotherapy group [12]. A nationwide study from Korea showed that there was less granulocyte-colony stimulating factor administration in the neoadjuvant treatment group than in the adjuvant treatment group, and OS was better in the neoadjuvant treatment group with a 23% risk reduction after propensity score matching [13]. Our results did not support the mentioned data. It might be related to small case numbers, especially responsiveness to the neoadjuvant treatment group.
A recent investigation showed that adjuvant chemotherapy was not standard for residual disease after surgery with neoadjuvant chemotherapy [14]. This approach might benefit survival, but the patients with node-positive disease following surgery had limited survival [15, 16]. We had two patients, and a small number prevented us from evaluating this point.
Alternative neoadjuvant regimens examinations are ongoing. Neoadjuvant pembrolizumab was evaluated in a phase 2 trial for MIBC patients with variant histology. Pathological complete response was 37%, and ≤ pT1 was %55 [17]. None of our patients received immune checkpoint inhibitors, either neoadjuvant or adjuvant setting.
Inside our study group, the poorer outcomes belonged to the unresponsive group. Cha et al. [18] supported our findings. They concluded that lymph node positivity after neoadjuvant chemotherapy and surgery was associated with worse outcomes and that 3-year recurrence-free survival was 26%. Another study from Norway found that neoadjuvant chemotherapy was indirectly associated with pathological downstaging and longer overall survival than no neoadjuvant chemotherapy [19].
We did not find any predictive factor or biomarker for downstaging after neoadjuvant chemotherapy. As far as we know, there is no evidence about this point.
The present study has several limitations. Retrospective analysis limited the recording quality. Also, the sample size is small, and the comparison of survival needed to be more satisfactory for analysis. Gender discrepancy may be observed from the literature due to retrospective assessment of a single-center experience. Less frequent subtypes are very small groups and could not be analyzed in more detail. There is no standardized method for pathological evaluation of response to treatment. A longer follow-up period is needed to clarify and answer the study question. Subgroups of different surgical procedures are quite small, and analyzing their success is challenging. To our knowledge, this study is the first comparison of responsive/unresponsive to neoadjuvant and adjuvant chemotherapies in a new way.
5 Conclusıons
Bladder cancer is still lethal, and neoadjuvant or adjuvant modalities are necessary. Our data show that patients who do not undergo downstaging have a poor prognosis, and neoadjuvant and adjuvant chemotherapies have similar survival rates. More large randomized clinical trials are needed. Prediction of downstaging and its factors need to be clarified for correct patient selection.
Availability of data and materials
Not available.
Abbreviations
- MIBC:
-
Muscle-invasive bladder cancer
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- ECOG-PS:
-
Eastern Cooperative Oncology Group performance status
References
Bladder Cancer—Cancer Stat Facts n.d. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 27 June 2023
NCCN (2023) Bladder Cancer version 3.2023
Grossman H, Natale R, Tangen C, Speights V, Vogelzang N, Trump D et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–866. https://doi.org/10.1093/med/9780190655341.003.0025
Dogan S, Hennig M, Frank T, Struck JP, Cebulla A, Salem J et al (2018) Acceptance of adjuvant and neoadjuvant chemotherapy in muscle-invasive bladder cancer in Germany: a survey of current practice. Urol Int 101:25–30. https://doi.org/10.1159/000487405
Lenis AT, Fero KE, Ojeaburu L, Lec PM, Golla V, Brisbane W et al (2021) The role of neoadjuvant chemotherapy, lymph node dissection, and treatment delay in patients with muscle-invasive bladder cancer undergoing partial cystectomy. Urol Oncol Semin Orig Investig 39:496.e17-496.e24. https://doi.org/10.1016/j.urolonc.2021.01.016
Arora A, Zugail AS, Pugliesi F, Cathelineau X, Macek P, Barbé Y et al (2022) Neoadjuvant chemotherapy does not increase peri-operative morbidity following radical cystectomy. World J Urol 40:1697–1705. https://doi.org/10.1007/s00345-022-04012-4
Strother MC, Kutikov A, Epstein M, Bochner E, Deng M, Handorf E et al (2022) Safety of neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer and malignant ureteric obstruction. BJU Int 129:364–372. https://doi.org/10.1111/bju.15410
Lehmann J, Franzaring L, Thüroff J, Wellek S, Stöckle M (2006) Complete long-term survival data from a trial of adjuvant chemotherapy vs control after radical cystectomy for locally advanced bladder cancer. BJU Int 97:42–47. https://doi.org/10.1111/j.1464-410X.2006.05859.x
Jue JS, Koru-Sengul T, Miao F, Velásquez MC, Sávio LF, Alameddine M et al (2021) Neoadjuvant versus adjuvant chemotherapy for muscle-invasive bladder cancer: a propensity matched analysis. Minerva Urol Nephrol 73:572–580. https://doi.org/10.23736/S2724-6051.19.03657-9
Del Bene G, Calabrò F, Giannarelli D, Plimack ER, Harshman LC, Yu EY et al (2018) Neoadjuvant vs. adjuvant chemotherapy in muscle invasive bladder cancer (MIBC): analysis from the RISC database. Front Oncol 8:1–9. https://doi.org/10.3389/fonc.2018.00463
Macleod LC, Fam MM, Yabes JG, Hale NE, Turner RM, Lopa SH et al (2020) Comparison of neoadjuvant and adjuvant chemotherapy in muscle-invasive bladder cancer. Clin Genitourin Cancer 18:201-209.e2. https://doi.org/10.1016/j.clgc.2019.12.011
Sawasdee A, Tanthanuch M, Bejrananda T (2022) Neoadjuvant versus adjuvant chemotherapy in patients with resectable muscle-invasive bladder cancer. Asian Pac J Cancer Prev 23:3641–3647. https://doi.org/10.31557/APJCP.2022.23.11.3641
Choi SY, Ha MS, Chi BH, Kim JW, Chang IH, Kim TH et al (2022) Neoadjuvant versus adjuvant chemotherapy in bladder cancer: a nationwide cohort study. J Cancer Res Clin Oncol 148:3135–3144. https://doi.org/10.1007/s00432-022-03926-1
Martinez Chanza N, Werner L, Plimack E, Yu EY, Alva AS, Crabb SJ et al (2020) Incidence, patterns, and outcomes with adjuvant chemotherapy for residual disease after neoadjuvant chemotherapy in muscle-invasive urinary tract cancers. Eur Urol Oncol 3:671–679. https://doi.org/10.1016/j.euo.2018.12.013
Seisen T, Jamzadeh A, Leow JJ, Rouprêt M, Cole AP, Lipsitz SR et al (2018) Adjuvant chemotherapy vs observation for patients with adverse pathologic features at radical cystectomy previously treated with neoadjuvant chemotherapy. JAMA Oncol 4:225–229. https://doi.org/10.1001/jamaoncol.2017.2374
Jeong H, Park KJ, Lee Y, Kim HD, Kim JH, Yoon S et al (2022) The prognosis and the role of adjuvant chemotherapy for node-positive bladder cancer treated with neoadjuvant chemotherapy followed by surgery. Cancer Res Treat 54:226–233. https://doi.org/10.4143/CRT.2021.365
Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R et al (2020) Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 77:439–446. https://doi.org/10.1016/j.eururo.2019.10.026
Cha EK, Sfakianos JP, Sukhu R, Yee AM, Sjoberg DD, Bochner BH (2018) Poor prognosis of bladder cancer patients with occult lymph node metastases treated with neoadjuvant chemotherapy. BJU Int 122:627–632. https://doi.org/10.1111/bju.14242
Møller CT, Støer NC, Blindheim A, Berge V, Tafjord G, Fosså SD et al (2022) Downstaging and survival after neoadjuvant chemotherapy for bladder cancer in Norway; a population-based study. BMC Cancer 22:1–8. https://doi.org/10.1186/s12885-022-10394-w
Acknowledgements
Authors thank Medical Oncology Team of the hospital.
Funding
None.
Author information
Authors and Affiliations
Contributions
SS was involved in the design, data interpretation, and literature review; GU contributed to the design, main idea, and language editing; IK assisted in writing, statistical analysis, and literature search; DB contributed to the literature search, data collection, and language editing; SAE was involved in the analysis, review, and design; IS performed language editing, writing, and data collection; MANS was involved in the design, main idea, and literature search; DU was involved in the design, data interpretation, and review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ankara Bilkent city Hospital Clinical Research Ethical Committee approved the study. Consent to participate is not applicable as the records of the patients were analyzed retrospectively.
Consent for publication
Not applicable.
Competing interests
All authors declare there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sekmek, S., Ucar, G., Karahan, I. et al. Comparison of responses to neoadjuvant and adjuvant chemotherapies in muscle-invasive bladder cancer. Afr J Urol 29, 66 (2023). https://doi.org/10.1186/s12301-023-00398-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00398-8