Abstract
Background
Quercetin, a naturally occurring flavonoid known for its potent antioxidant properties, has been investigated for its potential in counteracting the harmful effects of lead (Pb) toxicity, which induces apoptosis and oxidative damage in various human tissues. This study aims to assess the reparative effects of quercetin on lead-induced testicular damage.
Methods
Four groups, each comprising ten adult male albino rats, were randomly assigned as follows: Quercetin group, Pb group, Pb + Quercetin group, and control group. All treatments were administered orally via gavage daily for a duration of 30 days. Evaluation of sex hormone levels (serum testosterone, FSH, and LH), cytokines and inflammatory mediators (IL-1β, TNF-α, MCP-1), lead concentration, oxidative and antioxidant stress markers (superoxide anion [O2−], MDA, SOD, CAT, GSH), and sperm characteristics were carried out.
Results
The results demonstrated a significant decline in sex hormones and antioxidants, accompanied by an increase in lead concentrations, cytokines, inflammatory mediators, and oxidative stress indicators (O2−, MDA), while SOD, CAT, and GSH levels were reduced. The Pb-intoxicated group exhibited a substantial increase in dead and abnormal sperm, along with significant reductions in sperm concentration and motility. Morphometrically, a marked decrease was observed in spermatogonia, primary spermatocytes, spermatids, and sertoli cells per seminiferous tubule, as well as epithelial height. Furthermore, coadministration of quercetin exhibited notable benefits. It significantly elevated testosterone levels (P < 0.001), testicular SOD, CAT, and GSH activities, while decreasing MDA levels (P < 0.001). Quercetin also mitigated the deleterious effects of lead toxicity on sperm parameters and restored morphometric variations, including epithelial height.
Conclusions
Quercetin supplementation alongside lead exposure showed a potential for ameliorating degenerative changes caused by lead toxicity in the testicles. This cotreatment effectively reduced oxidative stress, cytokine levels, inflammatory mediators, and restored biochemical alterations, thereby improving morphometric parameters.
Similar content being viewed by others
1 Background
Lead (Pb) exposure is known to have detrimental effects on male reproductive health, acting as a spermicidal and abortifacient substance with cumulative effects [1, 2]. The endocrine system and spermatogenesis in the testicles are particularly susceptible to Pb toxicity [3]. Prolonged exposure disrupts the hypothalamic–pituitary–testicular axis and may lead to reproductive issues later in life [4]. Pb exposure is associated with teratospermia, hypospermia, asthenospermia, testicular atrophy, and hypofertility [5].
The adverse effects of Pb on the testicles include reduced cellularity, abnormal shape of mature cells, and shortened seminiferous tubules, indicative of impaired maturation. Fibrosis, thickening of basement membranes, and interstitial edema are also observed [5, 6]. Pb poisoning leads to oxidative stress, increased lipid peroxidation, necrosis, and apoptosis in cells. It activates lymphocytic response mechanisms and CD68, along with alterations in antioxidant enzyme activity such as catalase (CAT) and superoxide dismutase (SOD) [7]. Moreover, Pb exposure elevates malondialdehyde (MDA) levels [8].
Flavonoids, a group of naturally occurring compounds, have gained attention due to their diverse beneficial effects on human health. These compounds possess antiviral, antioxidant, anti-inflammatory, anticarcinogenic, liver-protecting, and immune system-activating properties [9]. Among the flavonoids, quercetin (3,5,7,3,4-pentahydroxy flavone) stands out as a well-known dietary antioxidant. Quercetin exhibits the ability to scavenge reactive species like hydroxyl radicals and peroxynitrite, thereby contributing to its positive health impacts [10].
Quercetin acts as an antioxidant by neutralizing free radicals and chelating metal ions [11]. It strengthens antioxidant defenses by promoting the activity of antioxidant enzymes [12]. By chelating metal ions and eliminating free radicals, quercetin exerts its antioxidant effects [13]. This compound safeguards body tissues by counteracting reactive oxygen species produced during cellular processes [14]. Additionally, quercetin has been shown to reduce testicular reactive oxygen species, diminish lipid peroxidation, and enhance sperm motility and count [15].
2 Materials and methods
2.1 Ethical approval
This work strictly adhered to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The King Abdulaziz University Faculty of Medicine's Scientific Ethics Committee granted approval for the animal experimentation process. (No: 221-19).
2.2 Chemicals
By dissolving this salt in DI H2O [8], lead acetate (PbAc, MilliporeSigma, Burlington, MA, USA) was created as a solution. Some 5N HCl was added to the PbAc solution to stop the precipitation of Pb salts. The quercetin was given by MilliporeSigma, Burlington, Massachusetts, USA. All additional chemicals and reagents were of the analytical quality and were acquired from regional businesses.
2.3 Animals
We used 40 adult male albino rats that weighed 215 g. They were kept in a typical laboratory environment (24 °C, 12/12-h light/dark cycle). A typical laboratory meal and fresh water were given ad libitum for one week as an acclimatization phase.
2.4 Experimental design
The rats were split into four groups with ten rats each:
The control group received distilled water for 30 days.
The Quercetin group received Quercetin 75 mg/kg BW daily for 30 days [16].
The Pb group received lead acetate 10 mg/kg BW daily for 30 days [17].
The Quercetin + Pb group received (75 mg/kg BW prior to 10 mg/kg BW of PbAc daily for 30 days. Oral gavages were used to administer all of the doses. The testes were then extracted from the rats after they were slaughtered.
2.5 Preparation of blood samples and serum
The animals were euthanized after 30 days, and blood samples were taken. To extract the serum, the samples were centrifuged at 3000 rpm for 10 min and then stored at − 20 °C in a clean Eppendorf tube for biochemical marker analysis [18].
2.6 Tissue sampling
At the end of the experiment, the testes were removed and perfused with phosphate-buffered saline (PBS). The right testis was stored in 10% neutral-buffered formalin for histopathology exams, whereas the left testis was retained at − 80 °C until the assay. In an ice-cold phosphate-buffer solution, tissue homogenates were prepared (pH 7.5). After homogenates were centrifuged at 12,000 g for 10 min, the supernatants were utilized for biochemical analysis [18].
2.7 Biochemical analysis
2.7.1 Estimation of sex hormones and cytokines and inflammatory mediator
The study measured LH (luteinizing hormone), FSH (follicle-stimulating hormone), and total testosterone in serum using ELISA kits from Cusabio and Calbiotech Inc. The levels of IL-1β, TNF-α, and MCP-1 were determined using ELISA kits for rat IL-1β, TNF-α, and MCP-1 from BioSource International USA. A microtiter plate reader from Fisher Biotech (Germany) was used to read the results of the ELISA assays [19, 20].
2.7.2 Estimation of lead concentration
The amount of lead in testes homogenates was determined using the atomic absorption spectroscopy method (μg/g tissue) [21].
2.7.3 Estimation of oxidative stress and antioxidant activity parameters
Superoxide anion (O2−) was determined using LumiMax superoxide anion detection kit supplied by Agilent Technologies, Canada [22]. MDA (malondialdehyde) was measured in testes tissue homogenate as a lipid peroxidation index [23] depending on the reaction with thiobarbituric acid-reactive substances spectrophotometrically. SOD (superoxide dismutase) activity was measured by reduction of nitroblue tetrazolium [24]. CAT (catalase) activity was determined based on the disintegration of H2O2 (hydrogen peroxide) [24]. GSH (reduced glutathione) level was assayed spectrophotometrically [25].
2.8 Sperm characteristics analysis
The right epididymis was cut open and placed in a petri dish containing 2 mL of phosphate-buffered saline solution at a temperature of 37 °C and with a pH of 7.4. The spermatozoa were released by puncturing the head of the epididymis into the buffer solution. Approximately 9 µL of the spermatozoa suspension was placed on a counting compartment. The computer-aided Sperm Analyzer (CASA; JH-6004 Sperm Quality Analyzer) was used to evaluate the sperm count and kinetics. Each sample had at least 200 cells counted in every investigation including sperm characteristics. Semen was collected and tested right away; sperm concentration, motility, and morphology) [26]. A negative stain was used to assess sperm morphology, with India ink as a contrasting reagent. Percentage of abnormal or dead spermatozoa were estimated where slides stained by the nigrosin–eosin stain using bright-field microscopy and any head, tail and entire sperm abnormalities were expressed as a percentage [27]. Sperm motility was determined visually [28] under light microscope at 400X magnification at 35 °C on a heated stage, and sperms were assigned either non-motile or motile [29]. Sperm concentration was calculated using a calibrated photo-colorimeter at 546 nm (Colorimeter 254, Ciba-Corning) [30] and light microscope at 200X magnification.
2.8.1 Light microscopic examination
The tissues were fixed in 10% neutral-buffered formalin (NBF). Tissues were stained with hematoxylin and eosin (H&E) as part of a regular histological procedure.
2.9 Image capture and quantitative morphometric analysis
The study used an Olympus BX53 microscope with a DP73 camera to analyze different sections. The diameters of each seminiferous tubule and the total and differential cell count were determined using short and long diameters that were perpendicular to each other. The diameter and epithelial height of each tubule were measured using photographs taken with a 10X and 40X objective lens and a high-resolution digital camera. The germinal epithelium was assumed and displayed at micrometers from the basement membrane to the last stage of germinal cells. Spermatogonia, primary spermatocytes, spermatids, and sertoli cells were counted for each tubule based on the form of their nuclei and cytoplasm. The images were viewed and recorded using an Olympus microscope with a digital camera and associated computer software. The study evaluated 25 tubules from 5 separate H&E-stained sections on different axes for each group. Only circular or nearly circular tubules were evaluated, and 10 non-overlapping fields were measured for each stained area to compute a mean value. The data were evaluated using Image-Pro Plus v6 (Media Cybernetics Inc., Bethesda, Maryland, USA) and ImageJ (National Institute of Health, Bethesda, MD, USA) (version 1.80) at a magnification of 100x.
2.10 Statistical analysis
The data were given as mean and standard deviation. To establish the significance of the mean between the groups, one-way analysis of variance (ANOVA) was used, followed by a Bonferroni post hoc test (SPSS 28). P-values of less than 0.05 were deemed statistically significant.
3 Results
Quercetin group shows nearly the same findings as control.
3.1 Sex hormones and cytokines and inflammatory mediator analysis
Quercetin coadministration reduced the levels of cytokines such as IL-1 β, TNF-α, and MCP-1 (P < 0.001), as well as inflammatory mediators such as MCP-1 (P < 0.001), and tended to raise the levels of sexual hormones such as testosterone (P < 0.001), FSH (P < 0.001), and LH (P < 0.001). Pb group showed significant elevations in the levels of IL-1β (P < 0.001), TNF-α (P < 0.001), and MCP-1 (P < 0.001), while the sexual hormone levels were significantly decreased testosterone (P < 0.001), FSH (P < 0.001) and LH (P < 0.01), indicating the integrity of testis structure (Table 1).
3.2 Pb-concentration and oxidative stress and antioxidant activity parameters
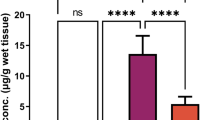
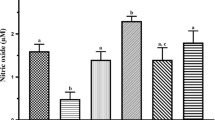
In Pb group, there is a significant increase of Pb-concentration (P < 0.001), superoxide anion O2- (P < 0.001), and MDA (P < 0.001). In addition, there is a significant decrease in SOD (P < 0.001), CAT (P < 0.001), and GSH (P < 0.001). Quercetin administration tended to decrease significantly the accumulation of Pb (P < 0.001) and oxidative stress: superoxide anion O2− (P < 0.001) and MDA (P < 0.001), and significantly increased the antioxidants: SOD (P < 0.001), CAT (P < 0.001), and GSH (P < 0.001) (Table 2).
3.3 Sperm characteristics
Sperm quality was significantly altered in Pb group: sperm concentration (P < 0.001), the sperm motility (P < 0.001), dead sperm (P < 0.001), and abnormal forms were significantly decreased (P < 0.001). When rats were treated with Quercetin, the sperm quality was slightly improved (P < 0.01), sperm motility (P < 0.001), dead sperm decreased (P < 0.001), and anormal forms (P < 0.001) (Table 3).
3.4 Quantitative morphometric analysis
Pb treatment induced a significant decline in seminiferous tubule epithelial height (P < 0.001), the count of total cells (P < 0.001) as spermatogonia (P < 0.001), primary spermatocytes (P < 0.001), spermatids (P < 0.001), and sertoli cells (P < 0.001). Concurrent administration of Quercetin significantly improved these parameters and the seminiferous tubule height, to near normal levels (P < 0.05) but have not completely restored the cellular organization of seminiferous tubule (P < 0.05) (Table 4).
3.4.1 Light microscopic results
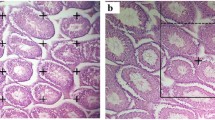
The seminiferous tubules with spermatogenic series were formed normally in the control section. Seminiferous tubules seemed to be spherical, with regular frameworks and a definite basement membrane surrounding them. Sertoli cells seemed stretched to the lumen and resting on the basement membrane. The large light nuclei and pale cytoplasm of these cells made them easily identifiable. Leydig's interstitial cells had a polygonal shape, eosinophilic cytoplasm, and a big spherical nucleus (Fig. 1).
A control: normal histoarchitecture Sg: Spermatogonia, Sp: Spermatids, Sz: Spermatozoa, B Pb group: variable degrees of degenerative changes in the seminiferous tubules. Tubules contain highly vacuolated cytoplasm (V) with deeply stained pyknotic nuclei. Exfoliated germ cells (Ex) appear in the lumina of the seminiferous tubules. Some tubules have completely degenerated, C Quercetin + Pb group: restoration of the histoarchitecture (H&E scale bar, 50 μm)
Few spermatogenic cells with pyknotic nuclei lined the seminiferous tubules in the Pb group, whereas others showed vacuolation and spermatogenic cell detachment. At the level of primary spermatocytes or round spermatids, there was also indications of retained spermatogenesis. The lumina of the seminiferous tubules contained less sperm. Due to abnormalities, the basement membranes of other seminiferous tubules looked to have deteriorated. Interstitial cell proliferation was observed as many cellular masses of vacuolated acidophilic cells with darkly colored nuclei. Furthermore, edema in the interstitial area appeared to be homogeneous eosinophilic exudates (Fig. 1).
In Quercetin coadministration, on the other hand, the histological structure of the seminiferous tubules was retained, which seemed normal with consistent skeletons and a large amount of sperm in their lumina. Vacuolation, spermatogenic cell detachment, and interstitial edema were detected despite the number of spermatogenic cells lining the tubules (Fig. 1).
4 Discussion
Lead exposure arises from various sources across different countries. For instance, in Nigeria, electronic waste, paint, and batteries are significant sources of exposure. In Mexico, lead exposure can occur from glazed ceramics, lead-contaminated utensils, and water. In India, traditional medicines and cosmetics contribute to lead exposure. In China, e-waste, traditional medicines, and industrial emissions are sources of lead exposure. In France, lead paint in older homes, imported ceramics and cosmetics, and industrial emissions are exposure sources. In Australia, lead exposure sources include paint, dust, imported toys, and traditional medicines. Finally, in the USA, paint, the industrial history of lead exposure, and batteries are the primary sources of lead exposure. [31]
When compared to controls, a significant concentration of Pb was found in the testis in the current investigation. However, cotreatment with Quercetin reduced Pb level in these tissues that agreed with previous works [32]. The orthophenolic groups on the Quercetin B ring can also chelate Pb by forming a coordination link with the Pb ions [33].
Pb causes a decrease in FSH, LH, and testosterone levels [32, 34]. This was due to a reduction in LH-binding sites in Leydig cells [35]. Quercetin partly regains these hormonal levels to acceptable levels. This might be ascribed to its antioxidant capability.
Quantitative measurement is an important predictor of typical harmonic germinal cell connections. The Pb group showed a significant reduction in tubular diameter, epithelial height, and total number of germ cells when compared to the control group. Seminiferous tubule atrophy, germinal cell sloughing, and cytoskeletal anchoring system breakdown are all signs of a specific disruption of cellular connections. These findings are similar to the authors' claims [4, 34, 36, 37].
Similarly, spermatogenic series count was decreased in Pb group; this agreed with previous finding [5]. This attributed to complete arrest of spermatogenesis with reduction in matured forms when compared to immature forms, observed in Pb group with significant reduction in spermatogonia and other germ cell populations [37]; this agreed with the current findings. Histopathological alterations noted included disintegrated shrunken seminiferous tubules, decreased cellularity, ad irregular wavy thickening of basement membrane. Current findings in Pb group are agreed with others, including abnormal ratio of spermatogenic cells and abnormalities in interstitial cells of Leydig [6, 38]. These findings are improved with the administration of Quercetin that guard against the Pb-induced histological variations [34].
Furthermore, vacuoles in between the spermatogenic series and sertoli cells seen in study [39] are agreed with the current findings. Other studies have found that sertoli cells in the Pb group are unaffected [37]. This is explained that Pb causes apoptosis of the germinal cells [40]. Furthermore, Pb caused a dose-dependent decrease in the activity of two key enzymes, alkaline phosphatase and Na–K ATPase [37].
In the current work, there are variations in sperm parameters detected due to Pb [38]. Likewise, reduction in sperm motility is accredited to rise of ROS-mediated damage. The fluidity and integrity of cellular membrane structures, particularly the cell membrane, are disrupted by ROS, which is important for sperm motility, organizational integrity, and ultimately sperm viability [41]. It was stated that Pb is able to encourage oxidative stress [6, 42].
Quercetin prevents cell death and oxidative harm by scavenging oxygen radicals, defending against lipid peroxidation, and chelating Pb ions, among other ways [8, 17]. Due to its ability to donate electrons or hydrogens, Quercetin is an excellent free radical scavenging antioxidant that scavenges hydroxyl groups, hydrogen peroxide, and superoxide anions [34].
Quercetin diffuses widely through basement membranes, allowing it to scavenge oxyradicals via the lipid bilayer in a number of sites [43]. It could be because of the pentahydroxy flavone structure, which allows it to chelate metal ions through the ortho-dihydroxy phenolic structure while simultaneously scavenging lipid peroxyl and alkoxyl radicals [44].
By blocking oxidative enzymes including NADPH oxidase, lipoxygenase, and xanthine oxidase, Quercetin may act as an antioxidant. Inhibiting these enzymes, which are important in the early stages of free radical-induced cellular damage, lowers oxidative stress [45]. Furthermore, Quercetin metabolites, like free Quercetin, have been shown to prevent peroxynitrite-mediated oxidation [46]. Apart from its direct hydrogen-donating characteristics, Quercetin's role in signaling cascades and indirect engagement with the endogenous antioxidant defense mechanism has recently received increasing attention [47]. According to one study, polyphenols interact with cellular defense mechanisms as detoxifying enzymes in stages I (mainly the CYP450 complex enzymes) and II (e.g., glutathione transferases and glucuronyltransferases) [48].
Flavonoids like Quercetin also boost the expression of c-glutamylcysteine synthetase, the rate-limiting enzyme in GSH synthesis [49]. The most effective of the flavonoids, quercetin, has been found to raise intracellular glutathione levels via increasing the expression of c-glutamylcysteine synthetase [50].
5 Conclusions
According to the results, Pb may act at the maturation level to cause discernible morphometric changes in the testicular tissues. Quercetin cotreatment prevents degeneration, lowers inflammatory mediators, cytokines, and oxidative stress and restores biochemical markers and sexual hormones.
Availability of data and materials
Not applicable.
References
Bhardwaj JK, Paliwal A, Saraf P (2021) Effects of heavy metals on reproduction owing to infertility. J Biochem Mol Toxicol. 35:e22823. https://doi.org/10.1002/jbt.22823
Raeeszadeh M, Karimfar B, Amiri AA, Akbari A, Jin H (2021) Protective effect of nano-vitamin C on infertility due to oxidative stress induced by lead and arsenic in male rats. J Chem 2021:1–12. https://doi.org/10.1155/2021/9589345
Gandhi J, Hernandez RJ, Chen A, Smith NL, Sheynkin YR, Joshi G, Khan SA (2017) Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 25:103–110. https://doi.org/10.1017/S0967199417000028
Adeyemi WJ, Abdussalam TA, Abdulrahim A, Olayaki LA (2020) Elevated, sustained, and yet reversible biotoxicity effects of lead on cessation of exposure: melatonin is a potent therapeutic option. Toxicol Ind Health. https://doi.org/10.1177/0748233720937199
Mabrouk A (2018) Therapeutic effect of thymoquinone against lead-induced testicular histological damage in male Wistar rats. Andrologia. 50:e13014. https://doi.org/10.1111/and.13014
Mustafa HN (2015) Potential alleviation of chlorella vulgaris and zingiber officinale on lead-induced testicular toxicity: an ultrastructural study. Folia Biol (Krakow) 63:269–278. https://doi.org/10.3409/fb63_4.269
Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H et al (2018) Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology 72:516–524. https://doi.org/10.1111/his.13379
Saleh HA, Abd El-Aziz GS, Mustafa HN, El-Fark M, Mal A, Aburas M, Deifalla AH (2019) Thymoquinone ameliorates oxidative damage and histopathological changes of developing brain neurotoxicity. J Histotechnol 42:116–127. https://doi.org/10.1080/01478885.2019.1619654
Arslan AS, Seven I, Mutlu SI, Arkali G, Birben N, Seven PT (2022) Potential ameliorative effect of dietary quercetin against lead-induced oxidative stress, biochemical changes, and apoptosis in laying Japanese quails. Ecotoxicol Environ Saf. 231:113200. https://doi.org/10.1016/j.ecoenv.2022.113200
Khodabandeh Z, Dolati P, Zamiri MJ, Mehrabani D, Bordbar H, Alaee S, Jamhiri I, Azarpira N (2021) Protective effect of quercetin on testis structure and apoptosis against lead acetate toxicity: an stereological study. Biol Trace Elem Res 199:3371–3381. https://doi.org/10.1007/s12011-020-02454-8
Dolati P, Zamiri MJ, Akhlaghi A, Khodabandeh Z, Mehrabani D, Atashi H, Jamhiri I (2021) Reproductive and embryological toxicity of lead acetate in male mice and their offspring and mitigation effects of quercetin. J Trace Elem Med Biol. 67:126793. https://doi.org/10.1016/j.jtemb.2021.126793
Xu D, Hu MJ, Wang YQ, Cui YL (2019) Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24:1123. https://doi.org/10.3390/molecules24061123
Durukan E (2020) Effects of (+) - catechin plus quercetin usage before exhaustion exercise on free radical and antioxidant enzyme levels. Prog Nutr 22:265–274. https://doi.org/10.23751/pn.v22i1.9041
Kicinska A, Jarmuszkiewicz W (2020) Flavonoids and mitochondria: activation of cytoprotective pathways? Molecules 25:3060. https://doi.org/10.3390/molecules25133060
Oyeyemi WA, Akinola AO, Daramola O-oO, Aikpitanyi I, Durotoluwa OT, Alele P-GO, Ogieriakhi IO, Okoro TD (2022) Vitamin E and quercetin attenuated the reproductive toxicity mediated by lead acetate in male Wistar. Bull Nat Res Centre 46:22. https://doi.org/10.1186/s42269-022-00709-z
El-Nekeety AA, Abdel-Azeim SH, Hassan AM, Hassan NS, Aly SE, Abdel-Wahhab MA (2014) Quercetin inhibits the cytotoxicity and oxidative stress in liver of rats fed aflatoxin-contaminated diet. Toxicol Rep 1:319–329. https://doi.org/10.1016/j.toxrep.2014.05.014
Mustafa HN, Hussein AM (2016) Does allicin combined with vitamin B-complex have superior potentials than alpha-tocopherol alone in ameliorating lead acetate-induced Purkinje cell alterations in rats? An immunohistochemical and ultrastructural study. Folia Morphol (Warsz) 75:76–86. https://doi.org/10.5603/FM.a2015.0076
Hegazy GA, Mustafa HN, Atahi RM, Yousef JM (2018) The Ameliorative potential of dexmedetomidine and benincasa cerifera extract in renal ischemia/reperfusion injury in a streptozotocin-induced diabetic model. Biomed Pharmacol J 11:285–303. https://doi.org/10.13005/bpj/1373
Zajkowska J, Hermanowska-Szpakowicz T, Pancewicz S, Kondrusik M, Swierzbińska R, Grygorczuk S (1995) Serum concentration of TNF-alpha and IL-1 beta in patients with chronic hepatitis C treated with interferon alpha–a preliminary report. Rocz Akad Med Bialymst 2002(47):276–286
Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima K, Sasayama S (1999) Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest 79:195–203
Beaty RD, Kerber JD (1978) Concepts, instrumentation and techniques in atomic absorption spectrophotometry. Perkin-Elmer, Waltham
Hassoun EA, Stohs SJ (1996) Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology 112:219–226. https://doi.org/10.1016/0300-483x(96)03404-x
Ohakawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Greenwald RA (2018) Handbook methods for oxygen radical research. CRC Press, Boca Raton
Avi-Dor Y, Lipkin R (1958) A spectrophotometric method for the determination of reduced glutathione. J Biol Chem 233:69–72
Gamboa S, Rodrigues AS, Henriques L, Batista C, Ramalho-Santos J (2010) Seasonal functional relevance of sperm characteristics in equine spermatozoa. Theriogenology 73:950–958. https://doi.org/10.1016/j.theriogenology.2009.11.023
Türk G, Ateşşahin A, Sönmez M, Çeribaşi AO, Yüce A (2008) Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril 89:1474–1481
Sonmez M, Turk G, Yuce A (2005) The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 63:2063–2072. https://doi.org/10.1016/j.theriogenology.2004.10.003
Gamboa S, Ramalho-Santos J (2005) SNARE proteins and caveolin-1 in stallion spermatozoa: possible implications for fertility. Theriogenology 64:275–291. https://doi.org/10.1016/j.theriogenology.2004.11.021
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93:141–154. https://doi.org/10.1385/BTER:93:1-3:141
Obeng-Gyasi E (2019) Sources of lead exposure in various countries. Rev Environ Health 34:25–34. https://doi.org/10.1515/reveh-2018-0037
Ali El-Toh A, Kamal AE (2014) Effect of vitamin E supplementation on testicular tissues of mice exposed to sub-chronic lead intoxication. Trend Med Res 9:33–43. https://doi.org/10.3923/tmr.2014.33.43
Bravo A, Anacona JR (2001) Metal complexes of the flavonoid quercetin: antibacterial properties. Transition Met Chem 26:20–23. https://doi.org/10.1023/a:1007128325639
Al-Omair MA, Sedky A, Ali A, Elsawy H (2017) Ameliorative potentials of quercetin against lead-induced hematological and testicular alterations in Albino rats. Chin J Physiol 60:54–61. https://doi.org/10.4077/CJP.2017.BAF440
Moustafa AH, Mohamed TM, Ali EM, Ahmed AA (2007) Effect of soybean on bone and gonad hormones in lead intoxicated rats. Egypt J Biochem Molecul Biol. https://doi.org/10.4314/ejbmb.v25i1.35947
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64. https://doi.org/10.1515/intox-2015-0009
Batra N, Nehru B, Bansal MP (2004) Reproductive potential of male Portan rats exposed to various levels of lead with regard to zinc status. Br J Nutr 91:387–391. https://doi.org/10.1079/BJN20031066
Asadpour R, Azari M, Hejazi M, Tayefi H, Zaboli N (2013) Protective effects of garlic aquous extract (Allium sativum), vitamin E, and N-acetylcysteine on reproductive quality of male rats exposed to lead. Vet Res Forum 4:251–257
Murthy RC, Saxena DK, Gupta SK, Chandra SV (1991) Lead induced ultrastructural changes in the testis of rats. Exp Pathol 42:95–100. https://doi.org/10.1016/s0232-1513(11)80054-x
Adhikari N, Sinha N, Narayan R, Saxena DK (2001) Lead-induced cell death in testes of young rats. J Appl Toxicol: JAT 21:275–277. https://doi.org/10.1002/jat.754
Wathes DC, Abayasekara DR, Aitken RJ (2007) Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 77:190–201. https://doi.org/10.1095/biolreprod.107.060558
El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA (2012) Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Exper Toxicol Pathol: Off J Gesellschaft fur Toxikologische Pathologie 64:753–760. https://doi.org/10.1016/j.etp.2011.01.012
Moridani MY, Pourahmad J, Bui H, Siraki A, O’Brien PJ (2003) Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radical Biol Med 34:243–253. https://doi.org/10.1016/s0891-5849(02)01241-8
Ravichandran R, Rajendran M, Devapiriam D (2014) Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem 146:472–478. https://doi.org/10.1016/j.foodchem.2013.09.080
Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G (2000) Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 468:166–170. https://doi.org/10.1016/s0014-5793(00)01211-4
Klotz LO, Sies H (2003) Defenses against peroxynitrite: selenocompounds and flavonoids. Toxicol Lett 140–141:125–132. https://doi.org/10.1016/s0378-4274(02)00511-8
Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW (2005) Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 521:9–20. https://doi.org/10.1016/j.ejphar.2005.08.005
Raucy JL (2003) Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metabol Disposit: Biol Fate Chem 31:533–539. https://doi.org/10.1124/dmd.31.5.533
Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO (2002) Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radical Biol Med 32:386–393. https://doi.org/10.1016/s0891-5849(01)00812-7
Moskaug JO, Carlsen H, Myhrstad MC, Blomhoff R (2005) Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 81:277S-283S. https://doi.org/10.1093/ajcn/81.1.277S
Acknowledgements
Not applicable.
Funding
There is no financial funding to report.
Author information
Authors and Affiliations
Contributions
The author contributed 100%. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work strictly adhered to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The King Abdulaziz University Faculty of Medicine's Scientific Ethics Committee granted approval for the animal experimentation process. (No: 221-19).
Consent for publication
I, the undersigned, give my consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“Material”) to be published in the above Journal and Article.
Competing interests
The author declares that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mustafa, H.N. Ameliorative potential of the quercetin on lead-induced testicular damage: morphohistometric and biochemical analysis. Afr J Urol 29, 36 (2023). https://doi.org/10.1186/s12301-023-00369-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00369-z