Abstract
Background
Despite the clinically apparent congenital urethra anomalies being one of the common causes of admission in pediatric urology, yet little is known about its associated factors, especially in third world countries. Understanding associated factors of clinically apparent congenital urethra anomalies is important in prevention and in genetic counseling that may help in reducing the incidence of their occurrence.
Methods
Hospital-based cross-sectional prospective study conducted among pediatric patients admitted to pediatric surgery unit at Muhimbili National Hospital from July 2021 to March 2022. Socio-demographic and clinical characteristics were collected from participant’s parent or guardian. Patients were examined thoroughly for clinically apparent congenital urethra anomalies and associated genital-urinary tract anomalies. Analysis was done using SPPS version 23 with descriptive statistics for categorical variables and univariate and multivariate logistic regression for association between presence of clinically apparent urethra anomaly and associated factors at 95% CI. A p-value of < 5 was considered statistically significant.
Results
Overall proportion of clinically apparent urethra anomaly was 24.4% (94 out of 386) with hypospadias being the commonest anomaly (23.6%); others were epispadias in 2 patients (0.5%) and bladder exstrophy in one patient (0.3%). Among hypospadias cases, sub-coronal (37.4%) and mid-shaft (29.6%) were the most prevalent. About 9.6% had associated anomalies cryptorchidism being the commonest in 8 (8.5%) patients. There was no any factor that was independently associated with development of clinically apparent congenital urethra anomalies. However, folic acid supplementation, maternal hypertension, environmental exposure to pesticides and familial history of congenital urethra anomalies were related to higher proportion of the anomalies despite no any significant relationship detected.
Conclusion
Hypospadias is the commonest clinically apparent congenital urethra anomalies with cryptorchidism being the most prevalent associated genital-urinary tract anomaly. No associated factor has shown significant relationship with clinically apparent congenital urethra anomalies; however, attention is called to maternal hypertension, environmental exposure, especially pesticides and familial history of congenital urethra anomalies for detailed study. Proper examination of newborns is encouraged for early detection of such anomalies and hence planning for early intervention.
Similar content being viewed by others
1 Background
Clinically apparent congenital urethra anomalies are birth defects involving urethra that can be identified during routine clinical examination of the patients. While some of the clinical apparent congenital urethra anomalies may be fatal due to incompatibility with life, others may lead to permanent handicaps which make the children often face psychological trauma peer or societal stigmatization.
Generally the etiology of birth defects including clinically apparent urethra anomalies remains unclear but is thought to be genetic (10–30%), environmental (5–10%), or due to multifactorial inheritance (20–35%), while (30–45%) are unknown [1]. Implicated maternal factors include age, lifestyle, and illness during pregnancy, medication use, and nonuse of periconceptual folic.
1.1 Hypospadias
Midline fusion defect of the male urethral results in a misplaced urethral meatus, most of the time associated with malformed prepuce (dorsal hooded with ventral deficiency) and sometime chordee. It is the most frequent genital malformation in male and estimates of its prevalence ranges from three to eight cases per 1000 live male births [1]. It is classified according to the location of urethral opening such as anterior (granular, coronal, sub-coronal), middle (distal, mid, shaft, and proximal penile), posterior (penoscrotal, scrotal, perineal).
1.2 Bladder exstrophy–epispadias complex
One of the most significant congenital urological anomalies characterized by a wide range of abnormalities affects the ventral body wall, urinary system, genitalia, bony pelvis, spine, and anus. Boys are twice as likely as females to develop the condition. However, other studies have revealed a substantial male preponderance, with a male to female ratio of 6:1 [2]. Maternal smoking and irradiation during the first trimester are both risk factors.
1.3 Epispadias
The failure of the urethral tube to tubularize on the dorsal aspect is a rare urogenital abnormality. It is frequently found as part of the bladder exstrophy–epispadias complex spectrum [3]. Isolated epispadias accounts for less than ten percent of all epispadias cases [3].
1.4 Megameatus intact prepuce (MIP)
Rare glanular hypospadias variant that occurs in about 3–6 percent of all hypospadias, many patients with MIP may not be diagnosed because it is not considered clinically relevant [4]. A sprayed urine stream may be observed in patients with the larger types of meatus.
1.5 Anterior urethral diverticulum
Saccular outpouching of the ventral surface of the anterior urethra can be found anywhere along the anterior urethral wall, although it is most common between the bulbous and mid-penile urethra. The cause is unknown, although theories include a corpus spongiosum developmental abnormality, cystic dilatation of the urethral glands, and sequestration of an epithelia nest after the urethral folds are closed [5].
Congenital anomaly prevalence changes with time and place, indicating a complex interaction between genetics and environmental variables [1]. Due to variances in genetics and other exposures such as infections and environmental exposures, the true prevalence of congenital malformations in Africa may differ from that in the developed world, but there is a scarcity of data in our situations. The goal of this study was to highlight the possible risk factors for clinically apparent congenital urethra anomalies so as to reduce the incidence of congenital urethra anomalies by controlling modifiable factors.
2 Methods
This was a hospital-based cross-sectional prospective study, included three hundred and eight six patients who were admitted at time of study. All patient’s care givers signed informed consent according to principles embodied in the Declaration of Helsinki for all investigations involving human materials and approved by Muhimbili University of Health and Allied Sciences ethical committee. Permission to conduct the study was also obtained from MNH Administration. Eligible study participant who their parent or guardian accepted to be involved in the study was given fully description of the study, and those who provided written consent were recruited. Those who were found to have associated anomalies apart from clinical apparent congenital urethra anomalies were referred to specific specialty for further management.
The study was carried out in Pediatric Surgery unit at Muhimbili National Hospital where Pediatric Urology Patients are usually admitted. Sample size was calculated as 385 patients based on Cochrane formula for cross-sectional studies, proportion was assumed to be 50% as no similar study done in our locality, marginal error was taken to be 5% and standard normal deviate was 1.96.
Each patient was assessed through history taking which included antenatally environmental exposure such as use of folic acids, maternal hypertension and diabetes, pesticides exposure, and cigarette smoking both active and passive. Fetal-highlighted factors are pregnancy status, gestation age at birth, birth weight, and family history of congenital urethra anomalies. Then thoroughly physical examination of the patient genitalia was conducted. All of this information was feed in standardized Swahili questionnaire.
2.1 Data analysis
Data were analyzed using computer program SPSS version 23. Descriptive statistics was used in analyzing demographic and clinical characteristics and presented in frequency table. For continuous data, e.g., age mean and range were calculated using descriptive statistics. Relationship between associated factors and presence of clinically apparent urethral anomaly was analyzed using univariate logistic regression and to deal with confounders multivariate regression that was done. P-value of < 0.05 was considered statistically significance with 95% confidence interval.
3 Results
3.1 Socio-demographic characteristics of study participants
This study involved 386 patients admitted to pediatric surgery unit at MNH with different surgical indications in 9-month duration. The mean age was 39.52 ± 30.27 months with age range of 1–120 months, 231 (59.8%) were aged between 13 and 60 months, while 74 (19.2%) were age between 0 and 12 months. Three quarters (66.6%) equivalent to 257 patients were males. In terms of residence, 256 (66.3%) came from Eastern zone regions (Dar Es Salaam, Coastal, and Morogoro regions), while 3 (0.8%) were from lake zone. Of all the participants, 244 (63.2%) were born with normal birth weight; 379 (98.2%) were singleton pregnancy with 325 (84.2%) being born at term. Only 14 (3.6%) had familial history of congenital urethral anomalies. More details are given in Table 1.
3.2 Proportion of clinically apparent congenital urethra anomalies among patients admitted to pediatric surgery unit
Of all patients included in the study, 94 (24.4%) had apparent clinical urethral anomalies. Out of which, 91 (23.5%) had hypospadias with two (0.52%) having epispadias, while only 1(0.26%) patient had bladder exstrophy–epispadias complex. Thus, the overall proportional of apparent clinical urethral anomalies was 24.4% among patients admitted in pediatric surgery unit at MNH. For more details refer to Fig. 1.
3.3 Clinical profile of patients with clinically apparent congenital urethra anomalies
Among the 94 patients with clinically apparent congenital urethra anomalies, 91(96.8%) had hypospadias out of which 34 (37.4%) had anterior sub-coronal type, 27 (29.7%) had mid-shaft type, while only 1 (1.1%) had distal penile type. Other classifications of hypospadias identified are given in Fig. 2. The rest 3 patients, 2 (2.1%) had epispadias and both were penopubic with one (1.1%) patient having bladder exstrophy.
On age basis, majority (63.7%) of patients with hypospadias attended at the hospital at 13–48 months of age, while 5.5% were attended at the hospital at the age 0–12 months and 97–120 months each. On the other hand, a patient with bladder exstrophy was attended at 0–12 months of age, whereas both epispadias patients were attended at the hospital at age between 13 and 48 months. Refer Fig. 3.
3.4 Other clinical apparent genital-urinary anomalies associated with clinically apparent congenital urethra anomalies
Among patients who had clinical apparent anomalies, only 9 (9.6%) of them had associated anomalies: 8(8.5%) patients had undescended testis, and 1(1.06%) patient had micro-penis. However, all associated anomalies were only among patients with hypospadias; none of the other anomalies had associated anomalies (Fig. 4).
3.5 Factors associated with clinically apparent congenital urethra anomalies
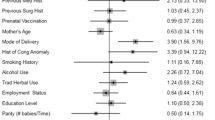
Out of 94 patients who had apparent clinical anomalies 91 (96.9%) of their mothers claimed to have taken folic acid during pregnancy, only one (1.1%) reported history of smoking, none of their mother was diabetic, 23 (24.5%) had history of hypertension either during pregnancy or prior to conception. On the other hand, 6 (6.4%) of their mothers reported history of pesticide exposure during pregnancy, while none of their mother reported history of radiation exposure.
Moreover, 63 (67.0%) of the patients had normal birth weight during their delivery; 81 (86.2%) of them were born at term with 92 (97.9%) being singleton. Only four (4.3%) had family history of urethral anomalies. However, none of the factors above was statistically significant as a risk factor for apparent clinical urethral anomalies; p-value was above 0.05 in both univariate and multivariate analysis. Refer to Table 2.
4 Discussion
This is the first study to assess the proportion of clinically apparent congenital urethra anomalies in our settings. Despite being one of the common encountered cases in pediatric urology yet only fewer issues have been studies. This study provides overall clinical profile of such anomalies with possible associated factors.
A total of 386 patients were included in the index study with only about a quarter (24.4%) of the overall patients having apparent clinical congenital urethra anomalies. This prevalence is much higher compared to Barakat et al. who studied 13,775 consecutive autopsies of general pediatric population and found 5.3% occurrence [6]. Springer and his colleagues in their systematic review had incoherent results; approximately, a mean prevalence of 19.9 in Europe, 34.2 in North America, 5.2 in South America, 0.6–69 in Asia, and 5.9 in Africa was reported (each expressed per 10,000 births); this is typically low compared to our study findings [7]. In another study an incidence of 1 case in 150–300 males was reported [8]. As shown in a study at a similar setting in Northern Tanzania having a somehow slightly higher prevalence of 4.8% compared to other studies despite being lower than our findings, this study was done at departmental level [9]. The reason for the high percentage in our study could be attributed by nature of our study population, which is pediatric surgery unit with one of the common causes of admission being hypospadias different from other studies where general pediatric population was used, hence accounting for the observed findings.
About 96.8% of the patient with anomalies in the index study had been hypospadias, with only two (2.1%) having epispadias and one 1(1.1%) with bladder exstrophy. These findings are in line with studies by Ogundoyin and his colleagues done in Nigeria and Van der Horst et al., which all showed the most common apparent congenital urethra anomalies to have been hypospadias [10, 11]. On the other hand, epispadias and bladder exstrophy have not been commonly reported in most of studies; in fewer studies, they have been reported to occur together as a complex [1, 12]. Spinoit et al. reported isolated epispadias being a rare malformation consistent with index study findings [13]. None of our study participants had megameatus intact prepuce, neither anterior urethral diverticulum nor prune belly syndrome which have been reported being rare in most of the studies [14].
Among patients with hypospadias, most of them had anterior sub-coronal type (37.4%), followed by mid-shaft type (29.7%) and only 1.1% with distal penile type. These findings are consistent with what was reported by Ogundoyin and his colleagues [10]. Interestingly, one descriptive study in Tanzania reported distal hypospadias in general as the commonest type of hypospadias different from the index study; however, their classification was generalized as proximal, mid-shaft, and distal [9]. Nyongole et al. reported 33.3% occurrence rate of sub-coronal type among 63 patients with hypospadias at the same settings which nearly the same as in this study [15]. A study in Israel found anterior and mid-shaft hypospadias being the common type among 163 hypospadias patients almost similar to the findings in this study [16].
Associated anomalies occurred in 9.6% of all the patients with clinically apparent congenital urethra anomalies. Moreover, all the associated anomalies seen in hypospadias none of other apparent anomalies had associated anomalies. Of the associated anomalies, 8.5% had undescended testis, 1.1% and 1.1% micro-penis. The 9.6% prevalence of associated anomalies seen in the index study is lower compared to findings by Ofodile et al. and Friedman with his colleagues where 52–72.3% had associated anomalies most of them being extra-urogenital [16, 17]. This study identifies the associated anomalies involving mainly the genitourinary system with cryptorchidism as the commonest. The finding is slightly lower with Friedman et al. with regard to urogenital-associated anomalies that were such anomalies accounted for 20.6% of associated anomalies with undescended testis and micro-penis being the commonest occurring by 3.7% each [16]. Similarly, Nyongole reports undescended testis as the only associated anomaly by 3.2% among patients with hypospadias; however, his study involved 63 patients about two-third of hypospadias patients in this study [15]. Similar findings have been reported in two other different studies at almost the same rate ranges [9, 18]. There is no clear explanation to why the cryptorchidism is the most common associated anomalies, but this could be attributed to their pathogenesis. Weidner et al. reported of cryptorchidism and hypospadias in animal models being related to prenatal estrogen exposure and some chemical used in farming [19].
Regarding factors associated with apparent clinical urethral anomalies from this study, none of the factors were significantly increased or reduced the risk for developing such anomalies. There have been controversial findings from other studies with regard to these factors. Some studies show association with certain factors such as genetic, environmental, and maternal factors, while in other studies no factors associated with such anomalies have been detected [20,21,22]. Despite no association with maternal hypertension nearly a quarter of patients’ mothers (24.5%) had history of hypertension either during pregnancy or prior to conception. It is well-known fact that hypertension is risk factor for congenital anomalies, and similar study by Chukwubuike and his colleagues reported that nearly one-fifth (19.4%) of the mothers whose children had congenital anomalies; gave a history of hypertension or gestational diabetes [23]. Similarly, Parimi et al. explained that up to 14% of congenital urinary anomalies could be eliminated if gestational diabetes is prevented [24]. In contrast, none of the mother in the current study was diabetic. Nevertheless, the two factors are still major high-risk predictors of congenital anomalies at large hence its of paramount importance that they are managed appropriately during maternal care if arises with proper screening of the neonates at birth.
One of the commonest reported factors associated with such clinically apparent anomalies, especially hypospadias, is genetic and environmental interactions. In the index study these were assessed based on history of smoking, pesticide exposure, and radiation exposure among patients’ mothers for environment interaction and familial history of urethra anomalies as a measure of genetic interaction. Only four patients (4.3%) had family history of urethra anomalies, and there was no significant relation to clinically apparent urethra anomalies. Moreover, 6.4% of those with anomalies had history of pesticide exposure, 1.1% had history of smoking, and none had history radiation exposure.
All the factors were not significantly related to development of clinically apparent congenital urethra anomalies. In other different studies, different findings have been documented pertaining to genetic and environmental exposures [21, 25, 26]. Thorup and his colleagues reported evidence of numerous lines suggestive of genetic component in development of apparent clinical urethral anomalies like hypospadias [25]. Similarly, familial aggregation has been accountable for approximately 10% of hypospadias cases which is the commonest apparent urethral anomaly [21]. Bouty et al. describe hypospadias and its related anomaly as a complex disorder with elements of both genetic and environment playing an important role [27]. Contrary to above findings, the index study does not, however, pinpoint any relationship between genetic and environmental exposures with the apparent urethral anomalies. It should be noted, in this study, none of the patients with anomaly was syndromic where genetic component has been reported to play great role. This might need further research to establish a better understanding on the genetic role in these anomalies possibly at molecular level.
Pertaining to environmental factors associated with such anomalies, pesticide exposure is one of the factors that has shown significant relationship reported in fewer studies. Kalfa et al. reported on raised suspiciousness of herbicides, fungicides, insecticides and by industrial products being related to hypospadias; however, none has been clearly identified as a risk factor [21]. Moreover, Weidner and his colleagues reported on chemical used in farming and gardening being related to development of urethral anomalies in animal models [19]. It has not been established for the case of human being. Interestingly, findings from this study demonstrated 6.4% of those with anomalies their mother’s reported history of pesticide exposure. It is very complex to establish a clear relationship between the assessed environmental factors and apparent clinical urethral anomalies as this might need a complex follow-up study to assess whether it were to be assessed possibly from conception period.
Low birth weight and prematurity have been associated with severe morbidity and congenital anomalies including genito-urinary system [28]. In one study Bhat et al. reported that low birth weight and preterm birth were significantly associated with genitourinary anomalies [29]. Similar findings were reported in other different studies [1, 28]. Contrary to our findings where majority (67.0%) of the patients had normal birth weight during their delivery, and 86.2% of them were born at term, these factors were not statistically significant risk factor, hence warranting further studies in our setting to provide evidence.
Majority (96.9%) mothers of patients with clinically apparent urethral anomalies claimed to have taken folic acid during pregnancy. However, there was no significant relationship detected. Nevertheless, the finding is alarming and further actions may be needed. Despite the fact that no association was demonstrated yet consistent results were reported by North et al. where vegetarian diet and iron supplementation in pregnant woman have been associated with higher risk of hypospadias which is one of the clinically apparent urethral anomalies [30]. Similarly, it was documented in one review that iron supplementation was suggested as one of the risk factors for the same urethral anomaly [31]. There is no clear understating to why folic or iron supplementation could increase the risk factor urethral anomalies; perhaps, this could be related to increased exposure to phytoestrogens, which interferes with normal endogenous estrogen during fetal development. Moreover, folic acid supplementation for a quite long time has been used among pregnant mother for growth and development of a fetus as well neural tube protection [32]. It is not yet established if disadvantages outweigh the benefits; however, these findings call for further research in this area specifically.
5 Conclusions
Nearly a quarter (24.4%) of admissions to pediatric surgery unit at Muhimbili National Hospital have clinically apparent urethral anomalies. Hypospadias is the commonest apparent clinical urethral anomalies identified with sub-coronal and mid-shaft subtype being the most prevalent. Other associated anomalies included undescended testis and micro-penis.
There was no identified associated factor that was significantly associated with development of clinically apparent urethral anomalies; however, maternal hypertension and use of folic acid supplementation during pregnancy showed higher proportion of patients with anomalies as well as detailed role of both genetic and environment interactions on such urethral anomalies.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BEEC:
-
Bladder exstrophy–epispadias complex
- IME:
-
Isolated male epispadias
- MIP:
-
Megameatus intact prepuce
- MNH:
-
Muhimbili National Hospital
- MUHAS:
-
Muhimbili University of Health and Allied Sciences
- SPSS:
-
Statistical Package for Social Sciences
- USS:
-
Ultrasound
References
Ajao AE, Adeoye IA (2019) Prevalence, risk factors and outcome of congenital anomalies among neonatal admissions in OGBOMOSO. Nigeria BMC Pediatr 19(1):1–10
Canning DA (1999) Hypospadias trends in two US surveillance systems. Rise in prevalence of hypospadias. J Urol. 161(1):366
Spinoit AF, Claeys T, Bruneel E, Ploumidis A, Van Laecke E, Hoebeke P (2016) Isolated male epispadias: anatomic functional restoration is the primary goal. Biomed Res Int 2016:1–5
Cendron M (2018) The megameatus, intact prepuce variant of hypospadias: Use of the inframeatal vascularized flap for surgical correction. Front Pediatr 6(March):1–5
Singh SK, Ansari M (2014) Congenital anterior urethral diverticulum. Turkish J Urol 40(3):182
Barakat AJ, Drougas JG (1991) Occurrence of congenital abnormalities of kidney and urinary tract in 13,775 autopsies. Urology 38(4):347–350
Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS (2016) Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 2020:1–10
Snodgrass W, Macedo A, Hoebeke P, Mouriquand PDE (2011) Hypospadias dilemmas: a round table. J Pediatr Urol 7(2):145–157
Massati M, Nyongole OV, Mteta AK (2014) A two years review of patients with hypospadias at Urology Department, Kilimanjaro Christian Medical Center in Moshi, Tanzania: what is the situation? East Cent African J Surg 19(3):39–44
Ogundoyin OO, Olulana DI, Lawal TA (2021) Experience with the management of anorectal malformations in Ibadan, Nigeria. Pan Afr Med J 38
van der Horst HJR, de Wall LL (2017) Hypospadias, all there is to know. Eur J Pediatr 176(4):435–441
Sandulescu SM, Vicol RM, Serban A, Carp AV, Cristian V (2018) Congenital anomalies of urinary tract and anomalies of fetal genitalia. Congenit Anomalies - From Embryo to Neonate
Spinoit AF, Claeys T, Bruneel E, Ploumidis A, Van Laecke E, Hoebeke P (2016) Isolated male epispadias: anatomic functional restoration is the primary goal. Biomed Res Int
Rodriguez MM (2014) Congenital anomalies of the kidney and the urinary tract (CAKUT). Fetal Pediatr Pathol 33(5–6):293–320
Nyongole OV, Mgaya DS (2020) A single center experience of Hypospadias Repair in Dar es Salaam. Tanzania J Med Res 6(5):225–229
Friedman T, Shalom A, Hoshen G, Brodovsky S, Tieder M, Westreich M (2008) Detection and incidence of anomalies associated with hypospadias. Pediatr Nephrol 23(10):1809–1816
Ofodile ferdinand (university of ID of surgery. hypospadia in nigeria 19978. ofodile1978.pdf.
Gohil A, Nema A (2018) A study of clinical profile of hypospadias cases at a medical college hospital of South Gujarat, India. Int Surg J 5(6):2127
Weidner IS, Møller H, Jensen TK, Skakkebæk NE (1998) Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Perspect 106(12):793–796
Frimberger D, Campbell J, Kropp BP (2008) Hypospadias outcome in the first 3 years after completing a pediatric urology fellowship. J Pediatr Urol 4(4):270–274
Kalfa N, Philibert P, Baskin LS, Sultan C (2011) Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol 335(2):89–95
Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ (2005) Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 159(10):957–962
Kevin EC (2021) Profile of congenital genitourinary anomalies in the two teaching hospitals in Enugu. Nigeria Biomed J Sci Tech Res 36(4):28725–28729
Parimi M, Nitsch D (2020) A systematic review and meta-analysis of diabetes during pregnancy and congenital genitourinary abnormalities. Kidney Int Reports 5(5):678–693
Thorup J, Nordenskjöld A, Hutson JM (2014) Genetic and environmental origins of hypospadias. Curr Opin Endocrinol Diabetes Obes 21(3):227–232
Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Christensen K, Wohlfahrt J et al (2008) Familial aggregation of hypospadias: a cohort study. Am J Epidemiol 167(3):251–256
Bouty A, Ayers KL, Pask A, Heloury Y, Sinclair AH (2015) The genetic and environmental factors underlying hypospadias. Sex Dev 9(5):239–259
Liu Y, Shi H, Yu X, Xiang T, Fang Y, Xie X et al (2022) Risk factors associated with renal and urinary tract anomalies delineated by an ultrasound screening program in Infants. Front Pediatr 9(January):1–10
Bhat A (2007) Extended urethral mobilization in incised plate urethroplasty for severe hypospadias: a variation in technique to improve chordee correction. J Urol 178(3):1031–1035
Reutter H, Boyadjiev SA, Gambhir L, Ebert AK, Rösch WH, Stein R, et al (2011) Phenotype severity in the bladder exstrophy-epispadias complex: Analysis of genetic and nongenetic contributing factors in 441 families from North America and Europe. J Pediatr 159(5)
van der Zanden LFM, van Rooij IALM, Feitz WFJ, Franke B, Knoers NVAM, Roeleveld N (2012) Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update 18(3):260–283
McLean W (2020) Folic acid supplementation and pregnancy – more than just neural tube defect prevention. Aust J Herb Naturop Med 32(3):115–119
Acknowledgements
Many thanks to Prof .S. Yongolo, Dr. K. Njiku, and Prof .M. Aboud for their support and advices and to all patients participating in this work for their time and support.
Funding
No source of funding could be declared.
Author information
Authors and Affiliations
Contributions
JJ contributed to the conception and design of the study, data acquisition and entry, analyzed and interpreted the data, drafted original manuscript, and revised the manuscript. SY & NK contributed to the supervision of the whole research and revised the manuscript. MA contributed in revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All patient’s care givers signed informed consent according to principles embodied in the Declaration of Helsinki for all investigations involving human materials and approved by Muhimbili University of Health and Allied Sciences ethical committee. Permission to conduct the study was also be obtained from MNH Administration.
Consent for publication
Not applicable in this section.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kahuruta, J.J., Yongolo, S., Kimu, N. et al. Factors associated with clinically apparent congenital urethra anomalies among pediatric patients attending Muhimbili National Hospital, Dar Es Salaam, Tanzania. Afr J Urol 29, 11 (2023). https://doi.org/10.1186/s12301-023-00343-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00343-9