Abstract
Background
Moonseed vine (Triclisia gilletii Staner) in the family Menispermaceae is a robust creeper of up to 10 cm diameter, of the lowland dense rain forest. In Ondo State, located in the South Western part of Nigeria, the plant which is usually called Peshe is used for the management of renal-related ailments. The present study was undertaken to explore the efficacy of Triclisia gilletii, a folkloric therapy in the management of renal-related ailment.
Results
Phenols, steroids, saponins, and flavonoids are present in the TGME with a total antioxidant capacity of (30.36 ± 1.90 (mg GAE/g extract), LD50 greater than 5000 mg/kg b.w., and in vitro anti-nucleation activity (iC50 = 7.09 mg/mL). Calcium oxalate stone formation as a result of oxalate from ethane-1,2-diol was evident by hypocalcemia, and further electrolyte imbalance and decreased glomerular filtration rate. The enhanced oxidative milieu in hyperoxaluria was evident by increased MDA and PC and decreased enzymatic and non-enzymatic antioxidants as well as renal membrane enzymes activities. The renal histopathological study further emphasized oxalate-induced damage and the ameliorative potential of TGME.
Conclusion
The abnormal biochemical, redox electrolyte, membrane integrity, and histological alterations were attenuated by TGME which affirms its usage as nephroprotectant.
Similar content being viewed by others
1 Background
Nephrolithiasis or urinary calculi or kidney stone disease is a pathological condition (-iasis) in which stones (lith-) are formed in the kidneys (nephro-). Many people all over the globe suffer from this pathological condition, and there is an increasing preponderance from the twentieth century until today [1]. It is a common disorder estimated to occur in approximately 12% of the population with a recurrence rate of 70–80% in male and 47–60% in female [2]. In Nigeria, earlier reports on nephrolithiasis showed low prevalence until recent studies indicated a rising incidence of urolithiasis [3].
The nature and type of stones depending on the constituents of the urine depend on the nature of the diet consumed in a given population [4]. About 85% of kidney stones are caused by the aggregation of calcium oxalate or uric acid crystals in the kidney tubules [5].
Ethane-1,2-diol popularly known as ethylene glycol (EG) can induce calcium oxalate (CaOx) kidney stones, inflammation, and renal damage following oxalate saturation, supersaturation, nucleation, crystal growth, aggregation, crystal retention, and stone formation [6]. CaOx and high oxalate load also cause oxidative damage and interstitial inflammation in the kidney, via production of reactive oxygen species, such as superoxide and hydrogen peroxide [7]. This ultimately leads to alterations in renal functional capacity.
Surgical endoscopic stone removal and extracorporeal shock wave lithotripsy have revolutionized the treatment of urolithiasis but do not prevent the likelihood of new stone formation aside acute kidney injury as a result of shockwave [8]. Also, therapies like thiazide as diuretic and alkali citrate commonly used in preventing the recurrence of hypercalciuria and hyperoxaluria which induce calculi are less effective [2].
The raised incidence of nephrolithiasis in our world today which could be as a result of increasing trend toward western diet and the various side effects accompanied with available therapies in managing the disease has gotten many researchers to probe into the good old traditional medicinal plants to find solutions since most of these fatal modern diseases were not dominant during the ages where medicinal plants were widely used [1].
The genus Triclisia comprises 20 species throughout the world with 12 in tropical Africa Region. Plants belonging to this genus possess potential therapeutic values known since ancient times to cure various ailments and infectious diseases such as malaria, venereal diseases, epileptic attacks, diarrhea, stomach problems, leprosy, mental health problems, dysentery, respiratory diseases, convulsive coughing, and renal-related ailments including edema, anemia, gout, urinogenital infections, and swelling of extremities [9,10,11,12].
Moonseed vine (Triclisia gilletii Staner) in the family Menispermaceae is a robust creeper of up to 10 cm in diameter, of the lowland dense rain forest. In Ondo State, located in the South Western part of Nigeria, the plant which is usually called ‘Peshe’ in Ondo City or ‘Ogbogan’ in Okeluse, Ondo State, Nigeria, and Akan-Akyem Sanhoma in Ghana is used locally for the management of swelling of extremities and other kidney-related diseases or infections either singly or in combination with other condiments by traditional practitioners. Tiam et al. [12] reported its usage for the management of edema and anemia which by our bet is closest to kidney function.
Various classes of bioactive compounds have been isolated and identified [12]. These include flavonoids (2′-hydroxy-4′-methoxyochnaflavone, myricetin, quercetin, and 3-methoxyquercetin), steroids (nonacosan-10-ol, stigmasterol, 3-O-β-d-glucopyranosylsitosterol, and 3-O-β-d-glucopyranosylstigmasterol), and triterpene (oleanolic acid). These various phytoconstituents have connection with nephroprotection.
This study establishes the protective property of Triclisia gilletii on ethane-1,2-diol-induced urolithiasis and renotoxicity in Wistar albino rats.
2 Methods
2.1 Drugs and chemicals
Ethane-1,2-diol was purchased from Titan Biotech Limited Bhiwadi-301019, Rajasthan, India. Tamsulosin hydrochloride was purchased from Sun Pharmaceutical Ind. Ltd. Dewas, India. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, creatinine, uric acid, bicarbonate, albumin, and total protein estimation kits and saponin were obtained from Sigma-Aldrich Co, St Louis, USA. Calcium, sodium, potassium, chloride, and glucose estimation kits were purchased from AGAPPE Diagnostics Switzerland GmbH. All other chemicals used in the experiment were of the highest grade commercially available.
2.2 Plant material
The leaves of Triclisia gilletii were collected from a location at Okeluse, Ose Local Government Area of Ondo State in May 2016. The plant was identified by Mr. Omomoh B.E., and a voucher specimen (IFE-17536) of the authenticated Triclisia gilletii leaves was deposited in the herbarium of the Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria. The leaves were separated for other extraneous matter and subjected to shade drying.
2.3 Preparation of aqueous methanolic leave extract of Triclisia gilletii (TGME)
The air-dried leaves were subjected to a coarse powder using a dry grinder. The powdered leaves were soaked in 80% methanol for 72 h and filtered using Whatman filter paper no. 1 to obtain the aqueous methanolic extract (TGME). The filtered extracts were concentrated in a rotary evaporator and further concentrated to dryness using freeze dryer. After drying, a dark brown aqueous methanolic extract (17.21% w/w) was obtained.
2.4 Preliminary phytochemical screening and quantitative estimation of phytoconstituents
Preliminary phytochemical screening [13] of TGME was carried out to detect the presence of phenolics, flavonoids, steroids, saponins, and tannins. The total phenolic content was determined spectrometrically [14] and expressed as milligram of gallic acid equivalents (GAEs) per gram of extract. Total flavonoid content was measured by aluminum chloride colorimetric assay [15] and expressed as milligram of quercetin equivalent per gram of extract. Total tannins content was measured according to the method described by Broadhurst et al. [16] and expressed as milligram of catechin equivalent per gram extract. Total saponins were determined according to the method of Brunner [17] and expressed as milligram saponin equivalent per gram sample.
2.5 Nucleation assay
The method as described by [18] was used to study oxalate crystallization. In this method, crystallization was studied without inhibitor (i.e., control) and with extract Triclisia gilletii (i.e., inhibitor). The result was expressed in percentage. The following reaction resulted in the growth of crystals [19]:
2.6 Animal studies
Sixty healthy male Wistar rats (age 6 weeks old; body weight 150–180 g) obtained from a private breeder in Akure metropolis of Ondo State, Nigeria, were used for the study. The animals were housed at standard housing condition (27 ± 3 °C) under 12-h light/dark cycle in polypropylene pathogen-free cages and fed standard rodent chow (Vita Feeds Nigeria Limited) and water ad libitum.
2.7 Acute toxicity study
Healthy Wistar rats, starved overnight, were subjected to acute toxicity studies to determine non-observable adverse effect dose level (NOAEL) by acute toxic class method [20]. The rats (n = 3) were orally administered TGME in the limit test dose of 5000 mg/kg orally and observed continuously for behavioral, neurological, and autonomic profiles for 2 h and after a period of 24 h for any lethality, moribund state, or death and approximate LD50 determination. Dosages between 0 and 1000 mg/kg were repeated every other day for 7 days. Animals were euthanized via cervical dislocation 24 h after the last administration, blood collected for serum biochemical analysis of AST, ALT, ALP activity, and urea, creatinine, and uric acid concentration using Randox diagnostic kits.
2.8 Grouping, treatments, and induction of urolithiasis
The rats (n = 6) were divided into 7 groups.
Group I Control—Animals in this group were allowed access to distilled water ad libitum and 1 ml/kg distilled water for 28 days.
Group II (Induced)—Animals in this group were allowed access to drinking water containing 1% ethane-1,2-diol ad libitum and 1 ml/kg distilled water for 28 days.
Group(s) III–V—Animals in these group(s) were allowed access to drinking water containing 1% ethane-1,2-diol ad libitum with co-administration of aqueous methanol leaf extract of Triclisia gilletii (50, 100, 200 mg/kg b.w.) orally for 28 days.
Group VI—Animals in this group were allowed access to drinking water containing 1% ethane-1,2-diol ad libitum with co-administration of tamsulosin hydrochloride (standard drug) orally for 28 days.
Group VII—Animals in this group were administered the highest dosage of the aqueous methanol leaf extract of Triclisia gilletii (200 mg/kg b.w.) orally for 28 days.
The groups II–VI received 1% ethane-1,2-diol in drinking water ad libitum for 28 days, respectively, to induce urolithiasis and generate CaOx deposition into kidneys. The experimental model of urolithiasis utilizes the toxic mechanism of ethane-1,2-diol poisoning. Following ingestion, ethane-1,2-diol is first hepatically metabolized to glycolaldehyde by alcohol dehydrogenase. Glycolaldehyde is then oxidized to glycolic acid, glyoxylic acid, and finally oxalic acid. Ethane-1,2-diol-induced urolithiasis model mimics the human clinical condition of lithiasis, whereby oxalate produced chelate calcium ions forming insoluble CaOx and ultimately leading to nephrotoxicity and renal failure [2].
2.9 General observations
During the study period, body weight, water intake, and animal health was observed regularly, so that stressed and unhealthy animals were excluded from the study.
2.10 Collection and analysis of urine
All animals were kept in individual metabolic cages, and 24-h urine samples were collected on 0, 7, 14, 21, and 28th day of calculi induction treatment for the assessment of volume and urinalysis using urinalysis reagent strips (ACON Laboratories Inc. San Diego, USA). Urine collected on the 28th day was assessed for creatinine, urea, uric acid, electrolytes (calcium, potassium, sodium, chloride, and bicarbonate), glucose, albumin, and total protein concentrations following methods in recommended kits.
2.11 Serum analysis
Blood was collected via cardiac puncture after euthanizing with cervical dislocation; serum was separated by centrifugation at 10,000 g for 10 min and analyzed for creatinine, urea, uric acid, electrolytes (calcium, potassium, sodium, chloride, and bicarbonate), glucose, albumin, and total protein concentrations using methods recommended in the kits.
2.12 Analysis of kidney homogenate sample
The abdomen was cut open to remove both kidneys from each animal. Isolated kidneys were cleaned off extraneous tissue and rinsed in ice-cold physiological saline. The kidney was finely minced, and 20% homogenate was prepared in Tris–HCl buffer (0.02 mol/L, pH 7.4). Total kidney homogenate was used for assaying antioxidant parameters such as lipid peroxidation [21], glutathione peroxidase (GPx) activity [22], superoxide dismutase (SOD) activity [23], estimation of reduced glutathione (GSH) level [24], protein carbonyl concentration [25], ferric reducing antioxidant power [26], anti-inflammation—myeloperoxidase activity [27], markers of kidney mitochondria integrity—complex I activity [28], glutamine synthetase activity [29], Na+K+ ATPase activity [30], lactate dehydrogenase (LDH) activity [31], and protein content [32].
2.13 Histopathological analysis
Histopathological analysis was carried out as described by Sikarwar et al. [2]. The slides were examined under a light microscope to study the light microscopic architecture of the kidney and calcium oxalate deposits using H and E (hematoxylin and eosin) staining.
2.14 Statistical analysis
The data were analyzed with one-way ANOVA followed by Duncan multiple comparison post hoc tests. A statistical difference of p < 0.05 was considered significant in all cases.
3 Results
3.1 Phytochemicals and antioxidant activities of the extract
The phytochemical screening of the aqueous methanolic leaf extract of Triclisia gilletii showed the presence of phenols (67.42 ± 0.69 mg GAE/g extract), flavonoids (87.78 ± 3.85 mg QE/g extract), tannins (8.33 ± 0.95 mg CE/g extract), cardiac glycosides, saponins (73.98 ± 1.56 mg SE/g extract), and steroids with total antioxidant activity of 30.36 ± 1.90 (mg GAE/g extract) as shown in (Table 1).
3.2 Antilithiatic activity of the extract in vitro
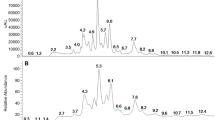
The aqueous methanol extract of Triclisia gilletii leaves prevented the nucleation of calcium chloride with sodium oxalate in a concentration dependent manner (Fig. 1), with percent inhibition iC50 7.09 mg/mL (Fig. 2).
3.3 Toxicological studies
The animals did not show any sign of toxicity even after the administration of TGME at the highest dosage (5000 mg/kg) (Table 2), except weakness within the first 24 h during the experimental period. Administration of TGME every other day for 7 days (10, 100, and 1000 mg/kg b.w.) representing 1/500th, 1/50th, and 1/5th of the highest dosage reduced (p < 0.05) the biochemical enzyme activities of ALT, AST, LDH, and concentration of uric acid with a marked increase in total protein concentration in the serum of animals administered TGME in a dose-dependent manner compared with control. No significant effect was observed in the concentrations of creatinine and urea in the animals administered TGME when compared with control (Table 3).
3.4 Nephroprotective study of TGME against ethane-1,2-diol-administered rats
3.4.1 Urine dipstick analysis, body weight, and urine volume
Bilirubin, urobilinogen, ketones, leukocytes, and protein concentration increased in the urine of ethylene-glycol-administered animals when compared with control on days 7, 14, 21, and 28. There was a significant reduction in the appearance of these molecules in the urine with co-administration of Triclisia gilletii. The pH of the animals administered ethane-1, 2-diol was slightly alkaline, and the specific gravity was observed to be lowered compared with control. Treatment with TGME ameliorated the effect of ethane-1,2-diol on pH and specific gravity (Table 4).
EG administration also affected the body weight and urine output of the animals with a significant reduction in weight gained and urine output on the 28th day of exposure when compared with control. Administration of varying dosages of TGME ameliorated the effect in a dose-dependent fashion (Figs. 3 and 4), respectively.
Effect of aqueous methanolic leaf extract of Triclisia gilletii on body weight gained per week in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); *p < 0.05, **p < 0.01, and ****p < 0.0001; * denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on urine volume/mL 24 h per week in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ****p < 0.0001; * denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
3.4.2 Urine and plasma biochemistry parameters
There was a significant (p < 0.05) decrease in creatinine clearance (Fig. 5) and urea clearance (Fig. 6). There was a decline in the concentrations of creatinine and urea in the urine of animals administered ethane-1,2-diol, compared with control. No significant difference was observed in uric acid concentration (Table 5). Plasma concentrations of creatinine and uric acid were significantly elevated in ethane-1,2-diol-administered animals compared with control, with no significant change in the concentration of urea (Table 6). Co-administration with TGME reversed the effect in a dose-dependent manner.
Effect of aqueous methanolic leaf extract of Triclisia gilletii on creatinine clearance in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); **p < 0.01, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on urea clearance in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Also, administration of ethane-1,2-diol-induced electrolyte imbalance favoring nephrotoxicity and is characterized by hypocalcemia, hyponatremia, hypochloremia, and hyperkalemia in the plasma (Table 6), with concomitant hypercalciuria, hypernatruria, and hypokaluria in urine (Table 5). Treatment with TGME significantly (p < 0.05) modulated the observed electrolyte imbalance.
A significant decrease p < 0.01 in plasma concentrations of total protein and albumin (Table 6), with concomitantly increased proteinuria and albuminuria (Table 5), is observed in the animals administered ethane-1,2-diol compared with control. Co-administration with TGME significantly protected the observed proteinuria and albuminuria.
No significant difference was observed in the plasma glucose concentration of animals administered ethane-1,2-diol when compared with control (Table 6). But increased glucosuria was observed in ethane-1,2-diol-administered animals when compared with control (Table 5). TGME significantly reduced the effect in a dose-dependent manner.
There was a significant p < 0.0001 increase in bicarbonate concentration in the plasma of animals administered ethane-1,2-diol compared with control. Co-administration with TGME significantly brought about a decrease in bicarbonate concentration (Fig. 7).
Effect of aqueous methanolic leaf extract of Triclisia gilletii on plasma bicarbonate concentration in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
3.5 Antioxidant and markers of membrane integrity parameters
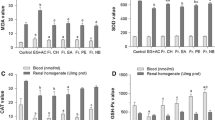
A significant increase was observed in the MDA produced (Fig. 8) and protein carbonyl concentration (Fig. 9) with a concomitant decrease in GSH (Fig. 10) and FRAP concentrations (Fig. 11) in the kidney homogenate of animals administered ethane-1,2-diol compared with control. Also, significant decreases were observed in the SOD (Fig. 12) and GPx activities (Fig. 13) in the kidney homogenate of animals administered ethane-1,2-diol when compared with control. TGME significantly brought about positive modulation in the antioxidant concentrations and antioxidant enzyme activities.
Effect of aqueous methanolic leaf extract of Triclisia gilletii on MDA produced in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, MDA malondyaldehyde
Effect of aqueous methanolic leaf extract of Triclisia gilletii on protein carbonyl concentration in animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on concentration of GSH in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); * < 0.05, ***p < 0.001, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, GSH reduced glutathione
Effect of aqueous methanolic leaf extract of Triclisia gilletii on concentration of FRAP in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); * < 0.05, ***p < 0.001, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, FRAP ferric reducing antioxidant power
Effect of aqueous methanolic leaf extract of Triclisia gilletii on SOD activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, SOD superoxide dismutase
Effect of aqueous methanolic leaf extract of Triclisia gilletii on GPx activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, GPx glutathione peroxidase
A significant increase p < 0.0001 was observed in the MPO activity in animals administered ethane-1,2-diol when compared with control. TGME significantly reduced the MPO activity in a dose-dependent manner (Fig. 14).
Effect of aqueous methanolic leaf extract of Triclisia gilletii on MPO activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); **p < 0.01, ***p < 0.001, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, MPO myeloperoxidase
There was a significant decrease p < 0.0001 in complex 1 activity (Fig. 15), Na–K ATPase activity (Fig. 16), glutamine synthetase activity (Fig. 17), and LDH activity (Fig. 18) in the kidney homogenate of animals administered ethane-1,2-diol when compared with control. Co-administration with TGME significantly brought about an increase in enzyme activity when compared with induced animals (Fig. 19).
Effect of aqueous methanolic leaf extract of Triclisia gilletii on complex 1 activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); **p < 0.01, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on Na–K ATPase activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ***p < 0.01, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on glutamine synthetase activity in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ns* > 0.05, *p < 0.05, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride
Effect of aqueous methanolic leaf extract of Triclisia gilletii on LDH activities in the kidney of animals administered ethane-1,2-diol. Data are expressed as mean ± SEM of 6 rats (n = 6); ns* > 0.05, *p < 0.05, and ****p < 0.0001; *denotes that data were compared with ethane-1,2-diol; TGME Triclisia gilletii aqueous methanolic extract, TH tamsulosin hydrochloride, LDH lactate dehydrogenase
3.6 Histopathology
From the histopathology analysis, no visible lesion was seen in the control animals. Some glomeruli and tubules were degenerated along with severe congestion of the interstitium with clumps of crystal casts found in the lumen of few tubules of animals administered ethane-1,2-diol (induced group). Several degenerated tubules with cellular debris and dark staining casts in the lumen were seen in the animals co-treated with (50 mg/kg) TGME. Also, few degenerate tubules with dark staining casts were seen in the animals co-treated with (100 and 200 mg/kg) TGME. Moderate interstitial congestion was seen in animals co-treated with standard (tamsulosin hydrochloride). No visible lesion was seen in the animals treated with 200 mg/kg TGME only.
4 Discussion
The kidneys are reddish brown, paired structures that lie on either side of the vertebral column in the lumbar region of the body. The kidney carries out its homeostatic mechanisms by maintaining the overall chemical composition of the intracellular environment and regulating the quantity of water, sodium chloride, potassium, phosphate, and numerous other substances in the body [33]. It is, therefore, a vital organ, and renal problems could be highly debilitating or even fatal as a result of waste or fluid accumulation and electrolyte imbalance.
Urinary supersaturation with respect to stone-forming constituents is generally considered to be one of the causative factors in calculogenesis. Previous studies indicated that 28-day administrations of 1% ethylene glycol to male albino rats resulted into the formation of renal calculi composed mainly of calcium oxalate [34,35,36,37]. Elevated urinary calcium is a favoring factor for nucleation of calcium oxalate from urine and further crystal growth basically through the sequestering power of oxalate produced from ethylene glycol metabolism and supersaturation in the kidney [38]. Calcium excretion progressively increased in calculi-induced animals. This chronic hypercalciuria results in massive crystalluria and subsequently stone formation [39].
In this present study, bicarbonate concentration which is a measure of acidity or alkalinity in the blood was high in ethane-1,2-diol-administered animals. This is not consistent with ethane-1,2-diol accidental toxicity in human. A lower concentration of ethane-1,2-diol in rats has been known to favor carbon dioxide elimination, while higher concentration favors the accumulation of glycolic acid, which usually results in metabolic acidosis. The proportion eliminated as oxalate is not dose dependent [40]. Therefore, the observed damage to the kidney in the animals is as a result of oxalate or calcium oxalate [41]. In this present study, TGME showed a protective effect in the damage caused by metabolic alkalosis due to excess bicarbonate generated from low-dose ethane-1,2-diol. Electrolyte imbalance observed as a result of administration of ethane-1,2-diol was modulated by co-administration of TGME. We suggest a possible inhibition of ethane-1,2-diol metabolism by the extract which calls for further studies.
Low 24-h urine volume can change the composition of urine and promote the risk of stone formation [42]. However, phytochemicals have proven to be effective in reducing stone formation [43], such as steroids, terpenoids, flavonoids, polyphenols, and tannins, those present in TGME. In most cases, they are acting as diuretics as observed in our present study with TGME.
In nephrolithiasis, the glomerular filtration rate decreases due to the obstruction to the flow of urine by stones in the urinary system as seen in the present study. Due to this, the waste products, particularly nitrogenous substances such as urea, creatinine, and uric acid, accumulate in the blood [34].
In calculi-induced rats, marked renal damage was seen as indicated by elevated serum levels of creatinine and uric acid, which are markers of glomerular and tubular damage. Although in the present study, ad libitum exposure of animals to 1% ethane-1,2-diol did not significantly increase the plasma urea concentration when compared with control, urea clearance per 24 h revealed that there was a significant difference in the functionality rate of the kidney in excreting urea. This suggests that accumulation of urea could be developing. Treatment with aqueous TGME showed an ameliorative effect on plasma concentrations of these markers by improving glomerular filtration. This could be ascribed possibly to the presence of phytoconstituents (saponins and flavonoids) in the TGME, as plants rich in the duo had been reported to protect against ethylene glycol-linked oxalate intoxication functioning as diuresis and possibly through an antioxidant mechanism [2].
Most proteins are too large to pass through the kidney filter into the urine unless the kidney is damaged. The main protein that is mostly filtered is albumin. Increased proteinuria, albuminuria, and glucosuria in this study indicate possible glomerular and tubular damage as a result of ethane-1,2-diol administration. The protectivity of TGME on the renal epithelial could be ascribed to the antioxidant power of quercetin and oleanolic acid present in the plant [12].
The development of tissue injury probably depends on the balance between the generation of reactive oxygen species (ROS) and the tissue antioxidant defense mechanism. Reduced tissue antioxidant enzymes activity is usually accompanied with increased free radical production in different stages of nephrolithiasis, which predispose the renal tissue under oxidative stress. The report that patients with kidney stones have less activity of antioxidant enzymes with increased lipid peroxidation further strengthened our finding [38].
Thamilselvan and Selvam [44] showed in their study that ethane-1,2-diol down-regulates kidney antioxidant system, which was also observed in the present study with 1% ethane-1,2-diol administration in Wistar rats. Co-treatment with TGME regimen up-regulates the antioxidant parameters. Phytochemical present in the plant (oleanolic acid) which is a triterpenoid saponin is known to preserve GSH [45], β-sitosterol [46], and stigmasterol [47], possessing antioxidant activity in different models of nephrotoxicity.
Neutrophil activation in the presence of CaOx stones is usually accompanied by increased MPO activity which is a predisposing factor for inflammation-enhancing nephrolithiasis [39]. Increased MPO activity in ethane-1,2-diol-administered animals in this study proves that inflammation accompanies stone formation, and the elevation in the creatinine and urea levels could be due to damage in the kidney tubules [48] as established by the marked changes in MPO activity.
Antioxidants have been known to decrease MPO activities in tissues owing to inhibition of neutrophil activation [49]. The decreased MPO activity observed in the animals co-administered with TGME cannot be farfetched from the potentials of its phytochemical constituents. Oleanolic acid is a known anti-inflammatory agent [50].
Mitochondrial function is important in regulating energy production and redox signaling which is usually impaired in patients with CaOx kidney stone disease [41, 51]. Hence, markers suggesting membrane damage were investigated in this study via complex 1 activity, Na–K ATPase activity, glutamine synthetase activity, and lactate dehydrogenase activity.
Complex 1 which is also known as NADH:ubiquinone oxidoreductase (EC 1.6.5.3) catalyzes movement of protons across the inner mitochondrial membrane which ultimately yields ATP for mitochondrial and cell function as well as movement of solute across the membrane. This enzyme (complex 1) is highly vulnerable to inactivation by free radicals and is probably the most susceptible component of the electron transport chain [52]. The increased activity of this enzyme in the kidney of animals administered TGME could be ascribed to the antioxidant potential of the extract as seen in the current study.
Also, (Na+–K+) adenosine triphosphatase (ATPase), which is localized to the basolateral membrane, is responsible for tubular re-absorptive process and regulated by direct interactions with membrane-associated cytoskeletal proteins that are also susceptible to oxidative damage [53]. The presence of calcium or calcium oxalate had been linked with the reorganization of the actin cytoskeleton, and it is believed to be important in the surface membrane structural, biochemical, and functional alterations in tubules [41].
Renal ammonia metabolism usually regulated by glutamine synthetase activity helps maintain acid–base balance or homeostasis [54]. Increased ammonia metabolism in the nephron is key to acid–base homeostasis in chronic kidney disease, until advanced chronic kidney disease is present [55], which is important for delaying progression of renal repair and for controlling skeletal muscle mass [56, 57].
Reduced activities of these markers of membrane stability enzymes including lactate dehydrogenase in the kidney homogenate signify progressive damage in the membrane integrity caused by oxalate or calcium oxalate stone [58, 59]. The protectivity of TGME cannot be farfetched from the antioxidant constituents in the plant.
5 Conclusion
The leaves of Triclisia gilletii exhibited a protective effect on experimentally induced urolithiasis and nephrotoxicity due to its effect on inhibition of crystal growth, modulation of electrolyte imbalance, and antioxidant and anti-inflammatory ability. The relatively safe property of the plant coupled with antiurolithiatic potentials possibly explains its usage as nephroprotectant in West Africa, particularly southwestern part of Nigeria (Ondo State). This investigation projects the therapeutic potential of the plant to be developed as an alternative herbal anti-urolithiasis drug.
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available because that is the policy of our university but are available from the corresponding author on reasonable request.
Abbreviations
- TGME:
-
Triclisia gilletii aqueous methanolic extract
- GAE:
-
gallic acid equivalent
- LD50 :
-
lethal dose
- MDA:
-
malondialdehyde
- PC:
-
protein carbonyl
- GSH:
-
reduced glutathione
- FRAP:
-
ferric reducing antioxidant power
- GPx:
-
glutathione peroxidase
- SOD:
-
superoxide dismutase
- MPO:
-
myeloperoxidase
- GS:
-
glutamine synthetase
- LDH:
-
lactate dehydrogenase
- CaOx:
-
calcium oxalate
- EG:
-
ethylene glycol
- AST:
-
aspartate aminotransferase
- ALT:
-
alanine aminotransferase
- ALP:
-
alkaline phosphatase
References
Amoah DO, Joson MB, Pareja MC (2017) Antiurolithiatic Potential of Eleusine indica Linn. (Goose grass) root extract on ethylene glycol induced nephrolithiasis in Rattus norvegicus (ALBINO RATS). Biomed Sci 3(5):99–108. https://doi.org/10.11648/j.bs.20170305.13
Sikarwar I, Dey YN, Wanjari MM, Sharma A, Gaidhani SN, Jadhav AD (2017) Chenopodium album Linn. leaves prevent ethylene glycol-induced urolithiasis in rats. J Ethnopharmacol 195:275–282
Emokpae MA, Gadzama AA (2012) Anatomical distribution and biochemical composition of urolithiasis in Kano, northern Nigeria. Int J Biol Chem Sci 6(3):1158–1166
Aji SA, Alhassan SU, Mohammed AM, Mashi SA (2011) Urinary stone disease in Kano North Western Nigeria. Niger Med J 52:83–85
Ankur C, Parasar A, Aadarsh C, Iyer D (2010) Potential of medicinal plants in kidney, gall and urinary stones. Int J Drug Dev Res 2(2):431–447
Aggarwal KP, Narula S, Kakkar M, Tandon C (2013) Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res Int 2013:1–21
Aslan Z, Aksoy L (2015) Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int Braz J Urol 41(5):1008–1013
Betanabhatla KS, Christina AJM, Sundar BS, Selvakumar S, Saravanan KS (2009) Antilithiatic activity of Hibiscus sabdariffa Linn. on ethylene glycol-induced lithiasis in rats. Nat Prod Rad 8:43–47
Asuzu IU, Okoro UO, Anaga AO (1995) The effects of the methanolic leaf extract of Triclisia subcordata. Oliv on smooth muscles. J Herbs Spices Med Plants 2(4):11–18
Abo KA, Lawal IO, Ogunkanmi A (2011) Evaluation of extracts of Triclisia subcordata Oliv and Heinsia crinita (Afz) G. Taylor for antimicrobial activity against some clinical bacterial isolates and fungi. Afr J Pharm Pharmacol 5(2):125–131
Samita F, Ochieng CO, Owuor PO, Manguro LOA, Midiwo JO (2017) Isolation of a new β-carboline alkaloid from aerial parts of Triclisia sacleuxii and its antibacterial and cytotoxicity effects. Nat Prod Res 31(5):529–536
Tiam ER, Ngono Bikobo DS, Abouem A, Zintchem A, Mbabi Nyemeck N, Moni Ndedi EDF, Betote Diboué PH, Nyegue MA, Koert U (2019) Secondary metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis. Nat Prod Res 33(5):642–650
Sofowora A (1993) Recent trends in research into African medicinal plants. J Ethnopharmacol 38(2–3):197–208
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall 40(3):255–260
Broadhurst RB, Jones WT (1978) Analysis of condensed tannins using acidified vanillin. J Sci Food Agric 29(9):788–794
Brunner JH (1984) Direct spectrophotometric determination of saponin. Anal Chem 34(396):1314–1326
Atmani F, Khan SR (2000) Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. BJU Int 85(6):621–625
Agarwal K, Varma R (2014) Ocimum gratissimum L. a medicinal plant with promising antiurolithiatic activity. Int J Pharm Sci Drug Res 6(1):78–81
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54(4):275–287
Varshney R, Kale RK (1990) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58(5):733–743
Haque ME, Asanuma M, Higashi Y, Miyazaki I, Tanaka KI, Ogawa N (2003) Overexpression of Cu–Zn superoxide dismutase protects neuroblastoma cells against dopamine cytotoxicity accompanied by increase in their glutathione level. Neurosci Res 47(1):31–37
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21(2):130–132
Jollow DJ, Mitchell JR, Zampaglione NA, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) [49] Determination of carbonyl content in oxidatively modified proteins. In: Methods in enzymology, vol 186. Academic Press, pp 464–478
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Van Der Vliet A (1998) Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391(6665):393
Birch-Machin MA, Turnbull DM (2001) Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol 65:97–117
Babu CS, Ramanathan M (2009) Pre-ischemic treatment with memantine reversed the neurochemical and behavioural parameters but not energy metabolites in middle cerebral artery occluded rats. Pharmacol Biochem Behav 92(3):424–432
Svoboda P, Mosinger B (1981) Catecholamines and the brain microsomal Na, K-adenosinetriphosphatase—I. Protection against lipoperoxidative damage. Biochem Pharmacol 30(5):427–432
Chan FKM, Moriwaki K, De Rosa MJ (2013) Detection of necrosis by release of lactate dehydrogenase activity. In: Immune homeostasis. Humana Press, Totowa, pp 65–70
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Hoenig MP, Zeidel ML (2014) Homeostasis, the milieu interieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9:1272–1281
Karadi RV, Gadge NB, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 105(1–2):306–311
Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48(4):1013–1018
Kr GU, Christina AJM (2011) Effect of Rotula aquatica Lour. on ethylene-glycol induced urolithiasis in rats. Int J Drug Dev Res 3(1):273–280
Emamiyan MZ, Vaezi G, Tehranipour M, Shahrohkabadi K, Shiravi A (2018) Preventive effects of the aqueous extract of Cichorium intybus L. flower on ethylene glycol-induced renal calculi in rats. Avicenna J Phytomed 8(2):170
Patel PK, Vyas BA, Joshi SV (2016) Evaluation of Antiurolithiatic effect of Pedalium murex fruit extract in ethylene glycol-induced nephrolithiasis in rat. Indian J Pharm Sci 78(2):230–239
Sener TE, Sener G, Cevik O, Eker P, Cetinel S, Traxer O, Tanidir Y, Akbal C (2017) The effects of melatonin on ethylene glycol-induced nephrolithiasis: role on osteopontin mRNA gene expression. Urology 99:287-e9
Fowles J, Banton M, Klapacz J, Shen H (2017) A toxicological review of the ethylene glycol series: commonalities and differences in toxicity and modes of action. Toxicol Lett 278:66–83
Patel M, Yarlagadda V, Adedoyin O, Saini V, Assimos DG, Holmes RP, Mitchell T (2018) Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line. Redox Biol 15:207–215
Gupta M, Bhayana S, Sikka SK (2011) Role of urinary inhibitors and promoters in calcium oxalate crystallisation. Int J Res Pharm Chem 1:793–798
Al-Snafi AE (2016) The medical importance of Cydonia oblonga—a review. IOSR J Pharm 6(6):87–99
Thamilselvan S, Selvam R (1997) Effect of vitamin E and mannitol on renal calcium oxalate retention in experimental nephrolithiasis. Indian J Biochem Biophys 34(3):319–323
Vyas N, Argal A (2012) Nephroprotective effect of ethanolic extract of roots and oleanolic acid isolated from roots of Lantana camara. Int J Pharmacol Clin Sci 1:54–60
Sharmila R, Sindhu G, Arockianathan PM (2016) Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J Basic Clin Physiol Pharmacol 27(5):473–482
Osuntokun OT, Oluduro AO, Idowu TO, Omotuyi AO (2017) Assessment of nephrotoxicity, anti-inflammatory and antioxidant properties of epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) derived from ethyl acetate stem bark extract of spondias mombin on Wistar rats using molecular method of analysis. J Mol Microbiol 1(1):103
Mahipal P, Pawar RS (2017) Nephroprotective effect of Murraya koenigii on cyclophosphamide induced nephrotoxicity in rats. Asian Pac J Trop Med 10(8):808–812
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. J Biomed Sci 7(6):444–458
Lee W, Yang EJ, Ku SK, Song KS, Bae JS (2013) Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 36(1):94–102
Ravi S, Mitchell T, Kramer PA, Chacko B, Darley-Usmar VM (2014) Mitochondria in monocytes and macrophages-implications for translational and basic research. Int J Biochem Cell Biol 53:202–207
Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP (2003) Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem 278(22):19603–19610
Coux G, Trumper L, Elı́as MM (2002) Renal function and cortical (Na ++ K +)-ATPase activity, abundance and distribution after ischaemia–reperfusion in rats. Biochimica et Biophysica Acta BBA-Mol Basis Dis 1586(1):71–80
Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID (2010) Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299(5):F1065–F1077
Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID (2007) Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293(4):F1238–F1247
Goraya N, Wesson DE (2012) Acid–base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens 21(5):552–556
Weiner ID, Verlander JW (2013) Renal ammonia metabolism and transport. Compr Physiol 3(1):201–220
Sudhahar V, Veena CK, Varalakshmi P (2008) Antiurolithic effect of lupeol and lupeol linoleate in experimental hyperoxaluria. J Nat Prod 71(9):1509–1512
Semangoen T, Sinchaikul S, Chen ST, Thongboonkerd V (2008) Altered proteins in MDCK renal tubular cells in response to calcium oxalate dihydrate crystal adhesion: a proteomics approach. J Proteome Res 7(7):2889–2896
Acknowledgements
Not applicable.
Funding
This study received no funding from any resource.
Author information
Authors and Affiliations
Contributions
OOS designed and performed the experiment, analyzed the data, drafted the article revising it critically for important intellectual content, and wrote the paper with revision. COO contributed to data analysis. OOE contributed to design and proofread the manuscript. ACA contributed to proofreading of the manuscript. MTO and AAA contributed to the design and proofreading of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experiments were performed in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines after the approval of the experimental protocol by the Institutional Animals Ethics Committee of the Department of Biochemistry, the Federal University of Technology Akure. At that time, there was no number to be given to the study, but the proposal number was adopted (Proposal No. BCH/07/1376). Approved consent was granted to purchase animals from a private colony in Akure, Ondo State, Nigeria.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Olayeriju, O.S., Crown, O.O., Elekofehinti, O.O. et al. Effect of moonseed vine (Triclisia gilletii Staner) on ethane-1,2-diol-induced urolithiasis and its renotoxicity in Wistar albino rats. Afr J Urol 26, 4 (2020). https://doi.org/10.1186/s12301-020-0018-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-020-0018-x