Abstract
Background
Benign prostatic hyperplasia (BPH) is a common problem in aging males which has a potential impact on patients’ health-related quality of life. In the present prospective study, we evaluated the effect of adding solifenacin to tamsulosin, compared to tamsulosin alone on overactive bladder symptoms scores (OABSS) and patients’ quality of life (QoL) in patients with filling lower urinary tract symptoms due to BPH.
Methods
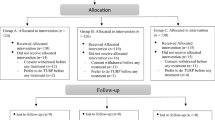
Patients included in our study were randomly assigned into 2 groups: group 1 included patients with BPH who received tamsulosin alone and group 2 included patients with BPH who received a combination of tamsulosin and sofinacin. Treatment period was 12 weeks in both groups. Quality of life and overactive bladder symptoms score questionnaires were obtained and compared in both groups before and after treatment.
Results
No significant differences were found between both groups before treatment. At the end of treatment period, The QoL score for Group 1 patients was significantly greater than the other group (mean rank was 138.98 in tamsulosin group versus 62.02 in the combination group, P-value < 0.01). Similarly, OABSS for tamsulosin only group was significantly higher than combined treatment patients (mean rank was 145.03 in tamsulin group versus 55.98 in the combination group, P-value < 0.01).
Conclusion
Adding solifenacin to tamsulosin was associated with an improvement of QoL and OABSS in patients with irritative urinary symptoms due to BPH when compared with tamsulosin monotherapy.
Similar content being viewed by others
1 Background
Benign prostatic hyperplasia (BPH) is a common benign disease affecting aging males. BPH can affect patients’ health-related quality of life. Irritative voiding symptoms secondary to BPH can lead to sleep disruption, depression, anxiety, increased falls, and sexual problems [1, 2].
Patients with BPH may be presented by irritative and/or obstructive symptoms. Irritative symptoms are in the form of urinary frequency, urgency, nocturia, and urinary incontinence, while obstructive symptoms can be in the form of hesitancy, intermittency, weak stream, or even urinary retention [3, 4].
Different options of treatment are available to treat BPH like, alpha 1 adrenergic blockers (α1-blockers), 5-α reductase inhibitors (5ARIs) or a combination of the both, anticholinergic agents, β3-adrenoceptor agonists, and phosphodiesterase type-5 inhibitors (PDE5i) which have been investigated by new studies for the treatment of BPH.
Recent studies have reported that the add-on effect of anticholinergic to patients already on α-blockers was associated with significant efficacy and health-related quality of life benefits over α-blockers monotherapy in men with LUTS/BPE [5,6,7].
The aim of our prospective study is to evaluate the effect of adding Solifenacin to tamsulosin on the overactive bladder symptoms scores (OABSS) and patients’ quality of life (QoL) in patients with filling lower urinary tract symptoms due to BPH.
1.1 Design
Prospective randomized study.
2 Methods
One hundred and seven patients aged 45 years or older, who were being treated for irritative voiding symptoms due to BPH in our urology outpatient clinic, were included in our prospective study. Patients were randomly assigned into 2 groups using opaque envelop technique. The first group (n = 52) was treated with 0.4 mg tamsulosin capsule once daily, and the other group (n = 55) was treated with 0.4 mg tamsulosin plus 5 mg solifenacin tablet once daily for 12 weeks. We obtained the quality of life questionnaire (Table 1) and overactive bladder symptoms score questionnaire (Table 2) and compared them in both groups before and at the end of treatment.
2.1 Sample size calculation
The sample size was calculated using G* power software version 3.1.9.4 and with test family (t-tests), type of power analysis (A priori: Compute required sample size—given α, power and effect size), input parameters, effect size = 0.64, α error = 0.05, power(1 − β) = 0.8, and with assuming allocation ratio N1/N2 = 1 resulting output parameters were sample size for each group 40 patients. This yield a total sample size of 80 patients.
2.2 Statistical analysis
Obtained data were presented as mean ± SD, numbers and percentages. Results were analyzed using Student t test and Chi square test (χ2 test). Statistical analysis was conducted using the IBM SPSS (Version 23, 2015) for Windows statistical package. P-value < 0.05 was considered statistically significant.
3 Results
Out of 52 patients who received 0.4 mg tamsulosin daily, only 48 patients had completed the study period as 3 patients were excluded from the study due to lost follow-up. The mean age of patients in this group was 63.25 ± 5.1 years old (mean ± SD). The quality of life was significantly improved by the administration of 0.4 mg tamsulosin. Significant improvements were observed in OABSS (Table 3). Out of 55 patients who received tamsulosin 0.4 mg plus solifenacin 5 mg daily, only 49 patients had completed the study period as 5 patients were excluded from the study due to lost follow-up. The mean age of patients in this group was 63.4 ± 5.69 years old (mean ± SD). The quality of life was significantly improved by the administration of 0.4 mg tamsulosin plus 5 mg solifenacin. Significant improvements were observed in OABSS (Table 4).
There was no significant difference between the 2 groups before treatment, but the quality of life score for patients after treatment with tamsulosin only is significantly greater than patients after treatment of tamsulosin plus solifenacin at significant level < 0.01 (Table 5). Also there was no significant difference in OABSS between 2 groups before treatment, but OABSS for group 1 was significantly greater than group 2 patients at significant level < 0.01 (Table 6).
4 Discussion
It is well known that the relief of urinary symptoms will improve the patients’ QOL. In our study, group (A) showed improvement in the QOL (question 8 of IPSS), 2.30 ± 0.50 after 12 weeks. The mean change in IPSS-QoL score from baseline to endpoint was (2 points). This improvement was statistically significant (P value < 0.001) and also was in agreement with Chapple et al. (2005) observation, (1.3 points; P = 0.0005) [8, 9].
In our study, group (B) showed improvement in QOL 4.37 ± 0.485 to 1.29 ± 0.498. This improvement was statistically significant (P value < 0.001) and in agreement with Van Kerrebroeck et al.(2013), who reported that the Change from baseline to endpoint in the QOL due to urinary symptoms with a combination of solifenacin 6 mg plus tamsulosin OCAS 0.4 mg was (1.3 points) (Fig. 1).
Significant improvements in IPSS QoL score were also reported (P < 0.001). Our study on Egyptian patients showed higher statistically significant differences than that in the NEPTUNE, which is explained by the preference of our patients to the medical treatment rather than other invasive interferences [10, 11].
In present study, combination therapy was associated with significant additional benefits (P value < 0.001) in QOL when compared with monotherapy with tamsulosin OCAS 0.4 mg; these result in agreement with van Kerrebroeck et al.(2013) who stated significant improvements in IPSS QoL score with combination therapy compared with tamsulosin OCAS (P < 0.05) [10].
Regarding overactive bladder symptom score (OABSS), in group (B) the combination therapy proved to be an effective treatment because of a statistically significant difference (P value < 0.001), as OABSS showed significant improvement from a mean ± SD of 11.43 ± 1.458 to 5.00 ± 1.064 after 3 months; these results were in agreement with Masumori et al. (2010), and Yamaguchi et al. (2011) who found also a significant improvement by addition of solifenacin to tamsulosin as an improvement from 8.0 ± 2.5 before addition of solifenacin to 4.8 ± 2.6 after 12 weeks of adding solifenacin [12]. In ASSIST study, OABSS also changes from baseline to end of treatment which was statistically significant in cases treated with tamsulosin 0.4 mg plus solifenacin 5 mg (P value < 0.001) [13, 14].
4.1 Study limitations
Our study lacks placebo group, as many patients at the beginning of the study (pilot study) refused to participate as a control group, and also most of patients were suffering from LUTS symptoms, so we found it unethical to deprive them from definitive treatment. So the decision was not to include placebo group at our study.
5 Conclusion
Our study concluded that in a short term of follow-up, the combination of solifenacin plus tamsulosin was associated with a benefit on QOL and OABSS in patients with mainly irritative symptoms due to benign prostatic hyperplasia compared to tamsulosin only.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BPH:
-
benign prostatic hyperplasia
- OABSS:
-
overactive bladder symptoms scores
- QoL:
-
quality of life
- α1-blockers:
-
alpha 1 adrenergic blockers
- 5ARIs:
-
5-α reductase inhibitors
- PDE5i:
-
phosphodiesterase type-5 inhibitors
- LUTS:
-
lower urinary tract symptoms
References
Unnikrishnan R, Almassi N, Fareed K (2017) Benign prostatic hyperplasia: evaluation and medical management in primary care. Clevel Clin J Med 84(1):53–64
Jung JH, MacDonald R, Kim J, Kim MH, Dahm P (2017) Silodosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 11(11):CD012615. https://doi.org/10.1002/14651858.CD012615.pub2
Gacci M, Carini M, Salvi M, Sebastianelli A, Vignozzi L, Corona G, Maggi M, McVary K, Kaplan S, Oelke M (2014) Management of benign prostatic hyperplasia: role of phosphodiesterase-5 inhibitors. Drugs Aging 31(6):425–439
Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, Jørgensen TM, Rittig S, Walle JV, Yeung C-K (2006) The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol 176(1):314–324
Sexton CC, Coyne KS, Kopp ZS, Irwin DE, Milsom I, Aiyer LP, Tubaro A, Chapple CR, Wein AJ (2009) The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int 103(s3):12–23
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A (2002) The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Am J Obstet Gynecol 187(1):116–126
Dimitropoulos K, Gravas S (2015) Solifenacin/tamsulosin fixed-dose combination therapy to treat lower urinary tract symptoms in patients with benign prostatic hyperplasia. Drug Des Dev Ther 9:1707
Chapple CR, Lorenz J, Mortensen R, Pauthner H, Reis MO, Schulman CC, van der Putten-Slob I (2005) Tamsulosin oral controlled absorption system (OCAS) in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): efficacy and tolerability in a phase 2b dose-response study. Eur Urol Suppl 4(2):25–32
Lepor H (1998) Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology 51(6):892–900
van Kerrebroeck P, Chapple C, Drogendijk T, Klaver M, Sokol R, Speakman M, Traudtner K, Drake MJ, N.S. Group (2013) Combination therapy with solifenacin and tamsulosin oral controlled absorption system in a single tablet for lower urinary tract symptoms in men: efficacy and safety results from the randomised controlled NEPTUNE trial. Eur Urol 64(6):1003–1012
Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G (2003) Combination treatment with an α-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized, controlled study. J Urol 169(6):2253–2256
Masumori N, Tsukamoto T, Yanase M, Horita H, Aoki M (2010) The add-on effect of solifenacin for patients with remaining overactive bladder after treatment with tamsulosin for lower urinary tract symptoms suggestive of benign prostatic obstruction. Adv Urol 2010:205251. https://doi.org/10.1155/2010/205251
Yamaguchi O, Kakizaki H, Homma Y, Takeda M, Nishizawa O, Gotoh M, Yokoyama O, Seki N, Yoshida M, N. S. Group (2011) Solifenacin as add-on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms—ASSIST, randomized controlled study. Urology 78(1):126–133
Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, Yamanishi T, Yamaguchi O, Takeda M, Nishizawa O (2006) Symptom assessment tool for overactive bladder syndrome—overactive bladder symptom score. Urology 68(2):318–323
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors have made a significant contribution to the findings and methods in the paper. ME worked on the idea and design. AI shared in the editing. ES helped in data collection and analysis. AK shared in the editing. AG contributed to revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed were in accordance with the ethical standards of the ethics committee IRB of Faculty of Medicine, Minia University, approved on February 18, 2018. Ethical Approval number: 236/2018. A written consent was taken from all participants after sufficient information about the study.
Consent for publication
Not applicable.
Competing interest
No competing interest is declared by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Elbadry, M., Ali, A.I., Saleh, E. et al. The impact of adding solifenacin to tamsulosin therapy for treatment of storage lower urinary tract symptoms owing to benign prostatic hyperplasia. Afr J Urol 26, 84 (2020). https://doi.org/10.1186/s12301-020-00094-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-020-00094-x