Abstract
Background
Plant cell walls have evolved precise plasticity in response to environmental stimuli. The plant heterotrimeric G protein complexes could sense and transmit extracellular signals to intracellular signaling systems, and activate a series of downstream responses. dep1 (Dense and Erect Panicles 1), the gain-of-function mutation of DEP1 encoding a G protein γ subunit, confers rice multiple improved agronomic traits. However, the effects of DEP1 on cell wall biosynthesis and wall-related agronomic traits remain largely unknown.
Results
In this study, we showed that the DEP1 mutation affects cell wall biosynthesis, leading to improved lodging resistance and biomass saccharification. The DEP1 is ubiquitously expressed with a relatively higher expression level in tissues rich in cell walls. The CRISPR/Cas9 editing mutants of DEP1 (dep1-cs) displayed a significant enhancement in stem mechanical properties relative to the wild-type, leading to a substantial improvement in lodging resistance. Cell wall analyses showed that the DEP1 mutation increased the contents of cellulose, hemicelluloses, and pectin, and reduced lignin content and cellulose crystallinity (CrI). Additionally, the dep1-cs seedlings exhibited higher sensitivity to cellulose biosynthesis inhibitors, 2,6-Dichlorobenzonitrile (DCB) and isoxaben, compared with the wild-type, confirming the role of DEP1 in cellulose deposition. Moreover, the DEP1 mutation-mediated alterations of cell walls lead to increased enzymatic saccharification of biomass after the alkali pretreatment. Furthermore, the comparative transcriptome analysis revealed that the DEP1 mutation substantially altered expression of genes involved in carbohydrate metabolism, and cell wall biosynthesis.
Conclusions
Our findings revealed the roles of DEP1 in cell wall biosynthesis, lodging resistance, and biomass saccharification in rice and suggested genetic modification of DEP1 as a potential strategy to develop energy rice varieties with high lodging resistance.
Similar content being viewed by others
Background
G protein (Guanine-nucleotide-binding protein) complex is a signal transduction molecule, consisting of Gα, Gβ and Gγ subunits, which widely exists on the plasma membrane of eukaryotic cells and plays important functions in various biological activities (Pandey 2019). Although the G protein signaling pathways in mammals are one of the best-understood pathways in the world, the underlying mechanisms related to G protein signaling in plants, especially in cell wall biosynthesis, have not been studied in depth. DEP1 (Dense and Erect Panicles 1) encodes a Gγ subunit in rice, and its gain-of-function mutation, dep1, plays a critical role in super rice breeding in Northern China (Huang et al. 2009; Botella 2012; Xu et al. 2016). The disruption of DEP1 has multiple positive effects on rice growth and development, including improvements in nitrogen utilization efficiency, panicle development, stress resistance, and lodging resistance (Huang et al. 2009; Sun et al. 2014; Xu et al. 2016; Zhao et al. 2016; Li et al. 2019; Cui et al. 2020). However, the mechanism underlying the positive effects of dep1 on those traits remains largely unknown.
Plant cells are surrounded by cell walls that provide mechanical strength for plant upright growth, determine cell shape, and protect plant cells against various environmental stresses (Zhang et al. 2021). The plant cell wall is mainly composed of polysaccharides, lignin, as well as minor wall proteins. Cellulose, a major wall polysaccharide, constitutes the load-bearing skeleton of cell walls and provides flexibility and mechanical strength for plants (Somerville et al., 2004). Cellulose is biosynthesized by the cellulase synthase (CESA) complex at the plasma membrane (Lampugnani et al. 2019). Hemicellulose is the most abundant amorphous polysaccharide in plant cell walls and plays an important role in cell wall formation by interacting with cellulose and lignin (Hatfield et al. 2017). Xylans are the principal hemicellulosic polysaccharides, which consist of a linear β-(1,4)-linked xylosyl residue chain substituted with diverse glycosyl residues (Zhong et al. 2019). Lignin, an amorphous polymer composed of phenylpropane units connected by carbon-carbon bonds and ether bonds, is primarily deposited in the secondary cell wall and provides plant stiffness (Vanholme et al. 2019). The synthesis and polymerization of lignin are required for over 90 genes derived from 10 super-families (Raes et al. 2003; Yoon et al. 2015).
Stem lodging resistance is an important and comprehensive agronomic trait that directly determines crop grain yield and quality. Stem lodging resistance is closely associated with breaking force of basal stem internode, fresh weight, and plant height (Li et al. 2015). The Green Revolution program aims to improve crop lodging resistance by breeding semi-dwarf varieties with reducing plant height. However, dwarfing breeding limits the further increase of crop grain yield. Hence, breeders have made efforts to breed rice varieties with high lodging resistance by improving mechanical strength without compromising on plant height. Since cell wall composition and structure fundamentally determine plant mechanical strength as well as biomass enzymatic saccharification, the genetic modification of cell walls is suggested as a potentially promising strategy to simultaneously improve crop lodging resistance and biomass saccharification.
Few studies have been conducted to investigate the connection between G protein and cell wall biosynthesis in Arabidopsis. Transcriptome analysis showed that differentially expressed genes in Gγ and Gβ mutants are highly enriched in cell wall biosynthesis and modification processes (Delgado-Cerezo et al. 2012). Klopffleisch et al. (2011) demonstrated that cell wall xylose metabolism-associated enzymes could interact with G protein detected by the yeast two-hybrid technology. Mutations in Gγ and Gβ lead to decreased xylose content in Arabidopsis (Klopffleisch et al. 2011; Delgado-Cerezo et al. 2012), whereas knockout of both Gɑ and Gβ results in an increase in the xylose proportion in cell walls (Klopffleisch et al. 2011). In addition, a 7TM (seven-transmembrane domain) protein, annotated as a putative GPCR (G protein-coupled receptor) response for regulation of G protein signaling, was characterized to be required for cellulose biosynthesis in Arabidopsis, particularly during cell wall stress (McFarlane et al. 2021). However, the roles of G protein signaling in cell wall production in grass remains elusive.
Although much progress has been made in the study of G protein signaling in mammals, the underlying mechanisms associated with G protein γ subunits in plants, especially in cell wall biosynthesis, have yet to be explored. In this study, we investigated the effects of DEP1 on cell wall biosynthesis, lodging resistance, and biomass saccharification in rice by characterizing DEP1 transgenic plants generated by the CRISPR/Cas9 approach. Our findings reveal that the DEP1 mutation alters cell wall composition and structure in rice, leading to large improvements in stem lodging resistance and biomass enzymatic saccharification. This study also suggests DEP1 as an elite gene resource for improving cell wall-relevant agronomic traits in rice.
Methods
Generation of Transgenic Rice and Growth Conditions
The DEP1 gene editing transgenic lines (dep1-cs) were developed by using the CRISPR/Cas9 approach, single-guide RNA for generation of dep1-cs1/2 (GCGCGAGATCACGTTCCTCA) and dep1-cs3/4 (GGGGGCTAGAGCAGTTGCAC) lines were designed with the web-based tool CRISPR-PLANT (http://omap.org/crispr/CRISPRsearch.html) and was respectively ligated to the pRGEB32 vector. The constructed plasmids were transferred into wild-type rice (Sasanishiki) using the Agrobacterium-mediated transformation method. Positive transgenic plants obtained from CRISPR/Cas9 editing were identified by examination of hygromycin and Cas9 genes through PCR technology. Then, the different variations of positive transgenic lines were sequenced. The homozygous transgenic lines of T2 generation were used for further analysis. All rice plants used in this study were grown in a paddy field of Shenyang Agricultural University (Shenyang, China) in natural growing seasons.
Gene Expression Analysis by Using the Real-Time Quantitative PCR (Q-PCR)
Total RNA extraction from fresh rice tissues and cDNA preparation were performed as described previously (Li et al. 2015). The primers used in this study for Q-PCR analysis are listed in Table S1.
Determinations of Agronomic Traits and Stem Properties
The plant height, grain number per panicle, panicle length, tiller number, setting rate, 1000-grain weight, grain yield, and dry biomass were determined at the mature stage. Dry biomass, including stem, leaves, and leaf sheath, was weighed after the samples were dried to constant weight in an oven at 55℃. The stem properties, including lodging index, breaking force, puncture force, extension force, stem wall thickness, and diameter of stem internode, were examined 25 days after flowering. The mechanical characteristics of the stem were detected using a digital force/length tester (RH-K300). The stem lodging index was calculated as previously described (Miao et al. 2023).
Analyses of Cell Wall Composition and Cellulose Crystallinity Index (CrI)
The rice stems were collected 25 days after flowering. The cell wall fractionation and quantification were performed as previously described (Dang et al. 2023) with minor modifications. In brief, the dry rice sample was ground into powder through a 60-mesh screen (0.25 mm × 0.25 mm) and then, respectively, removed soluble sugars, lipids, and starch by potassium phosphate buffer (pH 7.0), chloroform-methanol (1:1, v/v), and Dimethyl sulfoxide (DMSO) - water (9:1, v/v) step by step. After centrifugation at 3000 g for 5 min, the pellet was then washed twice with 75% (v/v) ethanol, and the residual pellet was dried to constant weight in an oven at 55℃ and was referred to as the alcohol insoluble residue (AIR). The pectin was then extracted from the AIR with the solution of ammonium oxalate (0.5% w/v) in a boiling water bath for 1 h. Following centrifugation, the hemicellulose was further extracted from the pellet with 4 N KOH (containing 1% NaBH4) for 1 h. After washed to be neutral with distilled water, the pellet, defined as crude cellulose, was dissolved in 72% H2SO4 (w/v). The hexose and pentose were determined as previously described (Li et al. 2013). The lignin content was determined as previously described (Li et al. 2014). The sugar composition of AIR was examined using GC-MS (7890 A-240; Agilent Technologies) as previously reported (Tang et al. 2022). All experiments were carried out in biological triplicate.
For the cellulose CrI assay, the crude cellulose obtained from cell wall fraction as described above was dried to constant weight and was used to examine cellulose CrI with the X-ray diffraction (Rigaku-D/MAX instrument, Ultima III; Japan) method as reported previously (Li et al. 2015).
Immunochemical Analysis of Cell Wall Sugars
The immunochemical analyses of hemicellulosic xylans and pectin polysaccharides were carried out with antibodies LM10, LM11, and JIM7 as previously reported by Li et al. (2018)
Analyses of Biomass Saccharification
The biomass enzymatic saccharification was quantified by calculating sugars released from mix-cellulases hydrolysis of biomass pretreated with 1% NaOH or 1% H2SO4, as previously described (Huang et al. 2012).
For observation of chemical-treated biomass residues in vitro, the biomass powder sample was treated with 1% NaOH for 2 h at 200 r/min in a shaking table at 50℃. After centrifugation at 3000 g for 5 min, the residual pellet was washed to be neutral with distilled water and dried to constant weight. The surface morphology of the dried sample was observed by scanning electron microscope observation (TM1000, Hitachi Ltd, Tokyo, Japan). All observations were conducted with three biological replicates, and each was observed three to five times, and a representative image was used in this study.
Cellulose Biosynthesis Inhibitor Treatments
The germinated rice seeds were grown under normal hydroponic culture. After four days, half of the seedlings of wild-type and dep1-cs were treated with 500 nM isoxaben or 1500 nM 2,6-Dichlorobenzonitrile (DCB) (both of those were dissolved in methanol) for four days, and the remaining seedlings were maintained in hydroponic culture supplemented with 0.03% methanol as a control. The length of roots of wild-type and dep1-cs plants was calculated to reflect the sensitivity of the DEP1 mutation to cellulose biosynthesis inhibitors.
RNA Sequencing Analysis
Total RNA was extracted from the fresh tissues of the second stem internode of wild-type and dep1-cs plants at the booting stage. RNA quality check, the next-generation sequencing, and the subsequent differentially expressed gene (DEG) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were carried out exactly as previously described by Ruan et al. (2022).
Statistical Analysis
Data were presented as the mean ± SDs and numbers (n) of biological replicates were described in figure legends. Significance levels were tested using Student’s t-test and one-way ANOVA followed by a post-hoc test in SPSS Statistics software v.19.0. P values are indicated or labeled with different letters.
Results
DEP1 is Highly Expressed in the Tissues Undergoing Cell Wall Biosynthesis
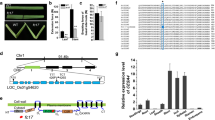
Previous studies demonstrated the important role of DEP1 in rice grain yield and nitrogen utilization (Huang et al. 2009; Sun et al. 2014). To further investigate whether DEP1 is involved in cell wall biosynthesis, we examined the expression pattern of DEP1 in various tissues and organs during rice growth. The DEP1 showed the highest expression during the panicle development process (Fig. 1A), which corresponds with its well-documented role in panicle development (Huang et al. 2009). Notably, the DEP1 gene is also highly expressed in the stem, which is enriched in cell walls. By contrast, the expression level of DEP1 was lower in leaves, calli, radicle, young root, and endosperm tissues, which contain lower levels of cell walls (Fig. 1A). These data implied that the DEP1 is closely associated with cell wall biosynthesis in rice. To investigate the potential function of DEP1 in cell wall biosynthesis, we examined the expression pattern of DEP1 during rice stem internode development, which has been established as a model for studying cell wall synthesis (Ruan et al. 2022). The stem internode of wild-type at the heading stage was divided into three sections (lower, middle, and upper, counting from bottom to the top), which represents the transition from the primary wall to the secondary wall (Zhang et al. 2017). The lower section contains cells undergoing elongation and the formation of primary and secondary cell walls, the middle section is in the state of rapid biosynthesis of secondary walls, and the cells in the upper section cease elongating and complete the secondary wall thicken (Zhang et al. 2017). Q-PCR analysis revealed that the DEP1 is highly expressed in all three sections with a relatively higher expression level in the lower and middle sections (Fig. 1B). By comparison, the CESA4 gene required for the secondary wall cellulose biosynthesis exhibited the highest expression level in the third section undergoing the secondary cell wall deposition (Fig. 1B). Collectively, these results suggest that DEP1 probably contributes to cell wall biosynthesis.
Generation of DEP1 Knockout Mutants by the CRISPR/Cas9 System
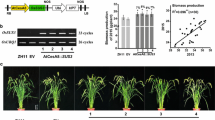
To genetically investigate the biological function of DEP1, four independent gene editing transgenic lines, dep1-cs1, dep1-cs2, dep1-cs3, and dep1-cs4, were generated on a japonica variety, Sasanishiki (wild-type) background by using the CRISPR/Cas9 system. The dep1-cs1 and dep1-cs2, had one base pair insertion in the first exon, leading to a premature stop codon and causing a very short truncated protein; while the dep1-cs3 and dep1-cs4 showed one base pair insertion in the cysteine-rich region of the fifth exon at the C-terminus, resulting in the loss of the cysteine‐rich domain (Fig. 2A; Fig. S1). Compared to wild-type plants, all dep1-cs lines showed a significant reduction in plant height and panicle length (Fig. 2B-E), consistent with previous reports (Li et al. 2019). The dep1-cs3 and dep1-cs4 plants, containing a truncated DEP1 protein without cysteine-rich region, displayed a shorter plant height than dep1-cs1 and dep1-cs2 lines containing completely knockout DEP1 (Fig. 2B, D). While panicle length and setting rate were significantly decreased without difference in 1000-grain weight (Fig. 2E-G), the grain number per panicle was significantly higher in dep1-cs lines relative to wild-type plants (Fig. 2H), resulting in a similar grain yield per plant (Fig. 2I). Additionally, tiller number was increased in the dep1-cs lines compared to wild-type plants (Fig. S2A), whereas no substantial differences observed in biomass production (Fig. S2B).
Phenotypes and agronomic traits of wild-type (WT) and DEP1 transgenic lines (dep1-cs) generated by the CRISPR/Cas9 system. A Generation of dep1-cs mutants. B, C Phenotypes (B) and panicle performances (C) of WT and dep1-cs plants. D-I Agronomic traits of WT and dep1-cs plants, including plant height (D), panicle length (E), setting rate (F), 1000-grain weight (G), grain number per panicle (H), and grain yield per plant (I). Values are means, error bars are SD; n = 12 biological replicates; Significant differences are indicated by different letters (P < 0.05)
The DEP1 Mutation Increases Stem Mechanical Strength for High Lodging Resistance
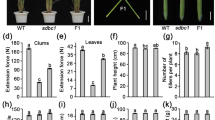
Notably, compared to the wild-type, the dep1-cs mutants showed a substantial decrease in the lodging resistance index, a parameter negatively correlated to plant lodging resistance ability (Fig. 3A). As plant lodging resistance is negatively determined by plant height and fresh weight and is positively determined by basal stem breaking force (Liu et al. 2018), we further compared the mechanical properties of dep1-cs lines with wild-type plants. As a result, the dep1-cs lines showed a significant increase in multiple mechanical properties of stem internode, including breaking force (Fig. 3B), puncture force (Fig. 3C), and extension force (Fig. 3D). Additionally, the stem wall thickness of basal internodes in dep1-cs lines was significantly increased compared to that in the wild-type plants, whereas no obvious alteration was observed in the diameter of basal stem internode (Fig. 3E, F). Hence, the substantially increased mechanical strength caused by the DEP1 mutation leads to a significant improvement in lodging resistance in dep1-cs lines.
Measurement of lodging index and mechanical strength associated properties of wild-type (WT) and dep1-cs plants. A Stem lodging index. B Breaking force, (C) puncture force, and (D) extension force of stem internode. E Stem wall thickness. F Diameter of stem internode. Values are means, error bars are SD; n = 12 biological replicates; Significant differences are indicated by different letters (P < 0.05)
DEP1 Affects Cell Wall Structure and the Content of Wall Polymers
The significant enhancement of stem lodging resistance and stem wall thickness by the DEP1 mutation promotes us to investigate the effect of DEP1 on cell wall composition and structure, which largely determines plant mechanical strength. The cross-sections of the second internodes (counting from the top) were harvested from wild-type and dep1-cs plants at the heading stage and were visualized by the SEM. Except for thicker stem walls, there was no significant change in the vascular structure of the stem in the dep1-cs lines relative to wild-type plants (Fig. 4A). Furthermore, we performed cell wall polymer extraction and analysis using the mature stem tissue harvested from the wild-type and dep1-cs plants. The cellulose, hemicellulose, and pectin content in the dep1-cs lines was significantly increased (30%, 26%, and 70%, respectively), while a 24% reduction was observed in the lignin level compared with the wild-type plants (Fig. 4B). Taken together, these data suggested that DEP1 is highly associated with the biosynthesis of cell wall polymers in rice.
Morphology of xylem cell walls and determination of cell wall polymers of wild-type (WT) and dep1-cs plants. A Morphology of xylem cell walls of WT and dep1-cs plants. B Determination of cell wall polymers of WT and dep1-cs plants. **P < 0.01. Student’s t-test, values are means, error bars are SD, n = 3 biological replicates
The DEP1 Mutation Affects Sugar Composition and Cellulose Structure
Since the DEP1 plays a role in the content of cell wall polymers, we further investigated whether the DEP1 mutation also affected sugar composition and cellulose features. The GC-MS technology was used to compare the monosaccharide composition of cell walls of mature stem tissues harvested from wild-type and dep1-cs plants. Compared to the wild-type plants, the dep1-cs lines showed a considerable increase in glucose, xylose, and arabinose, consistent with their increased content of cellulose and hemicellulose (Fig. 5A). In addition, the content of GlcA and GalA, two primarily monosaccharides of pectin, in the dep1-cs lines was also significantly increased (Fig. 5A). To confirm the alterations of sugar composition in dep1-cs lines, we performed immunochemical analysis of transverse sections of culm internodes labeled with antibodies LM11 and LM10 binding to xylan, and JIM7 directed to homogalacturonan. As a result, the fluorescent signals of all antibodies LM10, LM11, and JIM7 in the dep1-cs internodes were stronger than those in the wild-type plants (Fig. 5B), confirming the alterations of polysaccharides in the dep1-cs lines examined by the cell wall polymer fractionation and analysis. Moreover, we analyzed the CrI of cellulose extracted from mature stems of the wild-type and dep1-cs plants. The cellulose CrI of the dep1-cs plants was decreased by approximately 11% compared with that of the wild-type plants (Fig. 5C). Taken together, these results revealed an important role of DEP1 in the biosynthesis of polysaccharides.
Sugar composition and cellulose structural feature of wild-type (WT) and dep1-cs plants. A Monosaccharide composition of cell wall carbohydrates for WT and dep1-cs plants. AIR, alcohol-insoluble residues. *P < 0.05, **P < 0.01. Student’s t-test, values are means, error bars are SD, n = 3 biological replicates. B Immunochemical staining of cross-sections of WT and dep1-cs plant stem internodes. Representative micrographs of equivalent sections of mature culm internodes immunolabeled with antibodies directed to xylan (LM11 and LM10) and homogalacturonan (JIM7). PC, parenchyma cells; SC, sclerenchyma cells; VB, vascular bundle cells. C Determination of lignocellulose CrI of mature stems using the X-ray diffraction method
To further confirm the association of DEP1 with cellulose biosynthesis, we treated the three-day-old dep1-cs and wild-type seedlings with isoxaben and 2,6-Dichlorobenzonitrile (DCB), two cellulose biosynthesis inhibitors, for six days. Under unstressed conditions, there was no significant alteration in the root length between wild-type and dep1-cs plants (Fig. 6). By contrast, the dep1-cs lines displayed an increased sensitivity to both isoxaben and DCB. In detail, compared to wild-type plants, the root length was reduced by up to approximately 14% and 31% in dep1-cs seedlings exposed to isoxaben and DCB, respectively (Fig. 6). These data suggest an important role of DEP1 in cellulose biosynthesis, particularly, during cell wall stress.
The dep1-cs plants display increased sensitivity to cellulose biosynthesis inhibitors. A Representative images of five-day-old rice seedlings grown under hydroponic culture supplemented with 500 nM isoxaben or 1500 nM 2,6-Dichlorobenzonitrile (DCB). B Quantification of root lengths of seedlings represented in (A). n = 3 biological replicates, each comprising 6 plants, *P < 0.05, **P < 0.01, Student’s t-test
The DEP1 Mutation Leads to an Enhancement in Biomass Saccharification
The altered cell wall composition and reduced cellulose CrI promote us to investigate the biomass enzymatic saccharification of the dep1-cs mutants. We used two chemical reagents, alkali (1% NaOH) or acid (1% H2SO4), to pretreat the powders of the mature straw. We then compared the biomass saccharification by measuring hexoses released from mix-cellulases hydrolysis of pretreated mature straw. After NaOH pretreatment, both the dep1-cs lines displayed a significantly higher biomass enzymatic hydrolysis efficiency than the wild-type (Fig. 7A). By contrast, there was no obvious change in hexose yield released from enzymatic hydrolysis of biomass after H2SO4 pretreatment (Fig. 7B).
To explain the improved saccharification of the NaOH-pretreated biomass in the dep1-cs lines, we used SEM to scan the surface morphology of biomass residues under the control conditions or 1% NaOH treatment. There was no discernable difference in biomass residue surfaces without pretreatment between wild-type and dep1-cs plants (Fig. 7C). Notably, rougher surface of the NaOH-pretreated biomass residue in dep1-cs mutants was observed as compared with that in the wild-type (Fig. 7C). These data indicate that the DEP1 mutation produces a rougher surface of biomass residue under the alkali treatment, which increases the enzyme accessibility to lignocellulose for higher biomass saccharification in dep1-cs mutants.
The DEP1 Mutation Results in Substantial Transcriptional Changes in Cell Wall Biosynthesis-Related Genes
The RNA-Seq technology was used to compare transcriptional profiling of the stems of wild-type and dep1-cs3 plants at the heading stage. A total of 9955 DEGs (Differentially Expressed Genes) (P < 0.05, fold change ≥ 1.5) were found in the dep1-cs3 mutant compared to the wild-type, of which 5057 genes were upregulated and 4898 genes were downregulated (Fig. S3), corresponding to the involvements of DEP1 in many biological processes. Moreover, the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that the DEGs participate in up to 268 pathways, of which metabolisms of carbohydrates, amino acids, lipids, nucleotides, and vitamins were substantially affected (Fig. 8A). Among the carbohydrate metabolism-related DEGs, many genes have been recognized or annotated to be involved in cell wall polysaccharide formation. We further performed a Q-PCR analysis to examine the influences of the DEP1 mutation on the expression of genes involved in cell wall polymer biosynthesis and modification. The expression levels of CESA4/7/9 required for secondary wall cellulose biosynthesis in rice were significantly increased in the dep1-cs3 mutant, consistent with its higher cellulose content compared to wild-type plants (Fig. 8B). With regard to hemicellulose biosynthesis, we examined the expression of genes contributing to the elongation of the xylan backbone (IRX9, IRX10, IRX14) (Chiniquy et al. 2013; Dang et al. 2023), and the addition of sidechain (XAT2, XAT3) (Anders et al. 2012). All these genes were upregulated in the mutant, consistent with the increased contents of hemicellulose, arabinose, and xylose in dep1-cs lines (Fig. 8B). Based on the reduced lignin level in the mutant, we further analyzed the transcriptional level of lignin metabolism-related genes. The PAL6, PAL8, 4CL3, CCR10, and CAD7 genes were downregulated in the mutant, whereas the CAD2 and CAld5H1 were upregulated, implying a regulatory role of DEP1 in the biosynthesis of lignin monomers (Fig. 8B).
Transcriptome profile of stem internodes in wild-type (WT) and dep1-cs plants. A KEGG enrichment analysis was performed to identify the altered pathways of differentially expressed genes based on RNA-sequencing analysis. B The relative expression levels of genes involved in cell wall biosynthesis in WT and dep1-cs plants quantified by Q-PCR. *P < 0.05, **P < 0.01. Student’s t-test, values are means, error bars are SD, n = 3 biological replicates
Discussion
As a hub of signal reception and transfer, the G protein signaling is involved in the regulation of almost all biological processes in plants, including cell division, defense to stresses, switch of ion channels, perception of sugar signals, and various plant hormone signaling processes (Urano et al. 2013). However, the effect of G protein signaling on cell wall production, which fundamentally determines plant mechanical strength and biomass saccharification, is still unknown. In this work, we examined the roles of a G protein γ subunit, DEP1, in cell wall biosynthesis and cell wall-related traits in rice. We propose that the DEP1 mutation leads to cell wall remodeling by affecting expression levels of cell wall biosynthesis-related genes, which enhances biomass enzymatic saccharification and improves stem mechanical strength for high lodging resistance (Fig. 9).
DEP1 Plays a Role in Cell Wall Remodeling in Rice
In this study, we demonstrated that DEP1 functions in cell wall remodeling. The DEP1 gene is highly expressed in cell wall-rich tissues and the DEP1 mutation leads to altered expression levels of genes involved in cell wall biosynthesis. Similarly, a previous report revealed that the mutation in Gβ or Gγ leads to similar transcriptomic alterations, which are enriched in cell wall biosynthesis-related genes (Delgado-Cerezo et al. 2012). These data suggest that DEP1 is involved in cell wall production at the transcriptional level. Moreover, the dep1-cs lines showed a significant increase in sugar contents, including cellulose, hemicellulose, pectin, xylose, arabinose, and glucose, and a decrease in lignin, confirming the significant role of DEP1 in cell wall production. Consistently, the mutations in Arabidopsis G proteins result in altered contents of xylose and xylan (Klopffleisch et al. 2011; Delgado-Cerezo et al. 2012). Additionally, the dep1-cs lines exhibited more sensitivity to cellulose biosynthesis inhibitors, DCB, and isoxaben, relative to wild-type plants. A similar effect of G protein signaling on the regulation of cellulose synthesis was also observed in Arabidopsis (McFarlane et al. 2021). The mutation in the 7tm protein, interacting with the G protein complex, affects cellulose synthase complex (CSC) trafficking and thus impairs cellulose biosynthesis, particularly during cell wall stress (McFarlane et al. 2021). Furthermore, the DEP1 mutation also results in a significantly reduced cellulose CrI, an important structural parameter of cellulose. Hence, our findings demonstrate that DEP1 is involved in the biosynthesis and modification of cell wall polymers in rice.
The DEP1 Mutation Enhances Stem Mechanical Strength for Improved Lodging Resistance
Although positive roles of dep1 on rice growth performances in terms of panicle development, nitrogen utilization efficiency, and stress tolerance have been widely reported (Huang et al. 2009; Sun et al. 2014; Xu et al. 2016; Zhao et al. 2016; Li et al. 2019; Zhang et al. 2019; Cui et al. 2020), the underlying mechanisms connecting DEP1 with those traits remain largely unknown. In this study, we confirmed the negative role of DEP1 in stem lodging resistance by performing a genetic analysis. Notably, in addition to the reduction of plant height, the significantly increased mechanical strength caused by the DEP1 mutation-mediated reinforcement of cell walls should be another essential contributor to improved stem lodging resistance in dep1-cs plants. As the cell wall functions as a determinant of mechanical strength, the substantial increase in wall polysaccharides of dep1-cs lines enhances the breaking force for high lodging resistance. Taken together, the DEP1 mutation increases the contents of cell wall polymers, which reinforces the cell walls to enhance mechanical strength for a large improvement in stem lodging resistance.
The DEP1 Mutation Significantly Increases the Enzymatic Hydrolysis of Biomass After Alkali Pretreatment
It is well known that cell wall composition and structural feathers of wall polymers play essential roles in biomass saccharification. We found that the DEP1 mutation leads to a significant increase in biomass enzymatic hydrolysis under alkali pretreatment. The increased biomass saccharification in dep1-cs lines was supported by their altered cell walls. First, the DEP1 mutation substantially increases the contents of polysaccharides, especially cellulose, which provides more substrates for enzymatic hydrolysis reaction after pretreatment. Secondly, the reduction of cellulose CrI, a negative factor for biomass enzymatic hydrolysis, in dep1-cs mutants also contributes to the improvement of biomass saccharification by increasing cellulase accessibility to alkali-pretreated biomass.
Interestingly, the alkali pretreatment increased the yield of hexose from the dep1-cs biomass via enzymatic saccharification compared with that from the wild-type plants, whereas the sulfuric acid pretreatment did not. Inorganic alkali and acid treatments are two efficient pretreatment methods for biomass conversion, and they reduce the recalcitrance of lignocellulose, increasing the accessibility of cellulases with distinct mechanisms (Abraham et al. 2020). The acid treatment could dissociate the strong chemical bonds and partially release lignin monomers, hemicellulosic monosaccharides, and amorphous cellulose (du Pasquier et al. 2023). By comparison, alkali treatment could destroy cell wall polymers by eliminating crosslinked hemicellulose and lignin and decreasing cellulose CrI (Lee et al. 2015). As the dep1-cs plants contain a lower lignin content and a higher level of cellulose, the alkali treatment might further increase the proportion of amorphous cellulose and decrease cellulose CrI, resulting in significantly enhanced lignocellulose saccharification in mutants compared to wild-type plants. In contrast, the acid treatment might partially remove amorphous cellulose and lignin, reducing the advantage of lower cellulose CrI and lignin content for high biomass saccharification in dep1-cs plants, resulting in similar hexose yield from enzymatic hydrolysis of acid-treated biomass of dep1-cs compared to wild-type plants.
Conclusions
Our findings show that the DEP1 mutation improves both stem lodging resistance and biomass saccharification by remodeling cell wall deposition, including increased levels of cellulose, hemicellulose, and pectin, and reduced lignin content and cellulose CrI. This study provides a promising gene resource, DEP1, for genetic modification of cell walls to breed robust rice varieties with high straw biomass enzymatic saccharification.
Data Availability
No datasets were generated or analysed during the current study.
References
Abraham A, Mathew AK, Park H, Choi O, Sindhu R, Parameswaran B, Pandey A, Park JH, Sang BI (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725
Anders N, Wilkinson MD, Lovegrove A, Freeman J, Tryfona T, Pellny TK, Weimar T, Mortimer JC, Stott K, Baker JM, Defoin-Platel M, Shewry PR, Dupree P, Mitchell RA (2012) Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci USA 109(3):989–993
Botella JR (2012) Can heterotrimeric G proteins help to feed the world? Trends Plant Sci 17(10):563–568
Chiniquy D, Varanasi P, Oh T, Harholt J, Katnelson J, Singh S, Auer M, Simmons B, Adams PD, Scheller HV, Ronald PC (2013) Three novel rice genes closely related to the Arabidopsis IRX9, IRX9L, and IRX14 genes and their roles in xylan biosynthesis. Front Plant Sci 4:83
Cui Y, Jiang N, Xu Z, Xu Q (2020) Heterotrimeric G protein are involved in the regulation of multiple agronomic traits and stress tolerance in rice. BMC Plant Biol 20(1):90
Dang Z, Wang Y, Wang M, Cao L, Ruan N, Huang Y, Li F, Xu Q, Chen W (2023) The Fragile culm19 (FC19) mutation largely improves plant lodging resistance, biomass saccharification, and cadmium resistance by remodeling cell walls in rice. J Hazard Mater 15:458:132020
Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jordá L, Hernández-Blanco C, Sánchez-Vallet A, Bednarek P, Schulze-Lefert P, Somerville S, Estevez JM, Persson S, Molina A (2012) Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant 5(1):98–114
du Pasquier J, Paës G, Perré P (2023) Principal factors affecting the yield of dilute acid pretreatment of lignocellulosic biomass: a critical review. Bioresour Technol 369:128439
Hatfield RD, Rancour DM, Marita JM (2017) Grass cell walls: a story of cross-linking. Front Plant Sci 7:2056
Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41(4):494–497
Huang J, Xia T, Li, Yu B, Li Q, Tu Y, Zhang W, Yi Z, Peng L (2012) A rapid and consistent near infrared spectroscopic assay for biomass enzymatic digestibility upon various physical and chemical pretreatments in Miscanthus. Bioresour Technol 121:274–281
Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, Botella JR, Carpita NC, Carr T, Chen JG, Cooke TR, Frick-Cheng A, Friedman EJ, Fulk B, Hahn MG, Jiang K, Jorda L, Kruppe L, Liu C, Lorek J, McCann MC, Molina A, Moriyama EN, Mukhtar MS, Mudgil Y, Pattathil S, Schwarz J, Seta S, Tan M, Temp U, Trusov Y, Urano D, Welter B, Yang J, Panstruga R, Uhrig JF, Jones AM (2011) Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol 27:7
Lampugnani ER, Flores-Sandoval E, Tan QW, Mutwil M, Bowman JL, Persson S (2019) Cellulose synthesis - central components and their evolutionary relationships. Trends Plant Sci 24(5):402–412
Lee JW, Kim JY, Jang HM, Lee MW, Park JM (2015) Sequential dilute acid and alkali pretreatment of corn stover: sugar recovery efficiency and structural characterization. Bioresour Technol 182:296–301
Li F, Ren S, Zhang W, Xu Z, Xie G, Chen Y, Tu Y, Li Q, Zhou S, Li Y, Tu F, Liu L, Wang Y, Jiang J, Qin J, Li S, Li Q, Jing HC, Zhou F, Gutterson N, Peng L (2013) Arabinose substitution degree in xylan positively affects lignocellulose enzymatic digestibility after various NaOH/H2SO4 pretreatments in Miscanthus. Bioresour Technol 130:629–637
Li M, Si S, Hao B, Zha Y, Wan C, Hong S, Kang Y, Jia J, Zhang J, Li M, Zhao C, Tu Y, Zhou S, Peng L (2014) Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus. Bioresour Technol 169:447–454
Li F, Zhang M, Guo K, Hu Z, Zhang R, Feng Y, Yi X, Zou W, Wang L, Wu C, Tian J, Lu T, Xie G, Peng L (2015) High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol J 13(4):514–525
Li F, Liu S, Xu H, Xu Q (2018) A novel FC17/CESA4 mutation causes increased biomass saccharification and lodging resistance by remodeling cell wall in rice. Biotechnol Biofuels 11:298
Li X, Tao Q, Miao J, Yang Z, Gu M, Liang G, Zhou Y (2019) Evaluation of differential qPE9-1/DEP1 protein domains in rice grain length and weight variation. Rice 31(1):5
Liu S, Huang Y, Xu H, Zhao M, Xu Q, Li F (2018) Genetic enhancement of lodging resistance in rice due to the key cell wall polymer lignin, which affects stem characteristics. Breed Sci 68(5):508–515
McFarlane HE, Mutwil-Anderwald D, Verbančič J, Picard KL, Gookin TE, Froehlich A, Chakravorty D, Trindade LM, Alonso JM, Assmann SM, Persson S (2021) A G protein-coupled receptor-like module regulates cellulose synthase secretion from the endomembrane system in Arabidopsis. Dev Cell 56(10):1484–1497e7
Miao W, Li F, Lu J, Wang D, Chen M, Tang L, Xu Z, Chen W (2023) Biochar application enhanced rice biomass production and lodging resistance via promoting co-deposition of silica with hemicellulose and lignin. Sci Total Environ 855:158818
Pandey S (2019) Heterotrimeric G-protein signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 70:213–238
Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W (2003) Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol 133(3):1051–1071
Ruan N, Dang Z, Wang M, Cao L, Wang Y, Liu S, Tang Y, Huang Y, Zhang Q, Xu Q, Chen W, Li F (2022) Fragile culm 18 encodes a UDP-glucuronic acid decarboxylase required for xylan biosynthesis and plant growth in rice. J Exp Bot 73(8):2320–2335
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 24;306(5705):2206–2211
Sun H, Qian Q, Wu K, Luo J, Wang S, Zhang C, Ma Y, Liu Q, Huang X, Yuan Q, Han R, Zhao M, Dong G, Guo L, Zhu X, Gou Z, Wang W, Wu Y, Lin H, Fu X (2014) Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat Genet 46(6):652–656
Tang Y, Wang M, Cao L, Dang Z, Ruan N, Wang Y, Huang Y, Wu J, Zhang M, Xu Z, Chen W, Li F, Xu Q (2022) OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ 45(8):2492–2507
Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signalling in the plant kingdom. Open Biol 3(3):120186
Vanholme R, De Meester B, Ralph J, Boerjan W (2019) Lignin biosynthesis and its integration into metabolism. Curr Opin Biotechnol 56:230–239
Xu H, Zhao M, Zhang Q, Xu Z, Xu Q (2016) The DENSE AND ERECT PANICLE 1 (DEP1) gene offering the potential in the breeding of high-yielding rice. Breed Sci 66(5):659–667
Yoon J, Choi H, An G (2015) Roles of lignin biosynthesis and regulatory genes in plant development. J Integr Plant Biol 57(11):902–912
Zhang B, Zhang L, Li F, Zhang D, Liu X, Wang H, Xu Z, Chu C, Zhou Y (2017) Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat Plants 3:17017
Zhang D, Zhang M, Zhou Y, Wang Y, Shen J, Chen H, Zhang L, Lü B, Liang G, Liang J (2019) The rice G protein γ subunit DEP1/qPE9-1 positively regulates grain-filling process by increasing auxin and cytokinin content in rice grains. Rice 16(121):91
Zhang B, Gao Y, Zhang L, Zhou Y (2021) The plant cell wall: biosynthesis, construction, and functions. J Integr Plant Biol 63(1):251–272
Zhao M, Sun J, Xiao Z, Cheng F, Xu H, Tang L, Chen W, Xu Z, Xu Q (2016) Variations in Dense and erect panicle 1 (DEP1) contribute to the diversity of the panicle trait in high-yielding japonica rice varieties in northern China. Breed Sci 66(4):599–605
Zhong R, Cui D, Ye ZH (2019) Secondary cell wall biosynthesis. New Phytol 221:1703–1723
Acknowledgements
We would like to thank all colleagues at the Rice Research Institute of Shenyang Agricultural University for reading and participating in the discussions relating to the preparation of this manuscript.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (Grant 32272174).
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors are consent to the publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
12284_2024_712_MOESM1_ESM.pdf
Supplementary Material 1: Figure S1. Sequencing confirmation of the DEP1 gene in WT and dep1-cs transgenic plants generated by the CRISPR/Cas9 approach. Figure S2. Agronomic traits of wild-type (WT) and dep1-cs plants. A Tiller number per plant. B Dry biomass per plant. Values are means, error bars are SD; n = 12 biological replicates; Significant differences are indicated by different letters (P < 0.05). Figure S3. The visualization of differentially expressed genes (DEG) between wild-type and dep1-cs plants was performed by volcano plot, with the magnitude of the fold-change shown along the X-axis and the statistical significance (-log10 of q-value) shown on the Y-axis. Table S1. Primers used in this study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Wang, M., Yan, X. et al. The DEP1 Mutation Improves Stem Lodging Resistance and Biomass Saccharification by Affecting Cell Wall Biosynthesis in Rice. Rice 17, 35 (2024). https://doi.org/10.1186/s12284-024-00712-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-024-00712-0