Abstract
Heading date is an important agronomic trait of rice (Oryza sativa L.) and is regulated by numerous genes, some of which exhibit functional divergence in a genetic background-dependent manner. Here, we identified a late heading date 7 (lhd7) mutant that flowered later than wild-type Zhonghua 11 (ZH11) under natural long-day (NLD) conditions. Map-based cloning facilitated by the MutMap strategy revealed that LHD7 was on the same locus as OsPRR37 but exhibited a novel function as a promoter of heading date. A single-nucleotide mutation of G-to-A in the coding region caused a substitution of aspartic acid for glycine at site 159 within the pseudo-receiver (PR) domain of OsPRR37. Transcriptional analysis revealed that OsPRR37 suppressed Ghd7 expression in both ZH11 background under NLD conditions and the Zhenshan 97 background under natural short-day conditions. Consistently, the expression of Ehd1, Hd3a and RFT1 was enhanced by OsPRR37 in the ZH11 background. Genetic analysis indicated that the promotion of heading date and reduction in grain yield by OsPRR37 were partially dependent on Ghd7. Further investigation showed that the alternative function of OsPRR37 required an intact Ghd7-related regulatory pathway involving not only its upstream regulators OsGI and PhyB but also its interacting partner Hd1. Our study revealed the distinct role of OsPRR37 in the ZH11 background, which provides a more comprehensive understanding of OsPRR37 function and enriches the theoretical bases for improvement of rice heading date in the future.
Similar content being viewed by others
Background
The heading date (also known as flowering time) of rice is defined as the time from sowing date to the emergence of the first panicle. It largely determines the regional and seasonal adaptation of a specific variety. A complicated regulatory network of heading date in rice has been elucidated during the last two decades, among which Oryza sativa Pseudo-Response Regulator37 (OsPRR37), also known as Grain Number, Plant Height, and Heading Date7.1 (Ghd7.1)/Days to heading 7 (DTH7)/Heading date 2 (Hd2) (hereafter referred to as OsPRR37) is one of the most important components (Gao et al. 2014; Koo et al. 2013; Lin et al. 2000; Yan et al. 2013).
OsPRR37 encodes a pseudo-response regulator (PRR) protein that contains an N-terminal pseudo-receiver (PR) domain and a C-terminal CCT (CONSTANS, CO-like, and TOC1) domain. A previous study demonstrated that OsPRR37 delays heading date, increases plant height and enhances grain yield (Gao et al. 2014; Liu et al. 2015; Yan et al. 2013). Genetic diversity analysis revealed various non-functional variants including two frameshift mutations at 1515 bp and 1551 bp of the coding region between the PR and CCT domains, a gain of stop codon mutation at 2113 bp of the coding region in the CCT domain and a missense mutation at L710P in the CCT domain (Gao et al. 2014; Koo et al. 2013; Yan et al. 2013). In addition, other variants in the PR domain and the region between the PR and CCT domains were also detected, but their effects on the function of OsPRR37 were not demonstrated (Gao et al. 2014; Koo et al. 2013; Yan et al. 2013).
Natural variation analysis revealed that there are a total of 24 haplotypes of OsPRR37 among 178 cultivated rice varieties and 47 wild rice (O. rufipogon) accessions. Five functional haplotypes of OsPRR37 in cultivated varieties were also found in wild rice, but six rare defective haplotypes were not found, which suggested the pre-existence of genetic variations in wild rice accessions and the acquisition of mutations during domestication progression (Yan et al. 2013). Functional alleles of OsPRR37 were mainly found in central and southern China, while weak and defective alleles were found in areas from central to northern China (Yan et al. 2013). This distinct eco-geographical distribution pattern of OsPRR37 was further supported by the finding that japonica varieties harboring nonfunctional alleles of both OsPRR37 and Ghd7 flower extremely early and are adapted to the northernmost regions (Koo et al. 2013; Li et al. 2015; Ye et al. 2018). In addition to the contribution of OsPRR37 alone to the regional and seasonal adaptation of cultivars, gene combinations of OsPRR37, Ghd7 and Ghd8 were highly correlated with grain yield under different photoperiod conditions (Gao et al. 2014; Zhang et al. 2019a).
The transcriptional regulation of downstream targets of OsPRR37 has been reported. Some studies have shown that OsPRR37 suppresses the expression of Early heading date 1 (Ehd1) and thus suppresses Heading date 3a (Hd3a) and RICE FLOWERING LOCUS 1 (RFT1) (Gao et al. 2014; Yan et al. 2013), while other studies have suggested that OsPRR37 directly downregulates the expression of Hd3a (Koo et al. 2013). OsPRR37 acts downstream of rice Phytochrome B (PhyB) because mutation of phyB largely dampens the expression level of OsPRR37 under both long-day (LD) and short-day (SD) conditions (Gao et al. 2014). OsPRR37 is an ortholog of Arabidopsis PRR7 that is a crucial component of the circadian circuit. The functional allele of OsPRR37 complements the late-flowering phenotype of the prr7 mutant, which supports the conservative function between these two genes (Koo et al. 2013).
In addition to OsPRR37, other regulators such as Ghd7 and Hd1 also play important roles in the regulation of heading date. Ghd7 is another CCT domain-containing protein that acts as a strong heading date suppressor under LD conditions. The expression of Ghd7 is positively regulated by Oryza sativa GIGANTEA (OsGI) (Itoh et al. 2010), an ortholog of Arabidopsis GI, which acts as an output regulator of the circadian clock (Mizoguchi et al. 2005). OsGI is essential for setting the critical day length for the expression level of Hd3a by regulating Ghd7 and Ehd1 (Itoh et al. 2010). In addition, OsELF3 and Ehd3 were also reported as upstream regulators of Ghd7 (Matsubara et al. 2012; Matsubara et al. 2011; Saito et al. 2012; Yang et al. 2013). PhyB, a rice red/far-red light receptor, is involved in either transcriptional (Osugi et al. 2011) or post-transcriptional regulation of Ghd7 (Weng et al. 2014). Genetic analysis revealed the additive effects of Ghd7 and OsPRR37, which suggested their independent roles in the heading date regulation (Koo et al. 2013). Hd1, an ortholog of Arabidopsis CONSTANS, exhibits divergent and more complicated functions in heading date regulation in rice (Yano et al. 2000). Hd1 promotes heading date under SD conditions, but exhibits distinct effects under LD conditions. In the background with non-functional alleles of Ghd7 and OsPRR37, Hd1 consistently promotes heading date. However, alternative genetic effects are switched by combination with the functional allele of Ghd7 or OsPRR37 (Fujino et al. 2019; Subudhi et al. 2018; Zhang et al. 2019a; Zhang et al. 2017; Zhang et al. 2019b). Further study demonstrated that Hd1 interacts with Ghd7 and forms a complex that specifically binds to the cis-regulatory region in the Ehd1 (Nemoto et al. 2016).
In this study, OsPRR37 was found to exhibit alternative functions as a promoter of heading date in the ZH11 background under natural long-day (NLD) conditions. Transcriptional analysis revealed that OsPRR37 suppressed Ghd7 expression in both the ZH11 background under NLD conditions and the Zhenshan 97 background under natural short-day (NSD) conditions. Genetic analysis further revealed that the promotion of heading date by OsPRR37 partially relies on an intact Ghd7-related pathway involving not only its upstream regulators OsGI and PhyB, but also the Ghd7 interacting protein Hd1.
Materials and Methods
Plant Materials
The lhd7 (osprr37) mutant was identified in the M2 generation of an ethyl methane sulfonate-treated japonica rice cultivar, Zhonghua 11 (ZH11, Oryza sativa L.). The mutant of ghd7 with a G to A mutation in the coding region of variety ZH11 resulting in a premature stop codon was described in our previous study (Hu et al. 2019). Using the CRISPR method, OsGI, PhyB and Hd1 were individually knocked out in the both the ZH11 and osprr37 mutant backgrounds, and Ghd7 was knocked out in the osprr37 mutant background. The near-isogenic lines (NILs) Ghd7 Ghd8 Hd1, Ghd7 Ghd8 hd1, and Ghd7 ghd8 Hd1 were generated in the previous study (Zhang et al. 2019a). Briefly, these NILs were segregated and selected in a NIL-F2 population deriving from the NIL-F1 generated by crossing two NILs in the Zhenshan 97 (ZS97, Oryza sativa L. ssp. indica) background, NIL1 (Ghd7 Ghd8 osprr37 Hd1) and NIL2 (ghd7 ghd8 OsPRR37 hd1).
Plant Growth Conditions
The rice plants were examined under NLD (day length more than 13.5 h) conditions from mid-May to August in Wuhan (Huazhong Agricultural University, 114°21′ E, 30°28′ N) or NSD (day length less than 12.5 h) conditions from December to April in Lingshui, Hainan (110°2′ E, 18°30′ N). The plants used for expression analysis of flowering time genes were grown in chambers with controlled environment under LD (14 h light/10 h dark) conditions. Heading dates under NLD and NSD conditions were scored as the number of days from germination to the emergence of the first panicle.
MutMap Analysis of Heading Date Gene
The MutMap strategy (Abe et al. 2012) with some modifications was applied for map-based cloning of LHD7. Fifty extremely late heading individuals of the F2 population were bulked and sequenced with an Illumina HiSeq 1000 instrument. Sequence reads were filtered using Trimmomatic (version 0.36) (Bolger et al. 2014). Then the clean data were mapped against the MSU 7.0 rice genome with corresponding annotation by BWA (version 0.7.17) (Li and Durbin 2009), and sorted with SAMtools (version 1.8) (Li et al. 2009). These data were then analyzed with GATK (version 3.8) for variant calling (McKenna et al. 2010). The SNP index defined as the ratio of the number of reads of a mutant SNP to the total number of reads corresponding to the SNP (Abe et al. 2012) was calculated for each SNP. Finally, R-CMplot (https://github.com/YinLiLin/R-CMplot/) was used for visualization of the absolute value of Δ (SNP index) which indicates the difference in the SNP index between the bulked pool and ZH11.
Kompetitive Allele Specific PCR (KASP) Assays
In the progeny test, the KASP assay was used for genotyping SNPs in each individual plant. SNP-specific primers (Additional file 1: Table S1) were designed online at http://www.snpway.com/. For each reaction, 5–50 ng DNA of a specific individual was used in a total reaction volume of 5 μL, that contained 2.5 μL of KASP Master Mix (LGC Biosearch Technologies, Petaluma, California, USA), 0.075 μL (100 μM) of two allele specific primers and 2 μL (100 μM) of common primer. The PCR conditions were as follows: denaturation at 94 °C for 15 min followed by 10 cycles of 20 s at 94 °C and 1 min at 65–57 °C (decreasing 0.8 °C per cycle), followed by another 41 cycles of 20 s at 94 °C and 1 min at 57 °C. Once the thermal cycling was complete, the plates containing the PCR reactions were read with a BMG FLUOstar Omega (LGC Biosearch Technologies, Petaluma, California, USA). Finally, the data were analyzed with KlusterCaller software (LGC Biosearch Technologies, Petaluma, California, USA).
Vector Construction and Transformation
The coding sequence of OsPRR37 was amplified by PCR using the primers Ghd7.1-UF and Ghd7.1-UR (Additional File 1: Table S1) and inserted in into pU1301 with a Gibson assembly reaction (Gibson et al. 2009). To construct the CRISPR-Cas9 vector for Ghd7, the target sequence was designed online (http://crispr.hzau.edu.cn/CRISPR2/ (Lei et al. 2014)) and fused to the Ghd7-CRF and Ghd7-CRR primers (Additional File 1: Table S1). With a segment-overlapping PCR followed by a Gibson assembly reaction, the target sequence with a U3 promoter sequence was cloned into a pCXUN-Cas9 vector (He et al. 2017; He et al. 2018). Other CRISPR-Cas9 constructs for OsGI, PhyB and Hd1 were generated by the same method with corresponding primers (Additional File 1: Table S1). These vectors were induced into indicated acceptors with Agrobacterium-mediated transformation (Hiei et al. 1994).
RNA Sampling and Gene Expression Analysis
To analyze the transcriptional effects of OsPRR37 on other heading date genes, ZH11 and osprr37 mutant were grown under controlled LD conditions (14 h light/10 h dark). The leaves from 35-d-old plants were sampled every 4 h within a 24-h period, and three different individuals per time point were used as biological replicates. Total RNA was isolated with TRIzol reagent (Transgen Biotech, Beijing, China). For reverse transcription quantitative PCR (RT-qPCR), first-strand cDNA was synthesized using reverse transcriptase (Invitrogen), and qPCR was then performed using gene-specific primers (Additional File 1: Table S1), SYBR Master Mix reagent (Roche), and a Quant-Studio 6 Flex Real-Time PCR System (Life Science), according to the manufacturer’s instructions. The PCR conditions were as follows: 10 min at 95 °C followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. PCR amplifications were conducted in triplicate for each sample from three independent biological replicates, and a rice ubiquitin gene (Os02g0161900) was used for normalization.
Protein Sequence Alignments
Alignments were conducted with ClustalX (version 2.1) by using protein sequences of OsPRR37 (from ZH11 type), OsPRR73 (BAD38856 in DDBJ/EMBL/GenBank), OsPRR95 (BAD38857), OsPRR59 (AK120059), OsTOC1 (BAD38854) from Oryza sativa; PtPRR37 (XP_002311123.1), PtPRR73 (XP_002316333.1), PtPRR9a (XP_002320232.1), and PtPRR9b (XP_002301443.1) from Populus trichocarpa; AtPRR3 (BAB13744), AtPRR5 (BAB13743), AtPRR7 (BAB13742), AtPRR9 (BAB13741), and AtTOC1 (NP_200946) from Arabidopsis thaliana; and PpPRR1 (AB558266), PpPRR2 (AB558268), PpPRR3 (AB558267), and PpPRR4 (AB558269) from Physcomitrella patens.
Results
Phenotype of Late Heading Date 7 (lhd7) Mutant
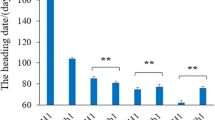
The late heading date mutant lhd7 was identified from the M2 plants of ethyl methane sulfonate (EMS)-treated rice cultivar ZH11. The lhd7 mutants (102.1 ± 3.0 d) flowered about 35 d later than that of control ZH11 plants (67.4 ± 1.0 d) under both natural long-day (NLD) and natural short-day (NSD) conditions (Fig. 1a, b). The lhd7 mutant exhibited longer and denser panicles (Fig. 1c, d) with more primary (Fig. 1e) and secondary branches (Fig. 1f). No significant difference (p = 0.16, t-test) of effective panicles was observed between lhd7 (8.1 ± 0.6) and WT (7.6 ± 0.5). Additionally, the lhd7 plants were found producing more yield per plant than that of ZH11 plants under NLD conditions (Fig. 1g).
Phenotype of lhd7 mutant compared with Zhonghua 11 (ZH11, Oryza sativa L. ssp. japonica). a Phenotype of whole plants of lhd7 and ZH11 grown under natural long-day (NLD) conditions. Scale bar, 25 cm. b Heading date performances of lhd7 and ZH11 under both NLD and natural short-day (NSD) conditions. c-g Comparison of length of the main panicles (c), phenotype of main panicle (d), number of primary branches (e), number of secondary branches (f), and yield per plant (g) between lhd7 mutant and ZH11 grown under NLD conditions. Data represent the mean ± standard deviation (SD), *, P < 0.05; **, P < 0.01; t-test, n = 16; Scale bar in B, 5 cm
Cloning of LHD7 with MutMap Strategy
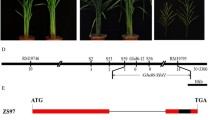
The lhd7 mutant was backcrossed with ZH11 to produce an F2 population in which the number of early heading plants to that of later heading plants fit a ratio of 3:1 (chi square = 0.37, df = 1, p = 0.54) (Fig. 2a). This result indicated that the variation in heading date is controlled by a single gene (Fig. 2a). Fifty extremely late heading individuals of the F2 population were bulked and used for MutMap analysis with next-generation resequencing (Abe et al. 2012). The single nucleotide polymorphism (SNP) indexes were calculated for each SNP. A distinct Δ (SNP index) peak of 1 harboring a cluster of four SNPs was detected at the end of the long arm of chromosome 7 (Fig. 2b, Table 1). To further analyze the causal SNP associated with the lhd7 phenotype, a kompetitive allele specific PCR (KASP) assay was applied to the F2 population with 498 individuals (Fig. 2c), within which five recombinants between marker SNP-UBA and SNP-37 were identified (Fig. 2d, Additional File 1: Table S1). Progeny tests of these recombinants showed a co-segregation relationship between heading-date phenotypes and marker genotypes of SNP-37 (Fig. 2c), which is located in the coding sequence of OsPRR37 that was previously identified as a strong suppressor of heading date (Gao et al. 2014; Koo et al. 2013; Yan et al. 2013). The G to A mutation at SNP-37 caused an amino acid substitution of glycine (G) to aspartic acid (D) at position 159 (G159D) within the PR domain of the OsPRR37 protein (Fig. 2e). Interestingly, the amino acid glycine at site 159 was highly conserved among PRR7 homologs in different organisms (Fig. 2f). To further validate that LHD7 was allelic to OsPRR37, a fragment containing the full-length coding region of OsPRR37 from ZH11 under the control of a maize ubiquitin promoter was transformed into the lhd7 mutant. Both the heading date and yield per plant of transgenic plants were restored to the level of ZH11 under NLD conditions (Fig. 2g, h). Thus, LHD7 was OsPRR37 (hereafter referred to as OsPRR37) but exhibited an alternative function as a promotor of heading date in the ZH11 background under NLD conditions.
Map-based cloning of LHD7. a Chi-square test of heading-date distribution of an F2 population derived from the cross between lhd7 mutant and ZH11. b Distribution of Δ index of single nucleotide polymorphism (SNP) across 12 chromosomes. Δ (SNP index) means the absolute value of the difference of SNP index between the bulked pool and ZH11. c Genotyping examples with SNP markers used in kompetitive allele specific PCR (KASP) assay of the F2 population. Dots in different colors and grouped together represent different genotype of the SNPs as indicated. d Progeny tests of 5 recombinants. Numbers of individual plant and information of SNP markers were listed on the left and above sides, respectively. Column chart on the right showed the phenotype of heading date of each plant. Bars with different colors represent different genotype as legends indicated below. e Protein structure and detailed mutation of OsPRR37 in lhd7 mutant. Bars with different colors represent different protein domains of OsPRR37 as indicated. Numbers near the bars represent the position of amino acid. f Conservation of pseudo-receiver (PR) domain of OsPRR37. Aliments of PR domain of Pseudo responsive regulator (PRR) proteins from different organisms. Red arrow indicates the amino acid Gly (G) at position 159 which mutated to Asp (D) in osprr37. g-h Phenotype of heading date and yield per plant of complementary lines compared with ZH11 and lhd7 mutant
OsPRR37 Suppresses the Expression of Ghd7 in the ZH11 and Zhenshan 97 Backgrounds under Different Day-Length Conditions
To further analyze the pathway through which OsPRR37 was involved in promoting heading date, the expression levels of multiple heading-date related genes were compared between the osprr37 mutant and ZH11. As expected, the expression of the flowering time integrator Ehd1 (Fig. 3a), and two florigen genes, Hd3a (Fig. 3b) and RFT1 (Fig. 3c), was strongly suppressed in the osprr37 mutant, especially at zeitgebers 2.5 and 22.5 when they were highly expressed in ZH11. Interestingly, the expression of Ghd7 at zeitgeber 2.5 was enhanced in the osprr37 mutant compared with ZH11 (Fig. 3d). Comparable expression patterns and levels of other flowering genes including OsPRR37 itself (Fig. 3e), Hd1, Ghd8, OsGI, OsELF3, OsMADS50, OsMADS51, OsMADS56, Oryza sativa CONSTANS 3 (OsCO3), CONSTANS LIKE 4 (COL4), OsCOL10, OsCOL13, DTH2, Ehd2, Ehd3, and Ehd4 were observed between osprr37 and ZH11 (Additional file 2: Figure S1). Moreover, in the background of Zhenshan 97, OsPRR37 showed distinct functions in different day-length conditions. OsPRR37 promoted heading date under NSD conditions, as in ZH11, but delayed heading date under NLD conditions (Fig. 3f-k). The expression of Ghd7 was also found to be significantly enhanced after dawn in near-isogenic lines harboring defective osprr37 under NSD but not NLD conditions (Fig. 3i-q). Together, these results implied that OsPRR37 may promote flowering through suppressing the expression level of Ghd7.
Expression analysis of heading date genes in osprr37 mutant and different NILs. a-e Log10-transforemed expression levels of indicated genes in leaves of 40-d-old plants under controlled LD conditions were determined by quantitative real-time PCR (qRT-PCR) and shown as mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization. f-k Comparing of heading date of near isogenic lines of OsPRR37 in Zhenshan 97 background harboring different alleles of Ghd7, Ghd8 and Hd1 under NLD (f-h) and NSD (i-k) conditions. i-q Comparing of Ghd7 expression in near isogenic lines indicated in f-k under controlled environments. Expression level of Ghd7 were detected 2.5 h before (BD) and after dawn (AD) and shown as Log10-transforemed style. Leaves of 40-d-old plants under indicated conditions was collected and used for quantitative real-time PCR (qRT-PCR). Data was shown as mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization. *, P < 0.05; **, P < 0.01; t-test
Heading Date Promotion by OsPRR37 Required Functional Ghd7
To analyze the relationship between OsPRR37 and Ghd7, we obtained the osprr37 ghd7 double mutant through knocking out Ghd7 in the osprr37 background with clustered regularly interspaced short palindromic repeats (CRISPR) strategy (Fig. 4a-c). The osprr37 ghd7 double mutants (#1: 65.8 ± 2.0 d, #2: 65.0 ± 1.6 d) flowered significantly earlier than the osprr37 mutant (102.1 ± 3.0 d), but later than the ghd7 mutant (54.7 ± 1.6 d) (Fig. 4d). The grain yield per plant of the osprr37 ghd7 double mutants (#1: 20.5 ± 1.6 g, #2: 20.0 ± 1.64 g) appeared to be intermediate between single mutants osprr37 (25.4 ± 4.2 g) and ghd7 (12.6 ± 2.1 g) (Fig. 4e). To gain further insight into the relationship between OsPRR37 and Ghd7, the expression levels of key genes in the regulation of heading date were investigated. At both testing points before and after dawn, the expression of Ehd1 showed distinct levels in these lines with the lowest in osprr37, the highest in ghd7 and the intermediate level in the double mutant osprr37 ghd7 (Fig. 4f). Hd3a and RFT1 showed similar expression patterns to Ehd1 in these lines (Fig. 4g, h), except that comparable expression of RFT1 was detected in osprr37 and osprr37 ghd7. The differences in the expression levels of these key flowering genes are consistent with that of the phenotypic changes. Thus, the promotion of heading date by OsPRR37 is partially attributed to the suppression of Ghd7 expression in the ZH11 background.
Genetic analysis between OsPRR37 and Ghd7. a Allele information of ghd7 mutant generated by clustered regularly interspaced short palindromic repeats (CRISPR) strategy. Black bars represent the exons, and solid line before, between and after the black bars represents the 5′ un-transcribed region, intron and 3′ un-transcribed region, respectively. Letters in the blue box is the protospacer adjacent motif (PAM) sequence. Blue letters indicate the targets sequence. Red letters indicate the mutation details. b-c Phenotype of whole plants (b) and main panicles (c) of ZH11, osprr37, ghd7 and double mutants osprr37 ghd7 (#1 and #2) grown under NLD conditions. Scale bar, 25 cm in b and 5 cm in c. d-e Heading date and yield per plant of ZH11, osprr37, ghd7, and the double mutants osprr37 ghd7 (#1 and #2) grown under NLD conditions. Data represent mean ± SD, n = 10. Different letters indicate significant differences, Duncan’s test. f-h Log10-transformed expression levels of Ehd1 (f), Hd3a (g) and RFT1 (h) in ZH11, osprr37, ghd7 and the double mutant osprr37 ghd7. Expression of indicated genes in leaves of 40-d-old plants under controlled LD conditions were determined 2.5 h before and after dawn by qRT-PCR and shown as mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization. Different upper- and lower-case letters indicate significant differences at P < 0.05 by using Duncan’s test to compare the expression levels of indicated genes before and after dawn, respectively
Effects of OsGI, PhyB and Hd1 on the Function of OsPRR37
The function of Ghd7 depends on intact elements involved in the Ghd7-related pathway including its upstream transcriptional regulators OsGI and PhyB and its physical interaction partner Hd1 (Itoh et al. 2010; Koo et al. 2013; Nemoto et al. 2016; Zhang et al. 2017). To validate the involvement of Ghd7 in the regulation of heading date by OsPRR37, single mutants of Ghd7-related genes (OsGI, PhyB and Hd1) and double mutants of OsPRR37 and these related genes were generated with the CRISPR method. OsPRR37 expression in the phyB mutant was significantly reduced as compared with that in ZH11, but no decline was detected in the osgi, hd1 or ghd7 mutants (Fig. 5a). Moreover, osgi (Fig. 5b), phyB (Fig. 5c) and hd1 (Fig. 5d) all headed earlier and exhibited decreased yield per plant compared with ZH11 under NLD conditions (Fig. 5e-k). Additionally, double mutants generated by knocking out OsGI (osprr37 osgi, Fig. 5b), PhyB (osprr37 phyB, Fig. 5c) and Hd1 (osprr37 hd1, Fig. 5d) in the osprr37 background displayed significantly earlier heading dates than the osprr37 mutant alone but delayed heading dates compared with their corresponding single mutants, osgi, phyB and hd1, respectively (Fig. 5i-k). Significant declined expression of Ghd7 at 2.5 h after dawn were detected in the osgi and phyB mutants but not the hd1 mutant (Fig. 6a-c). Also, at 2.5 h after dawn, an intermediate expression level of Ghd7 was observed in the osprr37 osgi double mutant compared with the osprr37 and osgi single mutants (Fig. 6a), while the osprr37 phyB double mutant displayed a comparable Ghd7 level to the phyB mutant (Fig. 6b). Mutation of Hd1 did not affect the expression of Ghd7 (Fig. 6c). Consistent with the phenotypic changes, the expression levels of the key heading date genes Ehd1, Hd3a and RFT1 in the osprr37 osgi, osprr37 phyB and osprr37 hd1 double mutants at 2.5 h after dawn were all higher than those in the osprr37 single mutant but lower than those in corresponding single mutants osgi, phyB and hd1, respectively (Fig. 6d-l). These results together suggested that the promotion of heading date by OsPRR37 requires an intact regulatory pathway of Ghd7 involving not only the upstream regulators OsGI and PhyB, but also the Ghd7-interacting protein Hd1.
Generation of knockout mutants for OsGI, PhyB and Hd1 with CRISPR strategy. a Comparison of expression of OsPRR37 among mutants ghd7, osgi, hd1, phyB, and control plant ZH11. Expression level of OsPRR37 in leaves collected 2.5 h after dawn from 40-d-old plants under controlled LD conditions were analyzed by qRT-PCR and shown as mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization. **, P < 0.01, t-test. b-d Mutation details of genetic materials of OsGI (b), PhyB (c) and Hd1 (d). Black bars represent the exons, and solid line before, between and after the black bars represents the 5′ un-transcribed region, intron and 3′ un-transcribed region, respectively. Letters in the blue box is the PAM sequence. Blue letters indicate the targets sequence of indicated gene. Red letters indicate the mutation details. e-h Phenotype of whole plants (e-g); and main panicles (h) of indicated genetic materials. Scale bar, 25 cm in e-g and 5 cm in h. i-k Genetic analyses between OsPRR37 and OsGI (i), PhyB (j) and Hd1 (k). Data represent mean ± SD, n = 16. Different letters indicate significant differences at P < 0.05, Duncan’s test
Expression analysis of key flowering genes in genetic materials related to OsGI, OsPhyB and Hd1. Log10-transformed expression of Ghd7, Ehd1, Hd3a, and RFT1 in the single mutants and double mutants of OsPRR37 and OsGI (a, d, g and j), OsPRR37 and PhyB (b, e, h and k), and OsPRR37 and Hd1 (c, f, i and l), respectively. Samples were collected 2.5 h before and after dawn from 40-d-old plants under controlled LD conditions. Data represent mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization. Different upper- and lower-case letters indicate significant differences at P < 0.05 by using Duncan’s test to compare the expression levels of indicated genes before and after dawn, respectively
Discussion
Alternative Function of OsPRR37 Is Partially Relying on Ghd7
In this study, the delay of heading date in the osprr37 mutant was due to the G159D mutation in the PR domain, which was highly conserved among its homologs in different organisms (Fig. 2e, f). PR domain is crucial for the function of PRR proteins. Through PR domain, PRR1/TOC1 interacts with ZTL, which targets PRR1/TOC1 for proteasome-dependent degradation (Kiba et al. 2007). In addition, PRR proteins could interact with each other and form heterodimers through their PR domains (Ito et al. 2003); this dimerization stabilizes the PRR protein and protects it from ZTL-dependent degradation (Para et al. 2007). Thus, it is possible that the G159D mutation might affect the interactions between OsPRR37 and other PRRs and the turnover of PRR proteins at the post-transcriptional level.
Our results demonstrated that OsPRR37 promotes heading date and decreases grain yield in the ZH11 background (Fig. 4d, e), in contrast to previous reports that OsPRR37 delays heading date and increases grain yield in genetic backgrounds of Zhenshan 97, Kita-ake, Milyang 23 and Dongjin under LD or NLD conditions (Gao et al. 2014; Koo et al. 2013; Yan et al. 2013). Similar effects of OsPRR37 on promoting heading date were also found in the Zhenshan 97 background but only under NSD conditions (Fig. 3i-k). This dual role of OsPRR37 is similar to that of Hd1, which promotes heading date under LD conditions in the ghd7-defective; otherwise, it delays heading date by interacting with Ghd7 and directly suppressing the expression of Ehd1 (Nemoto et al. 2016; Subudhi et al. 2018; Zhang et al. 2017). Apart from Ghd7, OsPRR37 also switches the effect of Hd1, which was revealed by analyzing the genetic effects of combinations of Hd1 and OsPRR37 in a segregating population (Fujino et al. 2019). Considering the dual role of Hd1 in heading date regulation, Hd1 might be the factor that converts the function of OsPRR37. To test this, genetic analysis was performed between OsPRR37 and Hd1. However, the osprr37 hd1 double mutant headed later than the hd1 single mutant (Fig. 5k), which went against with our expectation. Thus, Hd1 is not the converting factor.
Ghd7 expression was increased in the osprr37 mutant in the ZH11 background under NLD conditions (Fig. 3d) and near-isogenic lines with defective osprr37 in the Zhenshan 97 background under NSD conditions (Fig. 3o-q). Regardless of the day-length difference, these results together implied that OsPRR37 acts upstream of Ghd7. The osprr37 ghd7 double mutant in the ZH11 background showed significantly earlier heading date and reduced grain yield compared with the osprr37 single mutant (Fig. 4b-e), which further confirmed the involvement of Ghd7 in the pathway of heading date regulation by OsPRR37. However, the osprr37 ghd7 double mutant still headed later and produced more grains than the ghd7 single mutant (Fig. 4d-e), which suggested that the promotion of heading date by OsPRR37 is partially dependent on Ghd7 in the ZH11 background. The expression of Ghd7 was observed not affected at 2.5 h before dawn (Fig. 6a-c), while those downstream heading-date related genes, Ehd1, Hd3a and RFT1 were differentially expressed at this time point in ZH11, osprr37, osgi, phyB, hd1 and higher order double mutants (Fig. 6a-i). We believe that those effects could be explained by the previous finding that the repression of Ehd1 by Ghd7 depends on Ghd7 expression levels on the previous morning (Itoh et al. 2010). We previously investigated the heading dates of several near-isogenic lines with a combination of either functional or defective alleles of OsPRR37, Ghd7, Ghd8 and Hd1 in the Zhenshan 97 background (Zhang et al. 2019a). OsPRR37 was found consistently delays heading date regardless of other heading date genes under NLD conditions but exhibits a promoting effect under NSD conditions only in backgrounds with functional Ghd7. Therefore, the alternative promotion effect on heading date by OsPRR37 is dependent on Ghd7.
Upstream Signals from OsGI and PhyB are Essential for the Promotion Effect of OsPRR37 on Heading Date
Knocking out either OsGI or PhyB in the osprr37 background (osprr37 osgi and osprr37 phyB) promoted heading compared with osprr37, but the heading date of both double mutants was still later than that of the corresponding single mutants (Fig. 5i, j), which is consistent with the observation that the expression levels of Ehd1, Hd3a and RFT1 in double mutants were intermediate between those of osprr37 and the corresponding single mutants (Fig. 6d-l). However, the phyB line displayed an earlier heading date and a lower expression level of Ghd7 than ZH11 (Fig. 6b), which is contrary to previous results showing that higher expression of Ghd7 were detected in phyB compared with wild-type Nipponbare (Osugi et al. 2011). These different results could be attributed to the different tested tissues used because flag leaves were used here whereas the whole aboveground parts of plants were used in the previous study (Osugi et al. 2011). In addition, mutation of the circadian clock-related gene OsGI reduced the expression level of Ghd7 (Fig. 6a), which is in agreement with a previous report (Itoh et al. 2010). Therefore, the promotion effect of OsPRR37 on heading date requires an intact Ghd7 regulatory pathway involving not only circadian clock signals transduced by OsGI, but also light signal perception via PhyB.
Alternative Effect of OsPRR37 is Genetic Background Dependent
Our results here and in our previous study (Zhang et al. 2019a) demonstrated the promotion effect on heading date by OsPRR37 in different genetic backgrounds under different day-length conditions. Regardless of day-length conditions, OsPRR37 consistently promotes heading date in the ZH11 background under either NLD or NSD conditions (Fig. 1b). However, OsPRR37 exhibits completely different effects under NLD and NSD conditions in the Zhenshan 97 background (Fig. 3f-k). It promoted heading date under NSD conditions, but delayed heading date under NLD conditions. Although data of flowering-time under NSD and NLD in different years were not strictly comparable, it is also possible that an unknown daylength sensitive gene may exist and display functionality divergences in the ZH11 and Zhenshan97 backgrounds. Further investigation with a population deriving from crossing between ZH11 and near-isogenic line OsPRR37 Ghd7 Ghd8 Hd1 in the Zhenshan 97 background may facilitate the isolation of the unknown gene. We also observed that promotion effect of OsPRR37 partially depends on Ghd7 in the ZH11 background (Fig. 4d) but completely relies on Ghd7 in the Zhenshan 97 background (Zhang et al. 2019a). Thus, OsPRR37 may act in another pathway independent of Ghd7 to regulate heading date in ZH11.
Conclusions
Through map-based cloning and the MutMap strategy, we cloned a gene heading date gene LHD7 which is allelic to OsPRR37. Our results revealed the novel function of OsPRR37 in the promotion of heading date in the ZH11 background under both NLD and NSD conditions, which is opposite to the previous finding that OsPRR37 acts as a suppressor of heading date. Further genetic analysis demonstrated the promotion effect on heading date by OsPRR37 was partially dependent on Ghd7 and Ghd7-related pathway in the ZH11 background. Our finding not only revealed an alternative promotion function of OsPRR37 in the regulation of heading date, but also enriches the theoretical bases for improvement of heading date of rice in the future.
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- PR:
-
Pseudo-receiver
- PRR:
-
Pseudo-response regulator
- CCT:
-
CONSTANS, CO-like, and TOC1
- LD:
-
Long day
- SD:
-
Short day
- NLD:
-
Natural long day
- NSD:
-
Natural short day
- SNP:
-
Single nucleotide polymorphism
- KASP:
-
Kompetitive allele specific PCR
- CRIPSPR:
-
Clustered regularly interspaced short palindromic repeats
References
Abe A et al (2012) Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol 30:174
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Fujino K, Yamanouchi U, Nonoue Y, Obara M, Yano M (2019) Switching genetic effects of the flowering time gene Hd1 in LD conditions by Ghd7 and OsPRR37 in rice. Breed Sci 69:127–132. https://doi.org/10.1270/jsbbs.18060
Gao H et al (2014) Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci 111:16337–16342
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–U341. https://doi.org/10.1038/Nmeth.1318
He Y, Zhu M, Wang L, Wu J, Wang Q, Wang R, Zhao Y (2018) Programmed self-elimination of the CRISPR/Cas9 construct greatly accelerates the isolation of edited and transgene-free rice plants. Mol Plant 11:1210–1213. https://doi.org/10.1016/j.molp.2018.05.005
He Y et al (2017) Self-cleaving ribozymes enable the production of guide RNAs from unlimited choices of promoters for CRISPR/Cas9 mediated genome editing. J Genet Genomics 44:469–472. https://doi.org/10.1016/j.jgg.2017.08.003
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hu Y, Li S, Xing Y (2019) Lessons from natural variations: artificially induced heading date variations for improvement of regional adaptation in rice. Theor Appl Genet 132:383–394
Ito S et al (2003) Characterization of the APRR9 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol 44:1237–1245. https://doi.org/10.1093/pcp/pcg136
Itoh H, Nonoue Y, Yano M, Izawa T (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42:635
Kiba T, Henriques R, Sakakibara H, Chua NH (2007) Targeted degradation of PSEUDO-RESPONSE REGULATOR5 by an SCFZTL complex regulates clock function and photomorphogenesis in Arabidopsis thaliana. Plant Cell 19:2516–2530. https://doi.org/10.1105/tpc.107.053033
Koo B-H et al (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6:1877–1888
Lei Y, Lu L, Liu H-Y, Li S, Xing F, Chen L-L (2014) CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant 7:1494–1496
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li H et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Li X et al (2015) Combinations of Hd2 and Hd4 genes determine rice adaptability to Heilongjiang Province, northern limit of China. J Integr Plant Biol 57:698–707. https://doi.org/10.1111/jipb.12326
Lin H, Yamamoto T, Sasaki T, Yano M (2000) Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theor Appl Genet 101:1021–1028
Liu C et al (2015) OsPRR37 and Ghd7 are the major genes for general combining ability of DTH, PH and SPP in rice. Sci Rep 5:12803. https://doi.org/10.1038/srep12803
Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, Yano M (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53:709–716. https://doi.org/10.1093/pcp/pcs028
Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang ZX, Minobe Y, Yano M (2011) Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 66:603–612
McKenna A et al (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
Mizoguchi T et al (2005) Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17:2255–2270. https://doi.org/10.1105/tpc.105.033464%J
Nemoto Y, Nonoue Y, Yano M, Izawa T (2016) Hd1,a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J 86:221–233. https://doi.org/10.1111/tpj.13168
Osugi A, Itoh H, Ikeda-Kawakatsu K, Takano M, Izawa T (2011) Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice. Plant Physiol 157:1128–1137. https://doi.org/10.1104/pp.111.181792
Para A, Farré EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA (2007) PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 19:3462–3473. https://doi.org/10.1105/tpc.107.054775%J
Saito H et al (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short- and long-day conditions. Plant Cell Physiol 53:717–728. https://doi.org/10.1093/pcp/pcs029
Subudhi PK, De Leon TB, Tapia R, Chai C, Karan R, Ontoy J, Singh PK (2018) Genetic interaction involving photoperiod-responsive Hd1 promotes early flowering under long-day conditions in rice. Sci Rep 8:2081. https://doi.org/10.1038/s41598-018-20324-1
Weng X et al (2014) Grain number, plant height, and heading date7 is a central regulator of growth, development, and stress response. Plant Physiol 164:735–747
Yan W et al (2013) Natural variation in Ghd7. 1 plays an important role in grain yield and adaptation in rice. Cell Res 23:969
Yang Y, Peng Q, Chen GX, Li XH, Wu CY (2013) OsELF3 is involved in circadian clock regulation for promoting flowering under long-day conditions in rice. Mol Plant 6:202–215. https://doi.org/10.1093/mp/sss062
Yano M et al (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in Rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473–2483. https://doi.org/10.1105/tpc.12.12.2473%J
Ye J et al (2018) Divergent Hd1, Ghd7, and DTH7 alleles control heading date and yield potential of japonica rice in Northeast China. Front Plant Sci 9:35. https://doi.org/10.3389/fpls.2018.00035
Zhang B, Liu H, Qi F, Zhang Z, Li Q, Han Z, Xing Y (2019a) Genetic interactions among Ghd7, Ghd8, OsPRR37 and Hd1 contribute to large variation in heading date in rice. Rice 12:48. https://doi.org/10.1186/s12284-019-0314-x
Zhang Z, Zhang B, Qi F, Wu H, Li Z, Xing Y (2019b) Hd1 function conversion in regulating heading is dependent on gene combinations of Ghd7, Ghd8, and Ghd7.1 under long-day conditions in rice. Mol Breed 39:92. https://doi.org/10.1007/s11032-019-1001-8
Zhang Z et al (2017) Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions. Sci Rep 7:5388. https://doi.org/10.1038/s41598-017-05873-1
Acknowledgements
We thank Prof. Qi Sun in Cornell University for his kind help with the MutMap analysis. We are grateful to Dr. Yunde Zhao in University of California, San Diego for the kind donation of vectors for CRISPR.
Funding
The project was supported by the Projects of International Cooperation and Exchanges NSFC (32061143042), Special Projects of Technology innovation in Hubei Province (2018ABA083), and China Postdoctoral Science Foundation (2019 M652606).
Author information
Authors and Affiliations
Contributions
YX, AY and YH conceived and designed the research. YH, XZ and BZ performed the experiments. SL, XF, HL and QL contributed in the phenotyping and figure preparation. HZ and YH conducted the MutMap analysis. JZ and QH contributed in the preparation of genetic materials. YH and YX wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Primers used in this study.
Additional file 2: Figure S1
. Expression levels of indicated genes in leaves of 40-d-old plants under controlled LD conditions were determined by quantitative real-time PCR (qRT-PCR) and shown as mean ± SD of three replicates. Rice ubiquitin gene (Os02g0161900) was used for normalization.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Y., Zhou, X., Zhang, B. et al. OsPRR37 Alternatively Promotes Heading Date Through Suppressing the Expression of Ghd7 in the Japonica Variety Zhonghua 11 under Natural Long-Day Conditions. Rice 14, 20 (2021). https://doi.org/10.1186/s12284-021-00464-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-021-00464-1