Abstract
Background
Cannabis-related medical consultations are increasing worldwide, a non-negligible public health issue; patients presenting to acute care traditionally complain of abdominal pain and vomiting. Often recurrent, these frequent consultations add to the congestion of already chronically saturated emergency department(s) (ED). In order to curb this phenomenon, a specific approach for these patients is key, to enable appropriate treatment and long-term follow-up.
Objectives
This study reviews cannabinoid hyperemesis syndrome (CHS) and cannabis withdrawal syndrome (CWS), in a bid to help promote better understanding and handling of pathologies associated with chronic cannabis use. Following a literature review, we present a novel therapeutic algorithm aimed at guiding clinicians, in a bid to improve long-term outcomes and prevent recurrences.
Methods
Using the keywords “Cannabis,” “Hyperemesis,” “Syndrome,” “Withdrawal,” and “Emergency Medicine,” we completed a literature review of three different electronic databases (PubMed®, Google scholar®, and Cochrane®), up to November 2021.
Results
Although often presenting with similar symptoms such as abdominal pain and vomiting, cannabinoid hyperemesis syndrome (CHS) and cannabis withdrawal syndrome (CWS) are the result of two differing pathophysiological processes. Distinguishing between these two syndromes is essential to provide appropriate symptomatic options.
Conclusion
The correct identification of the underlying cannabis-related syndrome, and subsequent therapeutic choice, may help decrease ED presentations. Our study emphasizes the importance of both acute care and long-term outpatient follow-up, as key processes in cannabis-related disorder treatment.
Similar content being viewed by others
Background
The use of cannabis as a recreational substance has increased worldwide in the past 20 years, as its use becomes more socially accepted. Now regularly consumed by a large spectrum of the population, this trend has resulted in an increase in the number of cannabis-related medical consultations [1], making its consumption a non-negligible public health issue. In Switzerland, nearly 1/3 of the population over the age of 15 years has already tried cannabis for reasons other than medical purposes [2].
A well-recognized association of symptoms, abdominal pain, and vomiting is, in chronic users, generally attributed to cannabinoid hyperemesis syndrome (CHS). They are however also encountered in cannabis withdrawal syndrome (CWS), an often debated but officially (ICD and DSM) recognized withdrawal syndrome. Distinguishing between these two pathologies is important as the underlying mechanism and treatment options differ.
This study aims to present pathophysiological differences in a bid to help guide physician therapeutic decisions and optimize long-term patient outcomes.
Methods
Using the keywords “Cannabis,” “Hyperemesis,” “Syndrome,” “Withdrawal,” and “Emergency Medicine,” we performed an in-depth literature review of 3 electronic databases (PubMed®, Google scholar®, and Cochrane®), aimed at all articles containing any of the above keywords, until November 2021.
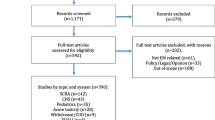
All levels of evidence were considered and reviewed. Publications in English and in French and full-text articles were considered. Three hundred ninety-three publications were identified, of which 42 were included for our study; we did not include individual case reports as these are associated with a low level of evidence, although we did consider one case series which included 98 patients, in light of the number of patients in the publication. Systematic reviews, randomized controlled trials, retrospective studies, and meta-analysis from 1998 to 2021 were included. Figure 1 presents the literature review selection and retention process.
Pathophysiology of abdominal pain and emesis
First identified in 1990, the innate endocannabinoid system serves multiple endocrine functions, through the activation of receptors (principally CB1 and CB2) within the central nervous system, as well as in bones, the gastrointestinal tract, hepatocytes, pancreatic cells, muscles, uterus, and adipose cells. When exposed to low doses of cannabinoid, the activation of this system principally has an antiemetic effect. Within the gastrointestinal tract, this interaction inhibits the opening of the gastro-esophageal sphincter, slows peristalsis (through its action on smooth muscle cells), and lowers acid secretion [3, 4]. In the CNS, activation of these receptors has a direct role in regulating the sympathetic and hypothalamic-pituitary-adrenal axis, preventing overstimulation.
Working in a dose-dependent and biphasic manner, progressive desensitization of CB1 receptors can occur when overstimulated, creating paradoxical effects. This overstimulation is thought to be multifactorial and relates in part to the lipophilic properties of cannabinoids, which store in body fat and are subsequently released in chronic users (whose reserves are elevated from continuous intake) [5, 6]. The fact that not all chronic users develop CHS suggests genetic predisposition, a feature recently described, via the identification of five gene mutations which seem to confer some level of protection from paradoxical effects of overstimulation of the endocannabinoid system [7].
In patients predisposed to symptoms, overstimulation of said receptors may result in increased gastric acid secretion and impaired gut motility and relaxation of the gastro-esophageal sphincter, as well as dysregulated basal sympathetic activity, altogether resulting in hyperemesis. Further to this, abdominal discomfort can also occur and is thought to be due to THC-induced splanchnic vasodilation (and cutaneous vasoconstriction), a phenomenon known as “cutaneous-stealing syndrome” [8]. The excessive self-administration of hot showers, a feature well-described in the literature [5], is thought to reverse this by inducing peripheral vasodilatation of the peripheries and redistributing blood flow away from the gastrointestinal tract [4]. Interaction of CB receptors with the TRPV1 receptor explains further this phenomenon and the therapeutic effect of capsaicin cream (see below), with high doses of cannabinoids causing hypothermia and low doses of hyperthermia [9].
In addition, chronic cannabis users often develop symptoms of addiction. One suggested reason is via the stimulation of the dopaminergic system by cannabinoids in the nucleus accumbens. While understimulation of this system leads to vomiting and abdominal pain, this reward pathway can also cause paradoxical effects when overstimulated, such as anhedonia and lower positive and higher negative emotionality scores [10], all symptoms regularly seen in chronic users. Sudden abstinence or drop in intake in patients whose dopaminergic pathway has been upregulated in response to high doses of cannabinoids could therefore theoretically cause symptoms of withdrawal. This has been well demonstrated in regularly THC-exposed animals and humans, whom, when administered Rimonabant© (a CB1 receptor blocker, used to treat obesity) [11, 12], rapidly develop abdominal pain and hyperemesis. In addition to these withdrawal symptoms, other disturbances have also been described in these patients, such as irritability, anxiety, sleep disturbances, and loss of appetite. It is believed these occur through decreased mesolimbic dopamine function [13].
Thus, chronic users seem to develop symptoms from stimulation of already overstimulated CB receptors (CHS) but can also develop symptoms upon cessation through decreased central nervous system stimulation (CWS). As mechanisms vary, correctly identifying which syndrome a chronic THC user is presenting is key to choosing the appropriate treatment.
Differentiating CHS from CWS
Bearing CHS and CWS in mind, patients who are chronic THC users presenting with hyperemesis and abdominal pain can have a multitude of other pathologies; Table 1 presents a non-exhaustive list of differential diagnoses illustrating the wide range of possibilities.
Clinical history
As with any patient, obtaining a detailed history is a vital component to correctly establish a differential diagnosis. In terms of abdominal pain and hyperemesis, this relies on excluding a wide range of differentials (see Table 1) thanks to a thorough exam and the use of paraclinical tests (see below), and focusing on certain differences may help differentiate CHS from CWS.
Epidemiologically, CHS occurs predominantly in males, whereas CWS affects women more frequently. A correlation between the time of onset of symptoms and last consumption has also been demonstrated, with CHS symptoms usually appearing within 24 h of last consumption (whereas CWS can occur anytime from 1 day to 10 days after last consumption). Patients presenting > 1 day after intake of THC are thus more likely to be suffering from CWS than CHS [14]. In terms of symptoms, the combination of abdominal pain and vomiting occurs more frequently in CHS than CWS (85.1% versus 8.3%), though this is unspecific. Hot showers, for reasons described above, exclude the diagnosis of CWS, being present in 92.3% of patients with CHS (versus 0% in CWS) [14, 15]. Psychological symptoms such as irritability, sleep difficulty, nervousness, restlessness, and depression tend to affect CWS patients more frequently, potentially significantly impairing daily life [14].
In terms of clinical course, patients suffering from CHS often describe three clear phases, which occur after a period of regular cannabis consumption, during which doses have been continuously increased to obtain desired effects (tolerance) [15]. An initial phase of morning sickness and dyspepsia, lasting anywhere from several months to years, is usually the prodromal phase, during which patients generally tend to increase their consumption to benefit from its antiemetic effect. This is then often followed by a second phase where patients experience recurrent episodes of hyperemesis with abdominal pain occurring in the hours after THC consumption. Each acute episode can last up from 24 to 48 h, and it is during this phase that patients are most likely to take hot showers for symptomatic relief and tend to present to the ED. Following this second phase, a recovery phase with two possible outcomes arises: either the patient continues THC consumption, in which case the symptoms appear again (as do the number of ED visits), or they will completely abstain, in which case, if said abstinence is maintained, CHS symptoms do not recur [4, 5, 16].
CWS, on the other hand, tends to present in chronic users within 1–10 days after last THC intake, with a peak incidence between days 2 and 6. No correlation has been established between symptoms severity and quantity (of THC) previously consumed, and initial presentation (to acute care) tends to vary, with a clinical course not well defined. Symptoms, which include nausea and vomiting as well as psychological and other somatic issues, generally worsen the further the patient is from last consumption, and can last up to 4 weeks. This likely corresponds to the time needed for CB1 receptors to return to their original state in the central dopaminergic pathways; this important feature is key to long-term management of these patients, who require ambulatory follow-up rather than simple symptomatic relief [13]. Table 2 summarizes the relevant clinical differences.
Paraclinical examination
While recurrent presentations may carry a bias, the initial presentation of a patient with hyperemesis and/or abdominal is usually associated with a wide range of paraclinical tests ordered, in light of the many differential diagnoses possible. A detailed history and clinical examination often renders these additional investigations obsolete, with little yielded benefit. Nonetheless, the severity of presentation may warrant a blood workup to exclude complications of vomiting-induced dehydration and dyselectrolytemia. An ECG is also generally advisable to exclude electrophysiological abnormalities (corrected QT interval, QRS interval, etc.), in light of differential diagnoses (e.g., inferior ST-Elevation Myocardial Infarct) and to anticipate for potential QTc modifying treatments [17]. Unfortunately, in addition to these tests, many patients undergo more invasive diagnostic tests such as CT, endoscopy, and occasionally exploratory laparoscopy, which often fail to find an alternative cause and expose the patient to potential complications [15, 18]. Screening of genetic predispositions may also provide some benefit in differential diagnosing, though this would not be of use in the acute setting; as of publication, 5 mutations have been identified as potentiators of CHS (COMT, TRPV1, CYP2C9, DRD2, and ABCA1) [7].
Official classification
Since the early 2000s, both CHS and CWS have been recognized by the ICD-10 (F12.241 and F12.30 of the 10th edition of the International Classification of Diseases, respectively). CHS is also included in the Rome IV definition as a functional gastrointestinal disorder, while CWS is encompassed in the DSM-5 (5th edition of the Diagnostic and Statistical Manual of Mental Disorders) [19, 20].
For CHS, cardinal symptoms are cyclic vomiting accompanied by abdominal pain following cannabis consumption. These symptoms can be alleviated by hot showers, and complete resolution of the syndrome requires complete abstinence [15]. For CWS, patients should at least have three DSM-5 symptoms, within 1 week of complete cessation or reduction in cannabis use; this should occur following a heavy or prolonged use. Symptoms include loss of appetite, hypothymia, irritability, restlessness, anxiety, and sleep disturbance. While no consensus exists pertaining to the minimal duration of exposure, one study demonstrated that smoking ≥ 6 marijuana joints/day over 12 months triples the odds of CWS (in comparison to smoking 1 joint/day over the same period) [13, 21]. It is of interest to note that abdominal pain and vomiting are not included in the diagnostic criteria for the DSM-5; this further reflects the importance of a thorough medical history in establishing a diagnosis.
To fulfill diagnostic criteria, in addition to the above conditions, in both cases, symptoms should not be attributable to another medical condition or mental disorder and should significantly impede the performance of everyday activities [13, 22]. A summary of validated criterion for CHS and CWS are listed in Table 3.
Therapeutic approach
Patients presenting with hyperemesis and abdominal pain should be thoroughly examined, the potential for dehydration, prerenal acute kidney injury, and dyselectrolytemia being on the initial preoccupations for acute care. With the added advantage that hydration and electrolyte substitution may reduce symptomatology [15], the next step is focusing on specifically addressing nausea and hyperemesis. In light of differing pathological processes, the choice of agent should be tailored to the suspected diagnosis, hence the importance of obtaining a thorough medical history.
-
a.
Cannabinoid hyperemesis syndrome
Conventional antiemetics, such as metoclopramide, ondansetron, or domperidone, seem to have little to no effect in CHS [23, 24]. Benzodiazepines (mostly lorazepam) seem to be effective, but the level of evidence associated with their use is low [23]. Many other agents have been tested (ex. antidepressants, opioids, neuroleptics), the most promising being haloperidol (0.05–0.1 mg/kg IV/IM) and droperidol (0.625 mg IV/IM) [25, 26]. Black box warning of long QT syndrome associated with butyrophenone neuroleptics should not prevent their use for CHS, in light of the doses used (see below) [27]; a pre-administration ECG is nonetheless advisable. In addition to its antiemetic properties, this pharmaceutical class has the added advantage of reducing agitation, which may also be present in CHS patients presenting to acute care.
If present, abdominal pain should be addressed with capsaicin. It is the active component that give chilies their spice; this molecule seems particularly effective in treating abdominal pain in CHS, in contrast to more conventional treatments (paracetamol, NSAIDs, etc.) [24] or opioids, which may worsen nausea and vomiting and have the potential to create dependency and increase the number of ED consultations in search of opioids [16, 24]. Through its interaction with TRPV1 receptors, topical capsaicin, applied to the forearms and abdomen, combats hypothermia and helps redistributing blood flow away from the gastrointestinal tract (see above) [9, 28, 29]. In addition, the use of a PPI reduces the risk of esophageal and gastric mucosal lesions, following excessive vomiting [19]. Hot showers, if available, should also be offered as this may help reduce anxiety and will help guide your diagnosis (as pathognomonic of CHS). Finally, the only known treatment for CHS is long-term abstinence [15]. Table 4 summarizes the studies relevant to therapeutic options for CHS.
-
b.
Cannabis withdrawal syndrome
CWS therapeutic options are more limited. Withdrawal symptoms seem to respond well to oral THC substitution [30], the concept being that these will interact with CB1 receptors, in a bid to counter severe symptoms due to rapid downregulation from withdrawal. Protocols using oral THC [31, 32], dronabinol [33], or nabiximols [34] have shown an improvement of withdrawal symptoms (altered mood and sleep, nausea and craving) and an increase in prolonged abstinence. Nabilone in association with zolpidem has also shown promising results [35]. Access to medical THC is however limited, and many countries (including Switzerland) have made access to these drugs very difficult, if not impossible. Alternative therapeutic options such as gabapentin [36] and behavioral therapies such as the twelve-step facilitation method (TFM), cognitive behavioral therapy (CBT), motivational enhancement therapy (MET), and contingency management (CM) [37, 38] (see Table 5) have all been shown to help improve symptoms while reducing relapse and should be sought after in countries where THC therapies are not yet available.
No studies have evaluated the treatment of abdominal pain, as its incidence in CWS is significantly less than in CHS. Furthermore, in light of the pathophysiological processes behind CWS, its presence may not be a direct consequence to THC but simply a response to emesis (and if present, may be a sign that the patient is experiencing CHS rather than CWS). Thus, if present, it should theoretically be manageable with conventional non-opioid analgesics and anticholinergics (such as butylscopolamine), the latter having the advantage of increasing dopamine concentrations in the brain. Regarding nausea, antipsychotics should be withheld as they tend to decrease central dopamine levels and may worsen withdrawal symptoms such as craving [42]. Table 5 summarizes the most relevant studies and levels of proof.
-
iii.
Abstinence and follow-up
In addition to abdominal pain, nausea, and vomiting, CHS and CWS are associated with many secondary issues, ranging from social isolation and financial difficulties (absence from work, cost of ED consults, water and electric bills in case of hot showers), as well as dermal burns from hot water in extreme cases of CHS [43].
In both syndromes, complete abstinence is the definitive treatment [15]. With a high potential for relapse (54% of patients achieving 2-week abstinence, and 71% relapse within 6 months [39]), follow-up of patients should be initiated, if possible, from acute care [39, 44]. This can be done through healthcare liaison officers, dedicated community outreach nurses, and/or group counseling sessions such as Marijuana Anonymous which works in a similar fashion to Alcoholics Anonymous, with sponsors and group discussions. These strategies have all demonstrated a reduction in relapse rates [13, 30, 37].
Discussion
Like many EDs worldwide, the normalization of cannabis consumption has led to an increase in the number of cannabis-related consults in the ED (positive delta from 2.3 to 13.3 cases per 100,000 ED visits in the USA from 2006 to 2013) [43]. In light of the severity of their symptoms, these patients often require increased monitoring and accompaniment. With average ED times of 13.9 h [26], these patients, who often do not fill the criterion for hospitalization, are bound to already chronically oversaturated EDs and add to the pressure on healthcare systems. Worse, frequent ED consultations have been shown to lead to cognitive bias from teams, which could trivialize symptoms and result in missed alternative diagnoses [45]. In light of these factors, and based on the above literature review, we proceeded to review the management of chronic cannabis users presenting to our ED with hyperemesis, nausea, and/or abdominal pain. In a bid to share with other acute care units, we will now present our internal guidelines, reflecting the current level of evidence.
Upon arrival to our ED, chronic cannabis users presenting with hyperemesis and nausea are first triaged to exclude any other conditions or signs of shock. If hemodynamically stable, they are redirected to a consult area with access to a nearby shower. IV access is set up, with bloods taken (a minimum of creatinine, urea, electrolytes, liver function tests, lipase, and full blood count are sent) and crystalloid-based rehydration started, as patients are usually unable to drink water due to nausea and/or emesis. An ECG is usually rapidly done, and the patient is given a PPI (PO, if tolerated). A thorough medical history is then obtained, and depending on whether CHS or CWS is suspected (the request for a warm shower is interpreted by us as diagnostic of CHS), a trial therapy with either 0.625 mg droperidol (IV) or haloperidol 0.05–0.1 mg/kg (IV/IM) for CHS or ondansetron (4 mg IV) for CWS is initiated. While currently not readily available in the author’s country of practice (available usually as a magistral preparation), the addition of capsaicin cream in the acute ED treatment may be highly beneficial at this stage: not only will the administration be demonstrated; the efficacy may actually improve ambulatory use.
The patient is then observed, and, depending on symptom progression, a second dose of butyrophenone neuroleptics may be added. If abdominal pain is present, standard analgesics are prescribed if CWS is suspected (for CHS, the use of topical capsaicin can again only be encouraged). During their time in our ED, these patients are generally seen by our frequent user (or complex case) team. Composed of specialized community outreach nurses, the aim is to identify what support systems the patient has in place in the community but also helps, in case of recurrent visits, determine what triggers have led to renewed consumption, to enable ambulatory follow-up and promote long-term abstinence. They are also trained, and take the time, to explain the diagnosis (including the pathophysiological pathways) and, for CHS, discuss the risk of developing a withdrawal syndrome (CWS) [44], all while promoting complete abstinence.
Upon resolution of acute debilitating symptoms, patients are usually discharged with a prescription for a magistral preparation of capsaicin cream (0.075%) (application on forearms and abdomen, 3–4 times daily), as well as a PPI, for CHS [26, 27, 40]. For suspected CWS, the only oral THC available in Switzerland is nabiximols (Sativex©) and is currently only licensed for multiple sclerosis [46]. Patients are therefore usually prescribed non-opioid analgesia, a reserve for gabapentin, with or without zolpidem; though after a review by our emergency psychiatry team, some are having psychological-associated symptoms. As there is always a risk for a missed diagnosis, prior to discharge, patients are reminded to present to the nearest healthcare center should their condition deteriorate or new symptoms develop. Our EMR system has been modified to automatically flag these patients when they present to our hospital, to ensure that healthcare teams have access to the long-term treatment plan (in patient notes). We believe this also helps reduce bias, as reading the notes illustrates the complexity of these syndromes.
Finally, after discharge, each patient is followed-up by our community outreach nurses, who review psychological, somatic, and social aspects of care. Follow-up is generally done on the phone, though community outreach nurses may see the patients within a dedicated clinic, to review progress, discuss symptomatic treatments, and prevent recurrent consultation to the ED [12]. Counseling may sometimes be required, though programs such as Marijuana Anonymous are currently non-existent in Switzerland, and so this is usually done with group behavioral therapy [30, 37]. A regular follow-up with the family doctor is also encouraged, as is physical activity, as this has been associated with prolonged abstinence [38, 40]. Should symptoms not resolve, or hospitalization be required for reasons other than purely symptomatic treatment (e.g., inadequate housing, previous psychiatric comorbidities, multiple failed attempts to quit), inpatient management including treatment (pain, nausea or insomnia for example), and psychosocial follow-up is governed according to a set protocol that includes a combination of the above [13].
Figure 2 summarizes our proposed emergency department therapeutic algorithm for cannabis use disorders.
Proposed emergency department therapeutic algorithm for cannabis use disorder. Rx, recipe; FBC, full blood count; U&Es, urea and electrolytes blood test; LFT, liver function tests; BGT, blood glucose test; PPI, proton-pump inhibitors; MET, motivational enhancement therapy; CBT, cognitive behavioral therapy
Limitations
While this paper covers many of the key pathophysiological and therapeutic possibilities for cannabis use disorders presenting to acute care, limitations arising from the retrospective nature of a literature review were identified during manuscript writing. We believe more research is needed regarding both acute and long-term treatment options.
Conclusion
CHS and CWS are rapidly becoming major public health issues and add to the caseloads of already chronically overburdened ED. Optimization of ED care is possible but requires understanding the pathophysiological differences of each syndrome. With the only known treatment being abstinence and the high risk of relapse, it is important to rely not solely on acute care but also on long-term follow-up strategies. Though rare, hospitalization may sometimes be required; we believe this can be reduced by a combination of specific acute care treatment choice and optimal community support programs.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CBT:
-
Cognitive behavioral therapy
- CHS:
-
Cannabinoid hyperemesis syndrome
- CM:
-
Contingency management
- CNS:
-
Central nervous system
- CVS:
-
Cyclic vomiting syndrome
- CWS:
-
Cannabis withdrawal syndrome
- ED:
-
Emergency department
- FBC:
-
Full blood count
- BGT:
-
Blood glucose test
- LFTs:
-
Liver function tests
- MET:
-
Motivational enhancement therapy
- PPI:
-
Proton-pump inhibitors
- Rx:
-
Recipe (prescription)
- THC:
-
Tetrahydrocannabinol
- TFM:
-
Twelve-step facilitation method
- U&Es:
-
Urea and electrolytes blood test
References
Baraniecki R, Panchal P, Malhotra DD, et al. Acute cannabis intoxication in the emergency department: the effect of legalization. BMC Emerg Med. 2021;21:32. https://doi.org/10.1186/s12873-021-00428-0.
OFSP - Office fédéral de la santé publique. Consommation de cannabis: faits et chiffres. https://www.bag.admin.ch/bag/fr/home/zahlen-und-statistiken/zahlen-fakten-zu-sucht/zahlen-fakten-cannabis.html. Accessed 05.2021.
Tramèr MR, Carroll D, Campbell FA, Reynolds DJM, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:6. https://doi.org/10.1136/bmj.323.7303.16.
Darmani NA. Cannabinoid-induced hyperemesis: a conundrum—from clinical recognition to basic science mechanisms. Pharmaceuticals. 2010;3(7):2163–77. https://doi.org/10.3390/ph3072163.
Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–70. https://doi.org/10.1136/gut.2003.036350.
Huestis, Marilyn A. Human Cannabinoid pharmacokinetics chemistry and biodiversity 4, no 8 2007; 1770-1804. DOI: https://doi.org/10.1002/cbdv.200790152
Russo EB, Spooner C, May L, Leslie R, Whiteley VL. Cannabinoid hyperemesis syndrome survey and genomic investigation. Cannabis Cannabinoid Res. 2022;7:336–44. https://doi.org/10.1089/can.2021.0046.
Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology. 2016;151(2):252–66. https://doi.org/10.1053/j.gastro.2016.04.015.
DeVuono MV, Parker LA. Cannabinoid hyperemesis syndrome: a review of potential mechanisms. Cannabis Cannabinoid Res. 2020;5:132–44. https://doi.org/10.1089/can.2019.0059.
DeVuono MV, Parker LA. Cannabinoid hyperemesis syndrome: a review of potential mechanisms. Cannabis Cannabinoid Res. 2020;5:132–44. https://doi.org/10.1089/can.2019.0059.
Lichtman A, Jl W, Kl LV, St N, Db A, Dm W, et al. Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur J Pharmacol. 1998;357(2-3):139–48. https://doi.org/10.1016/s0014-2999(98)00558-5.
Amir E, James MS, Paul DM. Cannabis in the arm: what can we learn from intravenous cannabinoid studies? Curr Pharm Des. 2012;18(32):4906–14. https://doi.org/10.2174/138161212802884618.
Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9–37. https://doi.org/10.2147/sar.s109576.
Gorelick DA, Levin KH, Copersino ML, Heishman SJ, Liu F, Boggs DL, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. 2012;123(1-3):141–7. https://doi.org/10.1016/j.drugalcdep.2011.11.007.
Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J Med Toxicol. 2017;13(1):71–87. https://doi.org/10.1007/s13181-016-0595-z.
Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid hyperemesis syndrome. Curr Drug Abuse Rev. 2011;4(4):241–9. https://doi.org/10.2174/1874473711104040241.
Chu F, Cascella M. Cannabinoid hyperemesis syndrome. Treasure Island (FL): StatPearls 2021. https://www.ncbi.nlm.nih.gov/books/NBK549915. Accessed 05.2021.
Sun S, Zimmermann AE. Cannabinoid hyperemesis syndrome. Hosp Pharm. 2013;48(8):650–5. https://doi.org/10.1310/hpj4808-650.
Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87(2):114–9. https://doi.org/10.1016/j.mayocp.2011.10.005.
Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–92. https://doi.org/10.1053/j.gastro.2016.02.011.
Livne O, Shmulewitz D, Lev-Ran S, Hasin DS. DSM-5 cannabis withdrawal syndrome: demographic and clinical correlates in U.S. adults. Drug Alcohol Depend. 2019;195(170-7). https://doi.org/10.1016/j.drugalcdep.2018.09.005.
Katz G, Lobel T, Tetelbaum A, Raskin S. Cannabis withdrawal - a new diagnostic category in DSM-5. Isr J Psychiatry Relat Sci. 2014;51(4):270–5.
Richards JR, Gordon BK, Danielson AR, Moulin AK. Pharmacologic treatment of cannabinoid hyperemesis syndrome: a systematic review. Pharmacother J Hum Pharmacol Drug Ther. 2017;37(6):725–34. https://doi.org/10.1002/phar.1931.
Lapoint J, Meyer S, Yu CK, Koenig KL, Lev R, Thihalolipavan S, et al. Cannabinoid hyperemesis syndrome: public health implications and a novel model treatment guideline. West J Emerg Med. 2018;19(2):380–6. https://doi.org/10.5811/westjem.2017.11.36368.
Ruberto AJ, Sivilotti MLA, Forrester S, Hall AK, Crawford FM, Day AG. Intravenous haloperidol versus ondansetron for cannabis hyperemesis syndrome (HaVOC): a randomized, controlled trial. Ann Emerg Med. 2021;77:613–9. https://doi.org/10.1016/j.annemergmed.2020.08.021.
Lee C, Greene SL, Wong A. The utility of droperidol in the treatment of cannabinoid hyperemesis syndrome. Clin Toxicol. 2019;57(9):773–7. https://doi.org/10.1080/15563650.2018.1564324.
Furyk JS, Meek RA, Egerton-Warburton D. Drugs for the treatment of nausea and vomiting in adults in the emergency department setting. Cochrane Database Syst Rev. 2015;9):CD010106. https://doi.org/10.1002/14651858.cd010106.pub2.
Pourmand A, Esmailian G, Mazer-Amirshahi M, Lee-Park O, Tran QK. Topical capsaicin for the treatment of cannabinoid hyperemesis syndrome, a systematic review and meta-analysis. Am J Emerg Med. 2021;43:35–40. https://doi.org/10.1016/j.ajem.2021.01.004.
McConachie SM, Caputo RA, Wilhelm SM, Kale-Pradhan PB. Efficacy of capsaicin for the treatment of cannabinoid hyperemesis syndrome: a systematic review. Ann Pharmacother. 2019;53(11):1145–52. https://doi.org/10.1177/1060028019852601.
Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacol. 2018;43(1):173–94. https://doi.org/10.1038/npp.2017.212.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86(1):22–9. https://doi.org/10.1016/j.drugalcdep.2006.04.014.
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacololgy. 2004;29(1):158–70. https://doi.org/10.1038/sj.npp.1300310.
Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1-3):142–50. https://doi.org/10.1016/j.drugalcdep.2010.12.010.
Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71(3):281–91. https://doi.org/10.1001/jamapsychiatry.2013.3947.
Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl). 2016; 233(13):2469-2478. DOI: https://doi.org/10.1007/s00213-016-4298-6
Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37(7):1689–98. https://doi.org/10.1038/npp.2012.14.
Kelly JF, Yeterian JD, Cristello JV, Kaminer Y, Kahler CW, Timko C. Developing and testing twelve-step facilitation for adolescents with substance use disorder: manual development and preliminary outcomes. Subst Abuse Res Treat 2016;10:55-64. DOI: https://doi.org/10.4137/SART.S39635
Budney AJ, Vandrey RG, Stanger C. Pharmacological and psychosocial interventions for cannabis use disorders. Revista brasileira de psiquiatria. 2010;32 Suppl 1(01):S46–55.
Mj Z, Dj P, Garey L, Manning K, Jbd H, Buckner J, et al. Perceived barriers for cannabis cessation: relations to cannabis use problems, withdrawal symptoms, and self-efficacy for quitting. Addict Behav. 2018;76(45-51). https://doi.org/10.1016/j.addbeh.2017.07.011.
Irons JG, Babson KA, Bergeria CL, Bonn-Miller MO. Physical activity and cannabis cessation. Am J Addict. 2014;23(5):485–92. https://doi.org/10.1111/j.1521-0391.2014.12135.x.
Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug and alcohol dependence. 2011;117(1):38–44. https://doi.org/10.1016/j.drugalcdep.2011.01.003.
Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2013;18:993–1002. https://doi.org/10.1111/j.1369-1600.2012.00461.x.
Bollom A, Austrie J, Hirsch W, Nee J, Friedlander D, Iturrino J, et al. Emergency Department burden of nausea and vomiting associated with cannabis use disorder: U.S. trends from 2006 to 2013. J Clin Gastroenterol. 2018;52(9):778–83. https://doi.org/10.1097/mcg.0000000000000944.
Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addict Abingdon Engl 2008; 103(5):787-799. DOI: 10.1111%2Fj.1360-0443.2008.02158.x
Ruger JP, Richter CJ, Spitznagel EL, Lewis LM. Analysis of costs, length of stay, and utilization of emergency department services by frequent users: implications for health policy. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11:1311–7. https://doi.org/10.1197/j.aem.2004.07.008.
OFSP - Office fédéral de la santé publique. Utilisation du cannabis à des fins médicales. https://www.bag.admin.ch/bag/fr/home/medizin-und-forschung/heilmittel/med-anwend-cannabis.html.
Acknowledgements
We would like to acknowledge the work of Mrs. Chantal Briones, a community care nurse, who took the time to review with us the different therapeutic options and discuss long-term follow-up.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
MR and EPH conceptualized the article, reviewed the literature, and drafted the first version. EPH, AE, and VDS reviewed the manuscript. All authors read, corrected, developed figures/tables, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Razban, M., Exadaktylos, A.K., Santa, V.D. et al. Cannabinoid hyperemesis syndrome and cannabis withdrawal syndrome: a review of the management of cannabis-related syndrome in the emergency department. Int J Emerg Med 15, 45 (2022). https://doi.org/10.1186/s12245-022-00446-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12245-022-00446-0