Abstract
Background
Tic disorder is a neuropsychiatric disorder characterized by involuntary movements or vocalizations. Previous studies utilizing diffusion-weighted imaging to explore white-matter alterations in tic disorders have reported inconsistent results regarding the affected tracts. We aimed to address this gap by employing a novel tractography technique for more detailed analysis.
Methods
We analyzed MRI data from 23 children with tic disorders and 23 healthy controls using TRActs Constrained by UnderLying Anatomy (TRACULA), an advanced automated probabilistic tractography method. We examined fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity, and mean diffusivity in 42 specific significant white matter tracts.
Results
Our findings revealed notable differences in the children with tic disorders compared to the control group. Specifically, there was a significant reduction in FA in the parietal part and splenium of the corpus callosum and the left corticospinal tract. Increased RD was observed in the temporal and splenium areas of the corpus callosum, the left corticospinal tract, and the left acoustic radiation. A higher mean diffusivity was also noted in the left middle longitudinal fasciculus. A significant correlation emerged between the severity of motor symptoms, measured by the Yale Global Tic Severity Scale, and FA in the parietal part of the corpus callosum, as well as RD in the left acoustic radiation.
Conclusion
These results indicate a pattern of reduced interhemispheric connectivity in the corpus callosum, aligning with previous studies and novel findings in the diffusion indices changes in the left corticospinal tract, left acoustic radiation, and left middle longitudinal fasciculus. Tic disorders might involve structural abnormalities in key white matter tracts, offering new insights into their pathogenesis.
Similar content being viewed by others
Introduction
Tic disorders represent a complex spectrum of neuropsychiatric conditions, characterized by involuntary, rapid, and recurrent motor movements or vocalizations, primarily observed in pediatric populations. These disorders, often preceded by distinctive premonitory sensations, typically manifest between the ages of 4 and 6, marking a critical developmental window in child neurology [3, 21]. The epidemiological profile of tic disorders is varied, with transient tics reported in 10–20% of school-aged children [38], while a comprehensive international meta-analysis indicates a global prevalence of 2.99% for transient tic disorder and 0.77% for chronic tic disorder [19, 37]. These figures underscore the public health significance of these conditions and highlight the need for a deeper understanding of their pathophysiology. Theoretical models elucidating the nature of tic disorders have evolved considerably over the years. The inhibition model, which posits a dysfunction in the cortico-basal ganglia-thalamo-cortical circuits, suggests impaired automatic inhibition of motor actions, while maintaining volitional control, as central to the development of tics [15, 31, 44]. However, while this model provides insights into the motor aspects of tics, it offers limited explanation of the sensory phenomena associated with them, such as premonitory urges [32, 36]. In contrast, the dopaminergic model offers a broader perspective, linking tic phenomenology to aberrations in dopamine neurotransmission. This model suggests that increased phasic dopamine release is intricately associated with both the generation of premonitory urges and the manifestation of tic symptoms [6, 24]. Further supporting this model are pharmacological studies that demonstrate the efficacy of dopamine antagonists in mitigating tic severity [38]. Additionally, neurochemical imaging studies have shown alterations in dopaminergic pathways in patients with tic disorders, reinforcing this hypothesis [8].
Diffusion tensor imaging (DTI) reveals the structural connectivity of the brain by measuring the diffusion of water molecules restricted by cell membranes of white matter tissue. The two common ways of analyzing DTI data are Tract-based spatial statistics (TBSS) and tractography. TBSS is an automated approach that allows whole-brain analysis of white matter in a voxel-wise manner, whereas tractography reconstructs 3-dimensional trajectories of specific white matter tracts, allowing a more detailed analysis of specific subpopulations of particular tracts. TRActs Constrained by UnderLying Anatomy (TRACULA) is one of the probabilistic tractography methods that has the advantage of being sensitive to the alterations of the predefined major white matter tracts [23, 46].
Previous studies on tic disorders have observed structural alteration in white matter. Increased fractional anisotropy (FA) in cerebello-thalamo-cortical tracts with an inverse linear relationship to tic severity [43], abnormalities among components of the fronto-striato-thalamic connections [25], and decreased FA in most of the major tracts including the internal capsule and corpus callosum were reported [33]. A study on adolescents also showed decreased axial diffusivity in various tracts including the corpus callosum, corticospinal tract, cingulum, anterior thalamic radiation, and inferior longitudinal fasciculus [41]. Adults with tic disorders showed lower FA values in the corpus callosum, particularly in fiber tracts connecting the bilateral primary and supplementary motor cortices [27]. Another structural network analysis study found reduced connectivity in the right hemisphere. The study also reported a positive correlation between global efficiency and YGTSS [39].
The presence of white matter abnormalities seems to be prominent among individuals with tic disorder. Furthermore, these pathophysiologic changes appear to continue from childhood through adulthood. Symptoms peak at around the ages of 10 to 12 and usually decline after puberty [20]. A plausible hypothesis regarding this subject suggests that puberty involves changes in white-matter structures [30], with particular emphasis on the corpus callosum [34]. Current study conducted on the adolescent sample to reflect the possible effect of the puberty of tracts in Tic disorder.
Yet, the specific tracts associated with the initiation and severity of tic disorder remain ambiguous. Previous studies adopted TBSS [33, 41], or ROI seeded probablistic tractography, only focused on tracts of interest or ROI from TBSS analysis [25, 27, 41, 43]. Given the practical difficulties of recruiting a sufficient number of participants in Tic disorders, restricting significant results to regions survived after multiple comparisons in a whole brain TBSS runs the risk of reducing power. An anatomically informed tracts-based approach is one alternative to address the issue considering the voxel-based nature of TBSS. Currunt study investigated white matter alteration in tic disorder using a novel method that can subdivide the major tracts in more detail. By adopting the novel tractography method allowing a more granular analysis of major white matter tracts, our approach seeks to delineate the specific neural pathways implicated in the onset and progression of tic disorders in more detail. This investigation is poised to enhance our understanding of the neurobiological basis of Tic disorder and potentially inform targeted therapeutic interventions.
Materials and methods
Participants
Patients were recruited from the department of psychiatry of Korea University Guro Hospital. Healthy controls were recruited from local schools and kindergartens. A total of 23 children with tic symptoms (mean age 10.40 ± 2.84 years) and 23 controls (mean age 10.00 ± 1.94 years) participated in the study. All participants had IQ scores above 70 on examination as measured by the Korean version of the Wechsler Intelligence Scale for Children fourth edition (K-WISC-IV), were free of psychotropic medication for at least 3 weeks, and had no history of neurological disorders including head trauma, tumors, or seizures. The demographics of all participants are summarized in Table 1, and there are no significant difference in demographic variables between two groups.
Diagnosis and clinical assessments
The diagnosis of tic disorders was made through clinical interviews by child and adolescent psychiatrists based on the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Tic severity was assessed using the Yale Global Tic Severity Scale (YGTSS) [22]. Patients were assessed for psychiatric comorbidities using the Korean version of the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) [18]. All research procedures were approved by the Institutional Review Board (IRB) of the Korea University Guro Hospital. Written informed consent was obtained from the parents or legal guardians of each participant. A more detailed description of the study procedures has been described in other published studies (e.g., [5]).
Brain imaging procedure
MRI data were collected at Korea University Guro Hospital using a Siemens 3 T Magnetom Prisma (SIEMENS Healthineers, Erlangen, Germany) with a 32 channel Head Coil. T1-weighted anatomical images were obtained using a T1-weighted magnetization-prepared rapid gradient-echo sequence (repetition time: 2,300 ms; echo time: 2.32 ms; inversion time: 900 ms; flip angle: 8°; field of view: 230 mm; voxel size: 0.9 mm isotropic; 208 slices; generalized auto-calibrating partially parallel acquisition acceleration factor: factor of two along the phase-encoding direction; received bandwidth per pixel: 200 Hz/pixel; echo spacing: 7.1 ms). Then, whole-brain diffusion-weighted images were obtained using a single-shot spin-echo echo-planar sequence with 64 gradient orientations (number of directions: 64; repetition time: 3,100 ms; echo time: 78 ms; flip angle: 90°; acquisition matrix: 112 × 112; field of view: 224 mm; voxel size: 1.0 × 1.0 × 2.0 mm; slice thickness, 2.0 mm; averages: 2). One image without motion probing gradient (b = 0 s/mm2) and 64 images with it (b = 1,000 s/mm2) were obtained for each subject.
Data processing
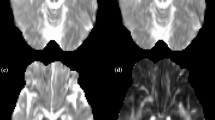
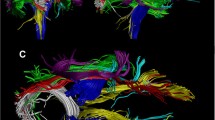
Cortical reconstruction and volumetric segmentation were performed with Freesurfer 7.3.2 (http://surfer.nmr.mgh.harvard.edu/). Automated segmentation of gray matter and white matter boundaries results were reviewed using Freeview for quality control and manually corrected when necessary according to the guidelines [14, 45]. Processed anatomical data is used for accurate white-matter reconstruction. Freesurfer TRACULA (TRActs Constrained by UnderLying Anatomy) software was used to reconstruct 42 major white-matter pathways using global probabilistic approach [23]. TRACULA calculates the probability distributions of neural pathways by combining a simplified model of diffusion called the “ball-and-stick” model with existing anatomical knowledge [46]. After determining the estimated distributions of the 42 major tracts, TRACULA derives four diffusion metrics for each tract: (1) fractional anisotropy (FA), which represents the fraction of diffusion that is directionally constrained; (2) axial diffusivity (AD), corresponding to the main direction of diffusion; radial diffusivity (RD), measuring the extent of diffusion perpendicular to the main direction; and mean diffusivity (MD), which indicates the overall magnitude of diffusion in the tissue. According to quality control pipeline [13], the resulting major 42 tracts were visually inspected to prevent the partially reconstruct tracts. No reconstruction failure or partially reconstructed tracts were found upon inspection.
Statistical analysis
First the analysis of covariance (ANCOVA) was conducted using averaged diffusivity scores of each reconstructed major tracts, while control the effect of age, biological sex, and IQ. Further analysis was conducted along the tracts shown significant difference between groups. For each diffusivity indices, we fit a general linear model (GLM) with the along-tract diffusivity value as the dependent variable, and diagnosis as independent variables, while same nuisance variables are controlled. The GLM analysis was performed with mri_glmfit for 1D data, then all the group analyses were corrected for multiple comparisons using Monte-Carlo Simulation with mri_glmfit-sim [12]. The cluster-forming threshold and the cluster-wise threshold for statistical significance were both set to p = 0.05, and 5,000 simulations were performed. Along with the p-value, effect sizes were estimated using Cohen’s d, and the Bayes Factor Bound (BFB) is calculated and reported for provide an approximate upper bound for Bayes Factor [1]. The correlation analysis was performed between the diffusion indices of significant tracts and YGTSS scores reflecting the severity of motor symptoms.
Results
The integrity of the white matter tracts
Relative to the control group, patients with tic disorder showed significantly lower FA values in the parietal part (p = 0.0010, d = 0.080, BFB = 53.26) and splenium of the corpus callosum (p = 0.0003, d = 0.387, BFB = 151.17), and in the left corticospinal tract (p = 0.0007, d = 0.794, BFB = 72.34). The patient group showed higher radial diffusivity (RD) values in the temporal part (p = 0.0011, d = 0.484, BFB = 49.09) and splenium of the corpus callosum (p = 0.0006, d = 0.209, BFB = 82.65), left corticospinal tract (p = 0.0009, d = 0.481, BFB = 58.28), and left acoustic radiation (p = 0.0010, d = 0.735, BFB = 53.26). The patient group also showed higher mean diffusivity (MD) values in the left middle longitudinal fasciculus (p = 0.0006, d = 0.178, BFB = 82.65, Table 2; Figs. 1 and 2).
Correlations between changes in the white matter and clinical scores
Significant correlations were observed between YTGSS motor scores and FA values of the parietal part of the corpus callosum (r= -0.493, p = 0.027) and RD values of the left acoustic radiation (r = 0.577, p = 0.008) while controlling for age, sex, and IQ (Fig. 3). No significant correlations were observed between YTGSS vocal scores and diffusivity indices.
Discussion
Our study’s findings of altered white matter integrity in the corpus callosum, corticospinal tract, acoustic radiation, and middle longitudinal fasciculus in tic disorder patients underscores the neuroanatomical changes associated with the condition. Since the study conducted on the adolescent participants of mean age 10.40, the changes of above tracts might be the important markers of the onset and prognosis of the Tic disorder.
The corpus callosum, as the largest white matter structure in the brain, plays a pivotal role in coordinating motor functions and cognitive processes between the two hemispheres. Its impairment could lead to the disrupted synchronization of neuronal activity, which is crucial for the smooth execution of voluntary movements. The corpus callosum connects the two cerebral hemispheres and plays an important role in interhemispheric inhibition of voluntary movements [10]. It is suggested that the tract is also integral to the compensatory neuroregulatory system, which involves prefrontal regulation of motor activity within the cortico-striato-thalamo-cortical (CSTC) circuits in tic disorder [16, 27, 35]. FA reduction in the corpus callosum has been repeatedly observed among children and adults with tic disorders [2, 4, 16, 33, 35]. Children with tic disorder had lower FA values in all five subregions of the corpus callosum, and the result remained stable even after excluding patient with comorbid ADHD or OCD, as well as patients on medication [35]. The observed lower FA and higher RD in the corpus callosum, particularly, align with the hypothesis that tic disorders involve disruptions in interhemispheric communication. This is consistent with the theory that tics are a result of dysregulated networks involving both cortical and subcortical structures [42].
Furthermore, the alterations in the corticospinal tract observed in our study are particularly intriguing. The corticospinal tract is a pathway that originates in the primary motor cortex of the cerebrum and extends down through the brainstem and spinal cord. It plays a critical role in voluntary movement by transmitting signals from the motor cortex to the muscles. This tract is fundamental in the initiation and modulation of voluntary movements. The changes in FA and RD we noted could reflect a structural basis for the motor symptoms characteristic of tic disorders. Prior research has highlighted alteration of the corticospinal tract in tic disorder. Specifically, a decrease in FA and an increase in RD have been documented [33]. These alterations could be indicative of a broader disruption in the motor network, potentially contributing to the involuntary movements seen in these patients.
The increased RD in the left acoustic radiation found in our study is another novel finding. This tract originates in the medial geniculate nucleus (MGN) and curves anteriorly through the white matter within the transverse temporal gyrus to the primary auditory cortex [40]. The acoustic radiation, involved in auditory processing, has not been traditionally associated with motor function. However, our results suggest a possible link between auditory processing pathways and motor symptom severity in tic disorders. This could be reflective of the multisensory integration issues often reported in these patients with neurodevelopmental disorders [9]. The medial geniculate nucleus, from which the acoustic radiation originates, is known to have connections with various cortical and subcortical areas involved in motor control. Thus, alterations in this pathway could contribute to the complex sensorimotor integration challenges observed in tic disorders.
The elevated MD values in the left middle longitudinal fasciculus (MdLF) observed in our patient group also warrant attention. The MdLF is an associative longitudinal fiber connecting the superior temporal gyrus and the inferior parietal lobule, specifically the angular gyrus [26, 29]. The MdLF was believed to perform language-related functions. However, an intraoperative study found that electrostimulation of MdLF did not interfere with picture naming tasks, and its surgical removal did not result in any language deficits [7]. Current hypotheses suggested that the MdLF, particularly on the left hemisphere, plays a role in the associative learning of verbal-auditory stimuli [20]. The MdLF, implicated in language processing and associative learning, may have a more nuanced role in tic disorders than previously thought. The association between structural changes in the MdLF and tic disorders could reflect underlying disruptions in the neural networks involved in complex cognitive functions. This is in line with recent studies suggesting that tic disorders are not merely motor disorders but involve a broader spectrum of cognitive and sensory processing anomalies [28]. In addition, the elevated MD values in the left MdLF observed in our patient group can be related to previous studies interpreting tic disorder as a problem at the temporo-parietal junction [11].
Several limitations of this study should be noted. Although this research found several differences between children with and without tic disorders, the sample size of the study is relatively small for general neuroimaging studies. Therefore, the results of this study should be interpreted with caution, considering the effect size is small to moderate.
Despite these limitations, the current findings highlight the difference in major white matter tracts of the children with Tic disorder compare to typically developed controls. The finding is in line with the recent studies conceptualize the Tic disorder as the disrupted network function [17, 47].
Conclusion
Taken together, our findings suggest that tic disorders are characterized by a complex pattern of white matter alterations, not limited to motor pathways but extending to sensory and cognitive processing networks. This aligns with the emerging perspective that tic disorders are a result of widespread neurodevelopmental anomalies affecting multiple brain networks. Future research should focus on longitudinal studies to understand the progression of these white matter changes and their relationship with clinical symptoms. Additionally, exploring the potential of these neuroimaging findings as biomarkers for early diagnosis and treatment response in tic disorders could be a promising avenue. The integration of neuroimaging with genetic and clinical data may also provide deeper insights into the etiology and pathophysiology of tic disorders, paving the way for more targeted therapeutic interventions.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Benjamin DJ, Berger JO. Three recommendations for improving the use of p-values. Am Stat. 2019;73(sup1):186–91.
Bharti K, Conte G, Tommasin S, Gianni C, Suppa A, Mirabella G, Cardona F, Pantano P. White matter alterations in drug-naive children with Tourette syndrome and obsessive-compulsive disorder. Front Neurol. 2022;13:960979.
Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. 2009;67(6):497–501.
Bruce AB, Yuan W, Gilbert DL, Horn PS, Jackson HS, Huddleston DA, Wu SW. Altered frontal-mediated inhibition and white matter connectivity in pediatric chronic tic disorders. Exp Brain Res. 2021;239(3):955–65.
Chi S, Mok YE, Kang J, Gim JA, Han C, Lee MS. Cytokine levels reflect tic symptoms more prominently during mild phases. BMC Neurosci. 2023;24(1):57.
Conceicao VA, Dias A, Farinha AC, Maia TV. Premonitory urges and tics in Tourette syndrome: computational mechanisms and neural correlates. Curr Opin Neurobiol. 2017;46:187–99.
De Hamer W, Moritz-Gasser PC, Gatignol S, Duffau P, H. Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum Brain Mapp. 2011;32(6):962–73.
Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, van der Doef TF, Boellaard R, Westenberg H, van Balkom A, Lammertsma AA, van Berckel BN. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23(11):1423–31.
Dionne-Dostie E, Paquette N, Lassonde M, Gallagher A. Multisensory integration and child neurodevelopment. Brain Sci. 2015;5(1):32–57.
Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005;28(4):940–6.
Eddy CM. 2016. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia(89), 465–77.
Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 2006;33(4):1093–103.
He X, Stefan M, Pagliaccio D, Khamash L, Fontaine M, Marsh R. A quality control pipeline for probabilistic reconstruction of white-matter pathways. J Neurosci Methods. 2021;353:109099.
Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, DeLorenzo C. Test–retest reliability of freesurfer measurements within and between sites: effects of visual approval process. Hum Brain Mapp. 2015;36(9):3472–85.
Jackson GM, Draper A, Dyke K, Pépés SE, Jackson SR. Inhibition, disinhibition, and the control of action in Tourette syndrome. Trends Cogn Sci. 2015;19(11):655–65.
Jackson SR, Parkinson A, Jung J, Ryan SE, Morgan PS, Hollis C, Jackson GM. Compensatory neural reorganization in Tourette syndrome. Curr Biol. 2011;21(7):580–5.
Jurgiel J, Miyakoshi M, Dillon A, Piacentini J, Makeig S, Loo SK. Inhibitory control in children with tic disorder: aberrant fronto-parietal network activity and connectivity. Brain Commun. 2021;3(2):fcab067.
Kim YS, Cheon KA, Kim BN, Chang SA, Yoo HJ, Kim JW, Cho SC, Seo DH, Bae MO, So YK, Noh JS, Koh YJ, McBurnett K, Leventhal B. The reliability and validity of kiddie-schedule for affective disorders and Schizophrenia-Present and Lifetime Version- Korean version (K-SADS-PL-K). Yonsei Med J. 2004;45(1):81–9.
Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90.
Latini F, Trevisi G, Fahlstrom M, Jemstedt M, Alberius Munkhammar A, Zetterling M, Hesselager G, Ryttlefors M. New insights into the anatomy, connectivity and clinical implications of the Middle Longitudinal Fasciculus. Front Neuroanat. 2020;14:610324.
Leckman JF. Tourette’s syndrome. Lancet. 2002;360(9345):1577–86.
Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–73.
Maffei C, Lee C, Planich M, Ramprasad M, Ravi N, Trainor D, Urban Z, Kim M, Jones RJ, Henin A, Hofmann SG, Pizzagalli DA, Auerbach RP, Gabrieli JDE, Whitfield-Gabrieli S, Greve DN, Haber SN, Yendiki A. Using diffusion MRI data acquired with ultra-high gradient strength to improve tractography in routine-quality data. NeuroImage. 2021;245:118706.
Maia TV, Conceição VA. Dopaminergic disturbances in Tourette syndrome: an integrative account. Biol Psychiatry. 2018;84(5):332–44.
Makki MI, Munian Govindan R, Wilson BJ, Behen ME, Chugani HT. Altered fronto-striato-thalamic connectivity in children with Tourette syndrome assessed with diffusion tensor MRI and probabilistic fiber tracking. J Child Neurol. 2009;24(6):669–78.
Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19(4):777–85.
Martino D, Delorme C, Pelosin E, Hartmann A, Worbe Y, Avanzino L. 2017. Abnormal lateralization of fine motor actions in Tourette syndrome persists into adulthood. PLoS ONE 12(7), e0180812.
Martino D, Ganos C, Pringsheim TM. Tourette Syndrome and Chronic Tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. 2017b;134:1461–90.
Menjot de Champfleur N, Maldonado L, Moritz-Gasser I, Machi S, Le Bars P, Bonafe E, Duffau A, H. Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151–7.
Menzies L, Goddings A-L, Whitaker KJ, Blakemore S-J, Viner RM. The effects of puberty on white matter development in boys. Dev Cogn Neurosci. 2015;11:116–28.
Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25(3):190–8.
Morand-Beaulieu S, Grot S, Lavoie J, Leclerc JB, Luck D, Lavoie ME. The puzzling question of inhibitory control in Tourette syndrome: a meta-analysis. Neurosci Biobehav Rev. 2017;80:240–62.
Neuner I, Kupriyanova Y, Stocker T, Huang R, Posnansky O, Schneider F, Tittgemeyer M, Shah NJ. White-Matter abnormalities in Tourette syndrome extend beyond motor pathways. NeuroImage. 2010;51(3):1184–93.
Piekarski DJ, Colich NL, Ho TC. 2023. The effects of puberty and sex on adolescent white matter development: a systematic review. Dev Cogn Neurosci, 101214.
Plessen KJ, Gruner R, Lundervold A, Hirsch JG, Xu D, Bansal R, Hammar A, Lundervold AJ, Wentzel-Larsen T, Lie SA, Gass A, Peterson BS, Hugdahl K. Reduced white matter connectivity in the corpus callosum of children with Tourette syndrome. J Child Psychol Psychiatry. 2006;47(10):1013–22.
Rawji V, Modi S, Latorre A, Rocchi L, Hockey L, Bhatia K, Joyce E, Rothwell JC, Jahanshahi M. Impaired automatic but intact volitional inhibition in primary tic disorders. Brain. 2020;143(3):906–19.
Robertson MM. The prevalence and epidemiology of Gilles De La Tourette syndrome. Part 2: tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res. 2008;65(5):473–86.
Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J obsessive-compulsive Relat Disorders. 2014;3(4):394–400.
Schlemm E, Cheng B, Fischer F, Hilgetag C, Gerloff C, Thomalla G. Altered topology of structural brain networks in patients with Gilles De La Tourette syndrome. Sci Rep. 2017;7(1):10606.
Siegbahn M, Engmer Berglin C, Moreno R. Automatic segmentation of the core of the acoustic radiation in humans. Front Neurol. 2022;13:934650.
Sigurdsson HP, Pepes SE, Jackson GM, Draper A, Morgan PS, Jackson SR. Alterations in the microstructure of white matter in children and adolescents with Tourette syndrome measured using tract-based spatial statistics and probabilistic tractography. Cortex. 2018;104:75–89.
Singer HS, Augustine F. Controversies surrounding the pathophysiology of tics. J Child Neurol. 2019;34(13):851–62.
Thomalla G, Siebner HR, Jonas M, Baumer T, Biermann-Ruben K, Hummel F, Gerloff C, Muller-Vahl K, Schnitzler A, Orth M, Munchau A. Structural changes in the somatosensory system correlate with tic severity in Gilles De La Tourette syndrome. Brain. 2009;132(Pt 3):765–77.
Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Feger J. Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord. 2015;30(9):1155–70.
Vahermaa V, Aydogan DB, Raij T, Armio RL, Laurikainen H, Saramäki J, Suvisaari J. FreeSurfer 7 quality control: key problem areas and importance of manual corrections. NeuroImage. 2023;279:120306.
Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics. 2011;5:23.
Zouki JJ, Ellis EG, Morrison-Ham J, Thomson P, Jesuthasan A, Al-Fatly B et al. Mapping a network for tics in Tourette syndrome using causal lesions and structural alterations. Brain Commun. 2023;5(3):fcad105.
Acknowledgements
The authors acknowledge H.M.C., and H.N.C. for their assistance in data collection and participant management.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C0012).
Author information
Authors and Affiliations
Contributions
Study concept and design: J.K., M.-S.L. Acquisition, analysis, or interpretation of data: J.K., M.-S.L., S.C., S.H.K., Methodology: J.K., M.-S.L., Drafting of the manuscript: J.K.,Y.E.M.,S.C., Critical revision of the manuscript for important intellectual content: J.K., M.-S.L., S.H.K., J.-A.G., Administrative, technical, or material support: J.K., M.-S.L, Supervision: J.K., M.-S.L. and J.-A.G.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The research processes were approved by the Institutional Review Board (IRB)of the Korea University Guro Hospital (2021GR0275). All research methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from parents or legal guardians (if parents not available) of all participants.
Consent for publication
Not applicable.
Competing interests
All authors report no financial interests or potential conflicts of interest that would bias the work reported herein.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kang, J.C., Chi, S., Mok, Y.E. et al. Diffusion indices alteration in major white matter tracts of children with tic disorder using TRACULA. J Neurodevelop Disord 16, 40 (2024). https://doi.org/10.1186/s11689-024-09558-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-024-09558-5