Abstract
Background
Continued dietary treatment since early diagnosis through newborn screening programs usually prevents brain-related complications in phenylketonuria (PKU). However, subtle neurocognitive and brain alterations may be observed in some adult patients despite early treatment. Nevertheless, neuropsychological and neuroimaging studies in the field remain scarce.
Objectives
This work aimed to determine possible neuropsychological and structural brain alterations in treated adult patients with PKU.
Methods
Thirty-five patients with PKU and 22 healthy controls (HC) underwent neuropsychological assessment and T1-weighted magnetic resonance imaging on a 3 T scanner. FreeSurfer (v.7.1) was used to obtain volumetric measures and SPSS (v27.0.1.0) was used to analyze sociodemographic, neuropsychological, volumetric, and clinical data (p < 0.05).
Results
Adult patients with PKU showed significantly lower performance than HC in Full Scale IQ (t = 2.67; p = .010) from the WAIS-IV. The PKU group also showed significantly lower volumes than HC in the pallidum (U = 224.000; p = .008), hippocampus (U = 243.000; p = .020), amygdala (U = 200.000; p = .002), and brainstem (t = 3.17; p = .006) as well as in total cerebral white matter volume (U = 175.000; p = .001). Blood phenylalanine (Phe) levels in PKU patients were negatively correlated with the pallidum (r = -0.417; p = .013) and brainstem (r = -0.455, p = .006) volumes.
Conclusions
Adult patients with early-treated PKU showed significantly lower global intelligence than HC. Moreover, these patients showed reduced global white matter volume as well as reductions in the volume of several subcortical grey matter structures, which might be related to the existence of underlying neurodevelopmental alterations. Higher blood Phe levels were also negatively correlated with pallidum and brainstem, suggesting a higher vulnerability of these structures to Phe toxicity.
Similar content being viewed by others
Background
Phenylketonuria (PKU) (OMIM #261600) and its milder variant hyperphenylalaninemia (HPA) (OMIM #261630) are inborn errors of amino acid metabolism. These conditions result from homozygous or compound heterozygous mutations in the phenylalanine hydroxylase gene (OMIM #612349), which encodes an enzyme responsible for the conversion of phenylalanine (Phe) into tyrosine. The disruption of this enzymatic pathway leads to a notable accumulation of Phe and a corresponding decrease in tyrosine levels. This imbalance manifests in elevated Phe concentrations in the brain, inducing various neuropathological outcomes. Among these neurotoxic effects [1], there is a relevant effect on the dopaminergic system [2], along with an adverse impact on oligodendroglia, causing defective myelin synthesis [3, 4].
Blood Phe concentration is the primary reported marker of metabolic control. Although target ranges are based on plasma Phe (the upper target for adults has been set at 600 μmol/L according to the European guidelines) [1], patients are routinely monitored using dried bloodspot (DBS) specimens due to the convenience of their collection [5].
PKU early diagnosis through newborn screening (NBS) programs and early treatment with a restricted low-protein diet together with an adapted nutritional formula, and sometimes tetrahydrobiopterin (BH4), prevents severe neurological damage and allows a good quality of life and long-term survival [2]. However, despite early treatment, cognitive, psychiatric and brain alterations have also been reported [6,7,8].
White matter alterations associated with PKU are the most consistent neuroradiological finding. Indeed, neuropathological studies of untreated patients showed an altered myelination pattern. Magnetic resonance imaging (MRI) (T2 weighted images and FLAIR images) allows the identification of impaired myelination as high-signal intensity in periventricular white matter. In a review including 312 individuals with PKU aged between 0.9 and 49 years, Anderson and Leuzzi [3] reported abnormal white matter in 93% of cases viewed on T2; and some years later Mastrangelo et al., also in a mixed age cohort, reported alterations in white matter mostly seen during adulthood [9].
There is clear evidence that the degree of white matter (WM) abnormalities, and specifically diffusivity in WM, is influenced by metabolic control [10] and thus, it could change according to diet adherence and/or clinical evolution even in treated patients [3, 11] due to individual sensitivity to Phe [12]. Diffusion tensor imaging (DTI) is a suitable technique to identify and quantify regional white matter changes through diffusion [13], with the posterior-anterior decrement in mean diffusivity (MD) being the most consistently reported finding in PKU patient samples [13,14,15,16,17,18,19,20,21,22,23].
On the other hand, studies on volumetric gray matter subcortical structure in PKU are scarce. An early study published in 2005 performed manual volumetry of brain structures in a sample of 31 adult patients with PKU and found hippocampal reductions but no differences in the caudate, nucleus lentiformis, or thalamic structures [24]. In 2006, Pérez-Dueñas et al. [25] studied a sample including children and adolescents with PKU using the voxel-based morphometry (VBM) technique and reported increased volume of the ventral striatum. Using the same neuroimaging approach but in a sample including only adults, Pilotto et al. [26] reported significant gray matter reductions in the putamen and thalamus. Nevertheless, only putamen nuclei remained significant after correcting for multiple comparisons. In a small sample of 13 participants with PKU, mixing children, adolescents, and adults and performing manual volumetry of basal ganglia, Bodner et al. [27] reported increased volumes of putamen and a significant positive correlation between current Phe levels and putamen volume. A similar positive correlation was reported by Brown et al. [28] using automatic volumetry. To summarize, these discrepant results may be explained by methodological differences and/or the inclusion of dissimilar study groups, sometimes including patients with immature brains. Currently, only one study has focused on adult patients exclusively, and this observed reductions in the putamen volume [26].
Although traditionally it was considered uncommon, recent studies are increasingly demonstrating cortical gray matter involvement in the PKU setting. Pfaendner et al. [24] described global reductions of estimated gray matter volume in adult patients with PKU, and Pérez-Dueñas et al. [25] found gray matter reductions in the motor cortex and the left premotor cortex using the VBM approach. Later, Christ et al. [29] obtained average gray matter volumetric measurements for each major cortical lobe and reported a decrease in parietal cortex volume in the group with PKU (19 patients with an age range from 9 to 33 years) compared with the control group. Subsequent subregional analysis revealed significant differences in parietal (e.g., left precuneus, dorsal supramarginal gyrus) and occipital regions (e.g., left inferior occipital gyrus, right posterior collateral sulcus). Later, Muri et al. [30] reported whole brain mean cortical thickness reduction in adult patients with PKU based on residual scores obtained by considering intracranial volume and age. However, surface area on a whole-brain level did not differ between patients and controls. At the lobar level, patients showed a significantly thinner cortex in the left and right temporal, parietal, and occipital lobar regions of interest (ROIs). Conversely, Hawks et al. [19] found no significant group differences in cortical whole-brain measures (cortical gray matter volume, total surface area, and average cortical thickness). To the best of our knowledge, there are no previous studies that have demonstrated a vertex-wise cortical thickness reduction in adults with PKU.
In summary, current research suggests a link between elevated Phe levels, which may indicate Phe toxicity, and anomalies in brain anatomy [10] and neuropsychological functioning, mostly when referred to subcortical structures, even in treated patients with PKU [3, 10]. Nevertheless, most studies have been performed on children or on mixed child and adult samples. Only four structural MRI-quantified studies have included adults only [26, 28, 30, 31]. As adulthood is the ideal condition in which to study structural changes associated with PKU as no significant neurodevelopmental variations are expected, we selected a relatively large sample of adults with PKU under early dietary treatment in the current study. We primarily aimed to investigate differences in cortical and subcortical gray matter and global volumetric measures in comparison to healthy controls. As a secondary objective, we tested the possible relationship between structural alterations and metabolic and neuropsychological variables.
Methods
Participants
Participants in this study were recruited from the Adult Inherited Metabolic Disorders Unit at the Hospital Clínic (Barcelona, Catalonia, Spain). The total sample comprised 57 participants, 35 of whom were early-treated PKU or hyperphenylalaninemia (HPA) patients; the remaining 22 participants were healthy controls (HC) matched according to age, sex, and body mass index (BMI).
The inclusion criteria for PKU patients were: (1) age above 18 years old and (2) genetic diagnosis of PKU or HPA. The exclusion criteria for patients were: (1) intelligence quotient estimation below 70 according to Wechsler Adult Intelligence Scale 4th edition (WAIS-IV) tests, (2) pregnancy or planning a pregnancy during the study period, (3) active cancer, (4) severe chronic hepatic disease, (5) acute cardiovascular event in the 6 months prior to study inclusion, (6) common MRI contraindications, (7) claustrophobia, (8) pathological MRI findings other than mild white matter hyperintensities in long-TR sequences, and (9) MRI artifacts. The exclusion criteria for HC were the same as those applied to the PKU group.
This study was approved by the Bioethics Committee of the University of Barcelona (IRB00003099) and Hospital Clínic of Barcelona (HCB/2020/0552) and was conducted in accordance with the basic principles of the Declaration of Helsinki, among other relevant regulations and guidelines. All participants in this study provided signed written informed consent, after a complete explanation of the procedures involved.
Clinical data
Sociodemographic information and clinical features of participants were also collected, including the date of PKU or HPA diagnosis, Phe monitoring using DBS, venous Phe, previous/current pharmacological treatments, use of adapted formulas (e.g., protein substitutes), consumption of dietetic supplements, subjective cognitive complaints through a clinical interview, and other medical diagnoses. Specific information regarding protein intake was also available, differentiating between natural protein intake and total (natural plus formula-derived protein) intake.
The Phe analysis was performed in DBS via tandem mass spectrometry (MS/MS) using the NeoBase™ 2 Non-derivatized kit (Revvity, Inc; Waltham, Massachusetts, U.S.). Briefly, to extract Phe from 3.2 mm of DBS, an organic compound solution that includes the deuterated Phe-d3 (internal standard) was added. Subsequently, 10 µL of this solution was directly injected into the MS/MS (Xevo-TQD; Waters Corp; Milford, Massachusetts, U.S.). Acquisition was performed in positive ionization and Multiple Reaction Monitoring modes using Masslynx software (Waters Corp). The run time was 2.5 min. The concentration of Phe was calculated based on the area relative to its internal standard, which has a known concentration, using Neolynx software (Waters Corp). The results are expressed in µmol/L.
Values of Phe obtained from DBS conformed to the index of dietary control (IDC), which was approximated using the median Phe levels measured in DBS recorded in the year prior to study inclusion (approximately 6–12 measurements per year for each patient) [30, 32].
Neuropsychological assessment
The participants included in this study (both HC and patients with PKU) underwent a comprehensive neuropsychological assessment selected according to the most affected domains reported in previous literature [33, 34].The neuropsychological battery included (1) the Vocabulary subtest (WAIS-IV) used to estimate premorbid functioning, (2) the Similarities subtest (WAIS-IV), (3) the Arithmetic subtest (WAIS-IV), (4) the Digit Span subtest (WAIS-IV), which includes Digit Forward, Digit Backward and Digit Sequencing, (5) Letter-Number Sequencing (WAIS-IV), (6) Block Design (WAIS-IV), (7) Matrix Reasoning (WAIS-IV), (8) Digit-Symbol Coding (WAIS-IV), (9) Symbol Search (WAIS-IV), (10) Rey’s Auditory Verbal Learning Test (RAVLT), including immediate, delayed recall after 20 min, and recognition, (11) parts A and B of the Trail Making Test (TMT), (12) semantic (animals) and phonemic (letter “P”) fluency tests, and (13) the Rey-Osterrieth Complex Figure (ROCF), including copy and immediate recall. Behavior and executive functioning were also assessed through the self-reported Behavior Rating Inventory of Executive Function for Adults (BRIEF-A).
MRI acquisition
High-resolution three-dimensional T1-weighted images were acquired in the sagittal plane (TR = 2400 ms, TE = 2.22 ms, TI = 1000 ms, flip angle 8°, 208 slices, FOV = 256 mm; 0.8 mm isotropic voxel) using a Siemens Magnetom Prisma 3 T scanner located at the Centre de Diagnostic per la Imatge of the Hospital Clínic de Barcelona (Catalonia, Spain). The scanning protocol used in this study also included T2-weighted images in axial orientation (TR = 3200 ms, TE = 563 ms; 512x307 matrix, flip angle 120°, slice thickness 0.8 mm with a 1.5 mm interslice gap) and an axial FLAIR sequence (TR = 9000 ms, TE = 125 ms; TI = 2500; 250×171 matrix, flip angle 150°, slice thickness 4 mm).
Cortical thickness and volumetric measures
Cortical thickness was estimated using the automated FreeSurfer stream version 7.1 available at https://surfer.nmr.mgh.harvard.edu/. The procedures in FreeSurfer included removal of non-brain data, registration to Talairach space, intensity normalization, and tessellation of the gray matter and white matter boundaries, automated topology correction, and accurate surface deformation following intensity gradients to identify tissue borders. Cortical thickness was calculated as the distance between the white and gray matter surfaces at each vertex of the reconstructed cortical mantle (https://freesurfer.net/fswiki/FreeSurferMethodsCitation). Results for each subject were visually inspected, and the appropriate manual corrections were performed to ensure the accuracy of registration, skull stripping, segmentation, and cortical surface reconstruction (https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData).
Deep gray matter mean volumes (e.g., in the thalamus, putamen, pallidum, caudate, hippocampus, amygdala, accumbens, and brainstem) and total cortical and subcortical gray matter were extracted through FreeSurfer version 7.1 [35].
The volumetric measures are presented in ratios, dividing the brain structure volume by the estimated total intracranial volume (ICV) and multiplying the result by 100.
Statistical analyses
Analyses of sociodemographic, neuropsychological, volumetric, and clinical data were performed using IBM SPSS Statistics 27.0.1.0. Continuous variables were analyzed using Student t-tests or Mann–Whitney U tests to accommodate both normal and non-normal distributions, while categorical variables were assessed through Pearson’s chi-squared test. The Mann–Whitney U test specifically addressed non-normal variables. However, significance in neuropsychological and volume data was reported exclusively for values that passed multiple comparison corrections that had survived family-wise error rate (FWE) correction and the false discovery rate (FDR) through MATLAB (v.R2020b). In all cases the significance threshold was set at a p-value < 0.05.
The study also explored relationships between volume variables, neuropsychological performance and metabolic control assessed by IDC, using pairwise Spearman’s rank correlation analyses, with a significance threshold of p < 0.05.
Intergroup cortical thickness analyses were conducted through a vertex-by-vertex general linear model (GLM) with FreeSurfer version 7.1, including cortical thickness as a dependent factor and group as an independent factor. Cluster-extent correction for multiple comparisons was tested using the Monte-Carlo simulation with 10,000 iterations implemented in FreeSurfer, in order to prevent false positives. Only the clusters that survived FDR with the statistical significance threshold set at p < 0.05 were reported.
The “ggseg” package in R was used for the graphical visualization of volumetric data.
Results
The sociodemographic and clinical characteristics of the participants are summarized in Table 1. There were no between-group differences in age (U = 311.500; p = 0.228), sex (X2 = 0.127; p = 0.722), parents’ education (U = 382.000; p = 0.961), or BMI (t = 0.889; p = 0.189), but there were differences in years of education (U = 218.500; p = 0.006), psychiatric comorbidities (X2 = 8.568; p = 0.003) and natural protein intake (U = 60.000; p = < 0.001).
Adult patients with PKU/HPA showed significantly lower performance than HC in the Verbal Comprehension Index (VCI) (t = 2.08; p = 0.043) and prorated Full Scale IQ (FSIQ) (t = 2.67; p = 0.010) from the WAIS-IV (Table 2). Only FSIQ survived FDR correction (F = 7.107; p = 0.035).
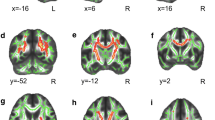
The PKU group showed significantly lower volumes than HC in the pallidum (U = 224.000; p = 0.008), hippocampus (U = 243.000; p = 0.020), amygdala (U = 200.000; p = 0.002), brainstem (t = 3.17; p = 0.006) and total cerebral white matter (U = 175.000; p = 0.001) (Table 3; Fig. 1). These results remained significant after adjustment for age.
PKU showed statistically significant lower volumes, presented as ratio scores (volume/intracranial volume*100), in the pallidum (U = 224.000; p = .008), hippocampus (U = 243.000; p = .020), amygdala (U = 200.000; p = .002), brainstem (t = 3.17; p = .006) and total cerebral white matter (U = 175.000; p = .001), in comparison with HC
IDC in PKU patients was negatively correlated with the pallidum (r = -0.417; p = 0.013) and brainstem (r = -0.455, p = 0.006) volumes (Fig. 2). No correlations were found between volume and neuropsychological performance or natural protein intake.
Discussion
We found gray matter reductions in subcortical gray matter in PKU. Specifically, patients differed from controls in the volumes of pallidum, hippocampus, amygdala, and brainstem. In addition, whereas total cerebral white matter volume was decreased, no changes were detected in the volumetric or thickness measures of cortical gray matter in early treated adult PKU.
Basal ganglia abnormalities have previously been reported in both directions i.e. increased [25, 27] and decreased [31] volumes. Since volumetric increases have only been seen in samples including children and adolescents, it can be argued that the effects of Phe in basal ganglia may depend on the degree of maturation and/or the long-term effects of Phe levels. Our results on basal ganglia are consistent with the only previous study performed in adults with PKU [31]. However, a discrepancy with our observations should be highlighted. Whereas Pilotto et al. [31] reported significant differences in the putamen, we found significant results in the pallidum. Both basal ganglia structures have a high density of dopamine receptors, as does the brainstem, which was also reduced in volume in our sample. In patients with PKU, intracerebral dopamine depletion has been postulated due to reduced Tyr uptake and altered dopamine synthesis [38]. Therefore, we speculate that Phe toxicity could be associated with specific abnormalities in dopaminergic structures. In this regard, brainstem reduction has also been described in neuropathological studies of non-treated patients with PKU [39]. Our study provides the first evidence on brainstem volume reduction in treated PKU patients using an automatic MRI segmentation procedure.
Moreover, we also found volumetric decreases in limbic structures i.e. the hippocampus and amygdala. Hippocampal reduction in adult PKU patients was reported by Pfaendner et al. [24] using a manual volumetry approach. They found a 14.5% reduction in patients compared to controls. Wesonga et al. [20] found that MD values of the hippocampus differed between patients with PKU and controls, and also reported increased values with aging in PKU individuals but not in controls. The age by group interaction was also significant. Similar results were reported by Hawks et al. [19] Thus, hippocampal involvement in PKU seems to be a consistent finding. Interestingly, we also found amygdala reductions that could be related to the emotional changes seen in some patients. This structure was not included in the ROI analyses [22, 24, 27]. Our results point to the need to pay special attention to this structure in further multicenter studies.
Moreover, we found no significant differences in whole brain gray matter volume, global cortical thickness, or cortical thickness maps. Whereas these results are consistent with the findings of Hawks et al. [19], who applied a similar MRI approach to our group, they conflict with recent results from Muri et al. [30], who suggested that cortical thickness is a particularly sensitive marker for gray matter alterations. It is noteworthy that previous results on cortical thickness were based on mean cortical thickness measures using global or ROI measurements. The ROI approach reduces problems related to multiple comparisons and is advantageous when an a priori hypothesis is clearly stated but not when the objective is the study of whole-brain cortical involvement. In this regard, no whole-brain vertex-wise approaches had been reported before the current study. Previous research has even identified variations in the gray matter of the cerebellum [40]; however, this region was not examined in our study.
Finally, our correlation analyses showed that IDC was negatively correlated with pallidum shrinkage. Of note, this correlation was congruent with the results obtained in group comparisons showing reductions in this subcortical structure. In our sample, patients with PKU presented a reduced brainstem volume, and this reduction was related to higher levels of Phe.
The strengths of our study lie in the inclusion of a relatively large sample of early-treated adults with PKU/HPA and well-matched HC, along with a detailed and comprehensive neuropsychological examination. Furthermore, we implemented a neuroimaging approach including both gray and white matter volumetric metrics, but also mean global measures and a surface-based approach to study whole-brain vertex-wise cortical changes. However, several limitations should also be acknowledged. First, although our cohort was more homogeneous (adults) compared to previous studies in the field, 26 individuals were diagnosed with NBS, and four were diagnosed between 2 months and 3 years old, so dietary treatment was initiated later for them. However, as patients with an estimated intelligence quotient < 70 (WAIS-IV) were not included, it is unlikely that our results were subjected to significant bias due to sample variability. Second, while classically the IDC is calculated as the median of Phe levels and the mean of all medians throughout the period of interest (childhood, youth, adulthood) [32], we focused on the median of Phe-DBS levels in the year prior to study inclusion. The average lifetime Phe levels for participants were not quantitatively available. This limitation arose because while pediatric history records were accessible for 18 participants, data were not available for all participants. As the calculated 1-year IDC allows the standardization of the same measure for the whole group and correction for sporadic decompensations (e.g., illness, dietary abandon), the selected approach offers a good picture of adults’ metabolic control. Finally, our study did not include an exhaustive assessment of executive functions such as the n-back task [17]. The executive functions in our study were assessed using the fluency test and the Trail Making test. In addition we used The BRIEF as a screening tool, but this is not able to detect the clinical phenotype of those individuals showing mild executive dysfunction or to detect the correlates of basal ganglia volumetric reductions. In this regard, previous studies reported consistent differences between groups in certain executive domains [34], and new toolboxes, which also integrate cognitive indices and additional motor evaluations, have recently been introduced for this purpose [41].
The incorporation of MRI and neuropsychological assessments is important in the initial evaluation and subsequent monitoring of PKU patients. These diagnostic tools may enhance our understanding of how metabolic control influences neuropsychological outcomes, to achieve individualized care strategies. Therefore, future research with multicenter cohorts is crucial, as it will enable the development of predictive models that can guide personalized treatment approaches more effectively.
In conclusion, this study expands on previous findings showing global white matter reduction but also gray matter abnormalities in adult patients with PKU. Early-treated PKU mainly gives rise to volumetric reductions in basal ganglia, brainstem, and limbic subcortical structures that are related to poorer metabolic control, without evidence of neocortical involvement.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- BH4:
-
Tetrahydrobiopterin
- BMI:
-
Body mass index
- BRIEF-A:
-
Behavior Rating Inventory of Executive Function for Adults
- DBS:
-
Dried bloodspot
- DTI:
-
Diffusion tensor imaging
- FEW:
-
Family-wise error rate
- FDR:
-
False discovery rate
- FSIQ:
-
Full Scale IQ
- GLM:
-
General linear model
- HC:
-
Healthy controls
- HPA:
-
Hyperphenylalaninemia
- ICV:
-
Intracranial volume
- IDC:
-
Index of dietary control
- MD:
-
Mean diffusivity
- MRI:
-
Magnetic resonance imaging
- MS/MS:
-
Mass spectrometry
- NBS:
-
Newborn screening
- Phe:
-
Phenylalanine
- PKU:
-
Phenylketonuria
- VBM:
-
Voxel-based morphometry
- VCI:
-
Verbal Comprehension Index
- WAIS-IV:
-
Wechsler Adult Intelligence Scale 4th edition
- WM:
-
Withe matter
References
Van Spronsen FJ, van Wegberg AM, Ahring K, Bélanger-Quintana A, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Rev Lancet Diabetes Endocrinol. 2017;5:743–56.
de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(SUPPL.):S86–9.
Anderson PJ, Leuzzi V. White matter pathology in phenylketonuria☆. Mol Genet Metab. 2010;99:S3–9.
Ferreira BK, Rodrigues MT, Streck EL, Ferreira GC, Schuck PF. White matter disturbances in phenylketonuria: possible underlying mechanisms. J Neurosci Res. 2021;99:349–60 John Wiley and Sons Inc.
Moat SJ, Schulenburg-Brand D, Lemonde H, Bonham JR, Weykamp CW, Mei JV, et al. Performance of laboratory tests used to measure blood phenylalanine for the monitoring of patients with phenylketonuria. J Inherit Metab Dis. 2020;43(2):179–88.
Blau N. Sapropterin dihydrochloride for phenylketonuria and tetrahydrobiopterin deficiency. Expert Rev Endocrinol Metab. 2010;5(4):483–94.
De Giorgi A, Nardecchia F, Manti F, Campistol J, Leuzzi V. Neuroimaging in early-treated phenylketonuria patients and clinical outcome: a systematic review. Mol Genet Metab. 2023;139:107588 Academic Press Inc.
van Spronsen FJ, Enns GM. Future treatment strategies in phenylketonuria☆. Mol Genet Metab. 2010;99:S90–5.
Mastrangelo M, Chiarotti F, Berillo L, Caputi C, Carducci C, Di Biasi C, et al. The outcome of white matter abnormalities in early treated phenylketonuric patients: a retrospective longitudinal long-term study. Mol Genet Metab. 2015;116(3):171–7.
Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn Reson Med. 2007;58(6):1145–56.
Dyer CA. Mental Retardation and Developmental Disabilities Research Reviews. Pathophysio of phenylketonuria†. 1999;5(2):104–12. https://doi.org/10.1002/(SICI)1098-2779(1999)5:2<104::AID-MRDD2>3.0.CO;2-7.
Nardecchia F, Manti F, Chiarotti F, Carducci C, Carducci C, Leuzzi V. Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: a longitudinal study. Mol Genet Metab. 2015;115(2–3):84–90.
Hellewell SC, Welton T, Eisenhuth K, Tchan MC, Grieve SM. Diffusion kurtosis imaging detects subclinical white matter abnormalities in Phenylketonuria. Neuroimage Clin. 2021;29:102555.
White DA, Antenor-Dorsey JAV, Grange DK, Hershey T, Rutlin J, Shimony JS, et al. White matter integrity and executive abilities following treatment with tetrahydrobiopterin (BH4) in individuals with phenylketonuria. Mol Genet Metab. 2013;110(3):213–7.
White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ, et al. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: a DTI study of the corpus callosum☆. Mol Genet Metab. 2010;99:S41–6.
Hood A, Antenor-Dorsey JAV, Rutlin J, Hershey T, Shimony JS, McKinstry RC, et al. Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol Genet Metab. 2015;114(1):19–24.
Hood A, Rutlin J, Shimony JS, Grange DK, White DA. Brain white matter integrity mediates the relationship between phenylalanine control and executive abilities in children with phenylketonuria. 2016. p. 41–7.
Peng H, Peck D, White DA, Christ SE. Tract-based evaluation of white matter damage in individuals with early-treated phenylketonuria. J Inherit Metab Dis. 2014;37(2):237–43.
Hawks Z, Hood AM, Lerman-Sinkoff DB, Shimony JS, Rutlin J, Lagoni D, et al. White and gray matter brain development in children and young adults with phenylketonuria. Neuroimage Clin. 2019;23:101916.
Wesonga E, Shimony JS, Rutlin J, Grange DK, White DA. Relationship between age and white matter integrity in children with phenylketonuria. Mol Genet Metab Rep. 2016;7:45–9.
González MJ, Polo MR, Ripollés P, Gassió R, Ormazabal A, Sierra C, et al. White matter microstructural damage in early treated phenylketonuric patients. Orphanet J Rare Dis. 2018;13(1):188.
Clocksin HE, Hawks ZW, White DA, Christ SE. Inter- and intra-tract analysis of white matter abnormalities in individuals with early-treated phenylketonuria (PKU). Mol Genet Metab. 2021;132(1):11–8.
Muri R, Maissen-Abgottspon S, Reed MB, Kreis R, Hoefemann M, Radojewski P, et al. Compromised white matter is related to lower cognitive performance in adults with phenylketonuria. Brain Commun. 2023;5(3):fcad155. https://doi.org/10.1093/braincomms/fcad155.
Pfaendner NH, Reuner G, Pietz J, Jost G, Rating D, Magnotta VA, et al. MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol. 2005;26(7):1681–5.
Pérez-Dueñas B, Pujol J, Soriano-Mas C, Ortiz H, Artuch R, Vilaseca MA, et al. Global and regional volume changes in the brains of patients with phenylketonuria. Neurology. 2006;66(7):1074–8.
Pilotto A, Zipser CM, Leks E, Haas D, Gramer G, Freisinger P, et al. Phenylalanine effects on brain function in adult phenylketonuria. Neurology. 2021;96(3):e399–411.
Bodner KE, Aldridge K, Moffitt AJ, Peck D, White DA, Christ SE. A volumetric study of basal ganglia structures in individuals with early-treated phenylketonuria. Mol Genet Metab. 2012;107(3):302–7.
Brown AA, Clocksin HE, Abbene EE, Ursery M, Christ SE. The relationship between metabolic control and basal ganglia morphometry and function in individuals with early-treated phenylketonuria. Mol Genet Metab. 2022;137(3):249–56.
Christ SE, Price MH, Bodner KE, Saville C, Moffitt AJ, Peck D. Morphometric analysis of gray matter integrity in individuals with early-treated phenylketonuria. Mol Genet Metab. 2016;118(1):3–8.
Muri R, Maissen-Abgottspon S, Rummel C, Rebsamen M, Wiest R, Hochuli M, et al. Cortical thickness and its relationship to cognitive performance and metabolic control in adults with phenylketonuria. J Inherit Metab Dis. 2022;45(6):1082–93.
Pilotto A, Blau N, Leks E, Schulte C, Deuschl C, Zipser C, et al. Cerebrospinal fluid biogenic amines depletion and brain atrophy in adult patients with phenylketonuria. J Inherit Metab Dis. 2019;42(3):398–406.
Vilaseca MA, Lambruschini N, Gómez-López L, Gutiérrez A, Fusté E, Gassió R, et al. Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr Hosp. 2010;25(1):60–6.
Romani C, Olson A, Aitkenhead L, Baker L, Patel D, Van SF, et al. Meta-analyses of cognitive functions in early-treated adults with phenylketonuria. Neurosci Biobehav Rev. 2022;143:104925.
Christ SE, Clocksin HE, Burton BK, Grant ML, Waisbren S, Paulin MC, et al. Executive function in phenylketonuria (PKU): Insights from the Behavior Rating Inventory of Executive Function (BRIEF) and a large sample of individuals with PKU. Neuropsychology. 2020;34(4):456–66.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation. Neuron. 2002;33(3):341–55.
van Spronsen FJ, J van Wegberg AM, Blau N, van Spronsen FJ, van Wegberg AM, Ahring K, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Rev Lancet Diabetes Endocrinol. 2017;5:743–56. Available from: www.thelancet.com/. [cited 2023 Oct 6].
Van Wegberg AMJ, MacDonald A, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12:1–56 BioMed Central Ltd.
Boot E, Hollak CEM, Huijbregts SCJ, Jahja R, van Vliet D, Nederveen AJ, et al. Cerebral dopamine deficiency, plasma monoamine alterations and neurocognitive deficits in adults with phenylketonuria. Psychol Med. 2017;47(16):2854–65.
Malamud N. Neuropathology of phenylketonuria. J Neuropathol Exp Neurol. 1966;25(2):254–68.
Aldridge K, Cole KK, Moffitt Gunn AJ, Peck D, White DA, Christ SE. The effects of early-treated phenylketonuria on volumetric measures of the cerebellum. Mol Genet Metab Rep. 2020;25:100647.
Christ SE, Clocksin HE, Zalik M, Goodlett BD, Sacharow SJ, Abbene EE. Neuropsychological assessment of adults with phenylketonuria using the NIH toolbox. Mol Genet Metab. 2023;139(1):107579.
Acknowledgements
The authors would like to express their gratitude to Dr. Antonia Ribes (International expert in IEM screening programs and diagnosis, Biomedical Diagnostic Center, Hospital Clínic, Barcelona, Spain) for their life-long devotion to the assistance of patients with metabolic disorders; to Carmen Cañueto and Tania Arellano for their valuable aid to precisely coordinate the participant’s schedule, and to the Consortium PKU.cat. The Consortium PKU.cat is an interdisciplinary healthcare team of basic and clinical researchers, who belong to universities, medical and research centers in Barcelona, Catalonia (Spain), devoted to the assistance of patients with phenylalanine disorders. PKU.cat final aim is to improve the quality of life of the affected patients and their families, to change the natural history of the disease and to gain further insight into the causes of poor clinical outcomes, both cardiovascular and neurological. We are also indebted to the Magnetic Resonance Imaging core facility of the IDIBAPS for technical support; and we acknowledge the CERCA Programme/Generalitat de Catalunya. The authors would also want to thank all the patients and healthy controls that freely participated in this clinical study providing new insights into the adult PKU/HPA setting to the scientific community. This work was supported by “Fundació La Marató de TV3, 2020”.
Consortium PKU.cat (Mendeley Data https://doi.org/10.17632/xr4m29cm29.1):
Argudo-Ramírez, Ana2; Barrau-Martínez, Blanca9; Cantó, Judith2; Campistol, Jaume10; Cardellach, Francesc2; Casals-Pascual, Climent2; Chiva-Blanch, Gemma2; García-Arenas, Dolores10; García-García, Francesc Josep2; García-Villoria, Judit2; González de Aledo-Castillo, José Manuel2; González-Rodríguez, Arnau9; Guitart-Mampel, Mariona2; Isern, Paula2; Jiménez, Amanda2; Laudo, Berta2; Llorach, Rafael9; Andújar-Sánchez, Félix2; López-Galera, Rosa2; Mª; Meavilla, Silvia10; Milisenda, José Cesar2; Morales, Blai2; Moreno-Lozano, Pedro Juan2; Moreno, Julián2; Nos, Mònica2; Ormazabal, Aida10; Ortega Ferrer, Montserrat2; Ortega, Emilio2; Padrosa, Joan2; Paredes, Abraham José2; Rubio, Elisa2; Tobías, Ester2; Torremade, Josep2; Urpi-Sarda, Mireia9; Valls, Laura2; Ventura, Roser2; Vergara-Gómez, Andrea2; Viaplana, Judith2; Viñals, Clara2
Affiliations:
1Institute of Neurosciences, Medical Psychology Unit, Department of Medicine, University of Barcelona, Barcelona, Spain
2Fundació de Recerca Clínic Barcelona-Institut d’Investigacions Biomèdiques August Pi i Sunyer (FRCB-IDIBAPS), Barcelona, Catalonia, Spain
3Biomedical Research Networking Center on Neurodegenerative Diseases (CIBERNED: CB06/05/0018-ISCIII), Barcelona, Catalonia, Spain
4Biomedical Research Networking Center on Physiopathology of Obesity and Nutrition (CIBEROBN), Barcelona, Catalonia, Spain
5Endocrinology and Nutrition Department, Adult Inherited Metabolic Disorders Unit (UECMA), Hospital Clínic de Barcelona, Barcelona, Catalonia, Spain
6Internal Medicine Department, Adult Inherited Metabolic Disorders Unit (UECMA), Barcelona, Catalonia, Spain
7Inherited Metabolic Diseases and Muscle Disorders Research, Centre de Recerca Biomèdica CELLEX – Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Faculty of Medicine and Health Sciences, University of Barcelona, Barcelona, Spain
8Biomedical Research Networking Center on Rare Diseases (CIBERER), Barcelona, Catalonia, Spain
9Nutrition, Food Science and Gastronomy Department, Xarxa d'Innovació Alimentària (XIA), Faculty of Pharmacy and Food Science, Food Science and Nutrition Torribera Campus, University of Barcelona, Barcelona, Catalonia, Spain
10Hospital Sant Joan de Déu – Fundació Sant Joan de Déu Barcelona, Barcelona, Catalonia, Spain
Informed consent
This study was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. All the participants of this study provided signed written informed consent, after a complete explanation of the procedures involved, and are available from the corresponding author upon reasonable request.
Funding
This study was sponsored by the Fundació La Marató de TV3 (202014-30-31-32), Generalitat de Catalunya (SGR 2021SGR00801), and supported by María de Maeztu Unit of Excellence (Institute of Neurosciences, University of Barcelona) CEX2021-001159-M. J.P. (PRE2021-099689) was supported by a fellowship from the Ministry of Science and Innovation.
Author information
Authors and Affiliations
Consortia
Contributions
B.S. and C.J. contributed to the design of the study. J.P., C.C.L., A.P., C.M., M.F., and G.G. provided data sets for their sites. J.P., C.C.L., and A.P. contributed to the collection of the data. J.P. and C.C.L. contributed to the analyses of the data. J.P., C.C.L., B.S., and C.J. contributed to the interpretation of the data. B.S. and J.P. contributed to the writing of the first draft and C.J. contributed to the final draft of the article. J.P., C.C.L., B.S., A.P., C.M., M.F., P.J.M., G.G., J.M.G.J., and C.J. revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Bioethics Committee of the University of Barcelona (IRB00003099) and Hospital Clínic of Barcelona (HCB/2020/0552) and was conducted in accordance with the basic principles of the Declaration of Helsinki, among other relevant regulations and guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pardo, J., Capdevila-Lacasa, C., Segura, B. et al. Volumetric brain reductions in adult patients with phenylketonuria and their relationship with blood phenylalanine levels. J Neurodevelop Disord 16, 33 (2024). https://doi.org/10.1186/s11689-024-09553-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-024-09553-w