Abstract
In this work, iron oxide (Fe3O4) magnetic nanoparticles (MNPs) and graphene oxide (GO) nanosheets were prepared via the co-precipitation technique and the Modified Hummer method. Fe3O4 MNPs and GO nanosheets were combined to prepare Fe3O4/GO nanocomposite and subsequently conjugated with Digitonin (DIG) in order to obtain a dual-targeted delivery system based on DIG/Fe3O4/GO nanocomposite. SEM images reveal the presence of Fe3O4 MNPs at a scale of 100 nm, exhibiting dispersion between the GO nanosheets. Aggregation of the DIG/Fe3O4/GO nanocomposite was observed at various size scales. The XRD structural analysis confirms the crystal structure of the prepared samples. The Fe3O4 MNPs demonstrated the main XRD-diffracted peaks. Also, GO nanosheets exhibit crystalline characteristics on the (001) and (002) planes. The predominant peaks observed in the DIG/GO/Fe3O4 nanocomposite are attributed to the crystal phases of Fe3O4 MNPs. The FT-IR vibrational modes observed in the GO/DIG/Fe3O4 nanocomposite indicate the presence of crosslinking between GO nanosheet layers and the Fe3O4 MNPs. The antioxidant activity of the prepared samples was measured and the DIG/GO/Fe3O4 nanocomposite demonstrated a significantly high antioxidant activity in both 2-diphenyl-1-picrylhydrazyl (DPPH·) and 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS·+) tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Antioxidant activity refers to the ability to restrict or prevent nutritional oxidation through oxidative chain reactions [1, 2]. Significant research in the field of nanobiotechnology has recently been conducted, with a particular focus on the investigation of antioxidant activities [3,4,5]. Antioxidants that mitigate oxidative stress are in increasing demand as a form of preventative therapy for conditions such as diabetes, cancer, cardiovascular disease, and neurological disorders [4].
Over the last several decades, significant advancements have been made in the field of nanomaterials and surface modification. These advancements have been detected and achieved in various fields such as biomedicine, biotechnology, catalysis, and magnetic chemistry [6, 7]. MNPs such as nickel, cobalt, and iron have garnered significant attention [8,9,10,11]. MNPs are frequently coated with molecules or polymers to improve their stability in aqueous and physiological environments. Untreated MNPs tend to aggregate when they interact with biological molecules. Utilizing the coating method can boost the colloidal stability of magnetic nanoparticles (MNPs), allowing them to persist and be difficult to remove during blood circulation for an extended duration [12]. The prolonged circulation time of magnetic nanoparticles (MNPs) within the bloodstream allows for extended interaction with biological components, potentially increasing opportunities for targeted binding or modifications. This increased exposure and interaction within the biological environment enhance the likelihood of MNPs undergoing functionalization processes for specific biomedical applications [5, 12]. Iron oxide (Fe3O4) MNPs, for example, are frequently prepared in different morphologies, such as spherical or hollow spherical structures of less than 100 nm in size, which enable drug delivery in the human body [13,14,15].
Graphene oxide (GO) has attracted attention in several research fields, such as solar cells, bio-sensing, membrane technology, and energy storage, due to its excellent chemical, physical, and mechanical characteristics [16,17,18]. Also, GO possesses a characteristic property such as amphiphilic, which enables it to penetrate cell membranes [19]. Furthermore, the non-covalent GO bonding facilitates the functionalization of its surface sheets and enhances the conjugation process due to π–π interaction [16].
GO has been synthesized via different processes such as chemical vapor deposition, thermal and mechanical exfoliation, radiation-based, and pyrolysis [20,21,22]. The modified Hummer method is widely acknowledged as one of the methods that have been used to synthesize GO, yielding multiple oxygen-containing groups [16, 23].
Due to the non-covalent bonding of GO, and functional groups including carboxylic, epoxide, and hydroxyl, the functionalization of GO with Fe3O4 is beneficial [24,25,26,27,28]. Furthermore, MNPs such as Fe3O4 coated with polymers such as chitosan and dextran enhance the aggregation and significantly decrease the composite surface energy [29]. GO/Fe3O4, as an example, was loaded with chitosan to prepare GO/Fe3O4/Chitosan nanocomposite by linking the amino groups of Fe3O4/Chitosan with the GO carboxylic group [30, 31]. GO/Fe3O4/Chitosan nanocomposite demonstrates a significant degree of bio-activity as an excellent platform for different drug delivery applications [32, 33].
Digitonin (DIG) shows a significant potential to be used in drug delivery applications [34, 35]. Therefore, A DIG-based GO/Fe3O4 nanocomposite may result in a reduction of the interlayer spacing between the MNPs and GO, which may enhance the nanocomposite effective surface area [36,37,38,39,40,41,42].
In this study, the GO nanosheets and the Fe3O4 were prepared using the modified Hummer and co-precipitation methods, respectively. DIG/GO and a dual-targeted platform of DIG/GO/Fe3O4 nanocomposite was prepared. The physiochemical properties of the synthesized samples were investigated, including the surface morphology, and structural and chemical characteristics. Furthermore, the antioxidant activity of the prepared samples was measured.
2 Experiment
2.1 Materials
Digitonin (DIG), sodium hydroxide (NaOH), sodium nitrate (NaNO3), hydrochloric acid (HCl), pure graphite, ferric chloride hexahydrate (FeCl3.6H2O), potassium permanganate (KMnO4), sodium tripolyphosphate (STP, Na5P3O10), ferrous chloride tetrahydrate (FeCl2.4H2O), hydrogen peroxide (H2O2), ethylene, DMF, and DMSO are obtained from Sigma-Aldrich, USA.
2.2 Preparation of Fe3O4 MNPs
The co-precipitation method was carried out to prepare the Fe3O4 MNPs. Initially, 2.59 g of FeCl3.6H2O and 1.59 g FeCl2.6H2O were dissolved individually in 100 ml HCl (37%) and mixed under mechanical stirring conditions at room temperature for about 15 min, the temperature was increased to 40 °C. Consequently, 20 ml of 3 M NaOH was gradually added dropwise for 1 h under continuous stirring. Finally, the resulting solution underwent centrifugation, leading to the magnetic collection of the precipitate. The precipitate was then subjected to washing with deionized water and ethanol, followed by drying in an oven set at a temperature of 65 °C for 24 h.
2.3 Preparation of GO nanosheets
The following steps are involved in producing GO nanosheets using the modified Hummer's method [43]. In the first step, 2.5 g NaNO3 was dissolved in 112 ml H2SO4 (96%) under mechanical stirring for 20 min. Subsequently, 4.6 g graphite powder was gradually added to the solution and continuously stirred for 10 min. In the second step, 15 g of KMnO4 was added to the mixture solution under vigorous stirring at a temperature of 0 °C for 1 h. Notably, the temperature was gradually increased to 40 °C while the mixture was then stirred for 2 h. In the third step, a solution of 20 ml of H2O2 (30%) and 240 ml DI water was slowly added to the final solution to remove the excess KMnO4. In the fourth step, a solution of yellow paste was produced and subsequently subjected to centrifugation at 6000 rpm for 10 min. The resulting paste was subjected to multiple washing processes using diluted HCl (10%) and DI water. Finally, after the completion of the washing process, a dark brown graphite oxide paste was successfully obtained. This paste was then subjected to a drying process in an oven with a temperature of 70 °C for 24 h. GO nanosheets were acquired in the form of a powdery substance.
2.4 Preparation of DIG/GO nanocomposite
The first solution was prepared by dissolving 200 mg of DIG in 0.10 ml AcOH and 5 ml of DI water. The second solution of GO was obtained by dissolving 25 mg GO in 10 ml DI water and sonicated for 40 min. The DIG solution was added to the GO solution under the influence of ultrasonic irradiation for 15 min. Finally, the DIG/GO nanocomposite solution was synthesized, centrifuged at 600 rpm for 15 min, and dried in the oven at 60 °C for a period of 2 h.
2.5 Preparation of DIG/GO/Fe3O4 nanocomposite
The DIG/GO/Fe3O4 nanocomposite preparation was carried out as follows:
First step, a mixture of 25 mg of Fe3O4 MNPs and 25 mg of GO nanosheets were added to 10 ml DI water under the influence of sonication probe for 30 min in order to prepare a homogenous solution of GO/Fe3O4 nanocomposite. Second step, 200 mg of DIG was dissolved in a solution composed of 0.10 ml AcOH and 5 ml of DI water to prepare a solution of DIG. In the third step, the GO/Fe3O4 nanocomposite solution was added to the DIG solution using the sonication probe for 20 min. The fourth step involved the preparation of a cross-linking solution. To achieve this, 1 g of Na5P3O10 was mixed in 100 ml of DI water. Subsequently, 83.3 ml of this solution was added to 16.7 ml of ethanol and the mixture was subjected to mechanical stirring for 1 h. In the fifth step, the solution obtained from the third step was added to the cross-linking solution from the fourth step under mechanical stirring for 1 h. Finally, the resultant solution, namely the DIG/GO/Fe3O4 nanocomposite, underwent centrifugation at 6000 rpm for 15 min and dried in an oven set at 60 °C for 2 h.
2.6 Characterization techniques
The structural and surface morphology properties of the prepared samples were analyzed using a Powder X-ray Diffractometer (XRD, Malvern Panalytical Ltd) and Scanning Electron Microscopy (SEM, Quanta FEG 450), respectively. The Fourier transform infrared spectroscopy was performed on the samples using the Thermo Scientific Nicolet iS10 FT-IR Spectrometer in the region of (250–4000) cm−1. Optical absorbance measurements were executed using Agilent Cary 5000 UV–Vis–NIR spectrophotometer.
3 Results and discussion
3.1 Surface morphology
The SEM images presented in Fig. 1 illustrate the morphology of the synthesized samples. Figure 1a, b provides insight into the formation of multilayer structures of GO nanosheets. Additionally, it is observed that flakes of different sizes formed after the drying process of GO nanosheets, particularly at the edges of the sheet structure. The presence of small-scale GO nanosheets and multiple sheets facilitates the dispersion of Fe3O4 MNPs within the stacked layers, thereby enhancing their potential for drug delivery. Figure 1c, d depicts the nanoscale aggregation of Fe3O4 MNPs, showcasing a range of various sizes. The average size of the Fe3O4 MNPs was calculated as 38.64 nm using the Gaussian distribution of the MNPs given inset in Fig. 1d using the SEM image. Figure 1e, f illustrates the surface morphology of DIG, indicating a low porosity and the existence of multiple stacked layers, as well as the presence of observed aggregates on the surface. Additionally, the SEM images show the formation of clusters of DIG molecules with varying sizes. Figure 2 illustrates the surface morphologies of the composite material DIG/GO before and after incorporating Fe3O4 MNPs. A uniform array of GO multilayers and DIG composite can be observed in Fig. 2a, b. A more detailed investigation reveals a substantial level of roughness detected on the surface, which suggests that DIG material is intercalated between the GO nanosheet layers. The structure serves as a platform for the dispersed and impeded Fe3O4 nanoparticles.
Figure 2c, d shows the presence of non-uniform composites resulting from loading Fe3O4 MNPs onto the DIG/GO nanocomposite. In addition, it is quite clear that grains of varying sizes are arranged in a stacked formation, resulting in a multi-layered array. At a smaller scale, the loading of Fe3O4 MNPs onto GO layers that are intercalated into the DIG material can be observed. The DIG material acts as a platform for intercalating GO sheets and incorporating Fe3O4 nanoparticles, which have a high molecular weight and large mass ratio, into preparing DIG/GO/Fe3O4 nanocomposite.
3.2 Structural analysis
The crystal structures of the samples were examined utilizing the Powder X-ray data analysis system, as illustrated in Fig. 3. Figure 3a demonstrates the GO nanosheet diffraction patterns. Sharp and weak peaks at 2θ = 10.5° and 21.3°, respectively, can be observed, suggesting the presence of crystallographic planes characterized by Miller indices (hkl) of (001) and (002) [23]. The sharp peak observed in the spectrum can be ascribed as the distinctive peak of the GO nanosheets, whereas the relatively weak peak, which is approximately 1/8th of the intensity of the characteristic GO peak, can be linked to a GO nanosheets [44]. The XRD patterns of Fe3O4 MNPs is shown in Fig. 3b. All of the diffraction patterns observed at 2θ = 30.3°, 35.6°, 43.3°, 53.6°, 57.3°, and 63.0°, which correspond to the Miller indices (220), (311), (400), (422), (511), and (440) respectively, are consistent with the diffraction patterns reported for Fe3O4 MNPs (JCDP card No.03-086) [45]. No impurity and secondary peaks were detected in the XRD analysis, indicating that the Fe3O4 MNPs in the system are free from contamination and confirming the successful formation of magnetite [45,46,47]. The XRD pattern of the DIG-GO nanocomposite, as presented in Fig. 3d, exhibits the major diffraction pattern of GO at an angle of 10.5°. Additionally, there are notable peaks observed at angles of 22.9°, 16.2°, and 14.9°, denoted by (∗), which indicate the presence of DIG with a higher intensity in comparison to the GO nanosheets. This result indicates that the DIG remained unchanged while forming the DIG/GO nanocomposite. Additionally, the GO nanosheets dispersed on DIG, resulting in a functionalization process between GO nanosheets and DIG. This process leads to the formation of the DIG/GO nanocomposite. Interestingly, the XRD patterns of the nanocomposite system DIG/GO/Fe3O4, as shown in Fig. 3e, demonstrate minimal alterations compared to the XRD pattern of Fe3O4 MNPs. This observation suggests that the Fe3O4 MNPs crystallinity structure remains unchanged after the composition process. Also, the dispersion of Fe3O4 MNPs on the DIG-GO is observed. Furthermore, Fig. 3e displayed a weak peak associated with GO structure, which is attributed to the low ratio of GO in the mixture. This observation also suggests to well dispersion of GO in the composite.
3.3 Physical analysis
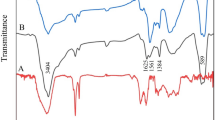
Figure 4 depicts the FTIR for GO, \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\), DIG, and \({\text{DIG}}/{\text{GO}}/{{\text{Fe}}}_{3}{{\text{O}}}_{4}\) samples. Figure 4a illustrates the FTIR transmission of the GO major bands between 3769 and 3000 cm−1 which corresponds to the hydroxyl groups and O–H stretching [48]. The peak located at 1735 cm−1 is assigned to the carbonyl and carboxylic groups presented in GO. Also, three peaks observed between 1038 and 1218 (cm−1) were associated with the alkoxy and epoxy groups [46, 49]. Figure 4b shows the FT-IR spectrum of the Fe3O4 MNPs. The main peak was observed at 542 cm−1, which is attributed to the stretching vibration mode attributed to the metal–oxygen Fe–O bonds in the crystalline lattice of \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\) [50, 51]. Moreover, the presence of hydroxyl groups is linked to a wide band at \(3480\,{{\text{cm}}}^{-1}\) and band at \(1624\,{{\text{cm}}}^{-1}\), were both associated to OH-stretching and OH-bending, respectively [52]. The FT-IR spectrum of \({\text{DIG}}\) as in Fig. 4c illustrates a broad band at \(3410\,{{\text{cm}}}^{-1}\) corresponding to –OH stretching vibration, and a band at \(2926\,{{\text{cm}}}^{-1}\) associated to C–H stretching and bending vibration [53, 54]. Also, two absorption peaks were observed at \(1452\, {{\text{cm}}}^{-1}\), and \(1372 \,{{\text{cm}}}^{-1}\) associated to C–H deforming vibration. The absorption peaks observed in the FT-IR profile of the \({\text{DIG}}\) in the spectral range of 1000–1200 cm−1 are associated to C–O–H stretching vibration, and C–O–C glycosidic band vibration [55]. The FT-IR spectrum of the as-prepared \({\text{DIG}}/{\text{GO}}\) nanocomposite as shown in Fig. 4d reveals major peak at \(3769\,{{\text{cm}}}^{-1}\) and \(575\,{{\text{cm}}}^{-1}\) demonstrating the presence of the GO nanosheets on the DIG surface [46]. The C=O stretching of the tertiary amide may be accountable for a new peak at \(1700\,{{\text{cm}}}^{-1}\), which demonstrates the successful functionalization of DIG with GO [46]. The \({\text{DIG}}/{\text{GO}}/{{\text{Fe}}}_{3}{{\text{O}}}_{4}\) spectrum as in Fig. 4e demonstrates the \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\) MNPs have been effectively loaded on the GO/DIG nanocomposite [46].
3.4 Optical absorbance
The investigation of the absorbance of the spectra of \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\) MNPs, \({\text{GO}}\), DIG, and \({\text{DIG}}/{\text{GO}}/{{\text{Fe}}}_{3}{{\text{O}}}_{4}\) composite with distinctive patterns were presented in Fig. 5. The spectra emerged, shedding light on the optical properties of these materials. Notably, the absorbance spectrum of \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\) MNPs illustrated a gradually increasing in the absorbance starting around 700 nm, with a sudden surge beyond 355 nm, demonstrating its distinct absorption behavior. Conversely, the absorbance spectrum of \({\text{GO}}\) showed a sharp onset at approximately 400 nm, indicative of its unique electronic transitions. DIG, On the other hand, exhibited a continuous rise in absorbance from 800 to 350 nm, culminating in a sudden increase. The absorbance spectrum of the \({\text{DIG}}/{\text{GO}}/{{\text{Fe}}}_{3}{{\text{O}}}_{4}\) composite revealed a nuanced interplay of the individual components with a gradual rise from 800 nm and a more increase from 700 nm, suggesting the influence of \({{\text{Fe}}}_{3}{{\text{O}}}_{4}\) MNPs and \({\text{GO}}\) in the visible region, while the composite exhibited absorbance characteristics positioned between the individual components.
3.5 Antioxidant activity
The determination of the antioxidant activity of the compounds is conducted using the 2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS)radical scavenging assay methods, as outlined in the literature [56,57,58]. The scavenging activity was computed by the equation given as [59]:
The absorbance of the control is denoted as Ac, while the absorbance of the extract is defined as As. The IC50 values were calculated by evaluating the relationship between the percentage of antiradical activity and the concentration of the compounds.
3.5.1 DPPH method
The DPPH of the extracts was carried out and compared to α-tocopherol and ascorbic acid in order to determine their total capacity [60]. In this manner, an amount of 2.0 ml of 0.1 mM of DPPH solution was added to 2.0 ml of the extract at various concentrations between 3.12 and 15.6 (\(\times\)10–3 mg/ml). The resultant solution was incubated in dark conditions for 30 min, and the absorbance was determined against methanol as a blank at wavelength of 517 nm.
3.5.2 ABTS method
The determination of antioxidant measurements was conducted according to the power of the ABTS·+ method [56,57,58]. The cation radical ABTS·+ was added to distilled water. After this, 3 ml of the resultant ABTS solution was mixed with 1 ml extract with different amounts between 3.12 and 15.6 (\(\times\) 10–3 mg/ml). The absorbance was measured at the wavelength of 734 nm using a UV–vis spectroscopic. The absorbance was carried out every 5 min. The ABTS capacity was compered to α-tocopherol and ascorbic acid standards. The concentration of the compound that causes a 50% reduction of the DPPH and ABTS (IC50) was measured to interpret the findings from both DPPH and ABTS methods. Using the linear regression, the IC50 was evaluated by plotting the percentage of the antiradical activity versus the concentration level of compounds as shown in Fig. 6.
In the present study, the assessment of the compounds' ability to scavenge free radicals was conducted using the ABTS and DPPH free radical scavenging methods. Screening results revealed that the antioxidant activities of all compounds exhibited a concentration-dependent pattern as in Fig. 6. The IC50 values for each compound are listed in Table 1. In comparison to the positive controls used in the present study, the four compounds exhibited significant antioxidant activity on both assay methods. Among the prepared samples, DIG/GO/Fe3O4 nanocomposite demonstrates a significant high antioxidant activity in both DPPH· and ABTS·+ tests. Furthermore, the results show that the DIG, GO, and Fe3O4 MNPs synergistically interact in terms of their ability to free radicals scavenging. This phenomenon can be attributed to the tendency of spherical Fe3O4 MNPs and lamellar GO to aggregate into significant clusters in aqueous solutions, thereby impeding their capability to effectively scavenge radicals [61]. When both DIG/GO/Fe3O4 nanocomposite were instantaneously exist in an aqueous solution, the spherical Fe3O4 MNPs might be successfully loaded onto the GO lamellae and intercalated to reduce agglomeration. This resulted in an increased number of active sites presented for interaction with free radicals, thereby enhancing the capacity for scavenging free radicals.
4 Conclusion
In the present study, the Fe3O4 MNPs and GO nanosheets were combined to form the GO/Fe3O4 nanocomposite. The GO/Fe3O4 nanocomposite was then consequently conjugated with DIG to prepare a nanocomposite platform system, known as DIG/GO/Fe3O4 nanocomposite. SEM images illustrate the formation of multilayer GO nanosheets, the dispersion of Fe3O4 MNPs within the GO layers, and the surface characteristics of the DIG. SEM images also showed the existence of a porous structure in the DIG/GO/Fe3O4 nanocomposite. In addition, the aggregation of the GO/Fe3O4 MNPs coated with DIG resulted in the formation of the DIG/GO/Fe3O4 nanocomposite. The validation of the structural analysis of the prepared samples was performed via XRD analysis. The main diffracted peaks for Fe3O4 MNPs are depicted. Moreover, the major characteristic peaks of GO nanosheets were observed and associated to the (001) and (002) crystallographic planes. The presence of crosslinking between the GO nanosheet layers and the Fe3O4 MNPs in the GO/DIG/Fe3O4 nanocomposite is demonstrated by the examined FT-IR vibrational modes. The antioxidant activity of the prepared samples was measured, and it was found that the DIG/GO/Fe3O4 nanocomposite exhibited significantly high antioxidant activity in both the DPPH· and ABTS·+ tests. Thus, the obtained results indicate that this novel composite has prospective uses in biomedical formulations due to its promising antioxidant activity, which may help address oxidative stress-related diseases. Additionally, its structural integrity suggests applicability in catalysis, sensing technologies, and environmental remediation.
Data availability
The data that support the findings of this study are available from the corresponding author, B.A., upon reasonable request.
References
Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–907.
Gülçin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innov Food Sci Emerg Technol. 2010;11:210–8.
Myint KZ, Yu Q, Xia Y, Qing J, Zhu S, Fang Y, Shen J. Bioavailability and antioxidant activity of nanotechnology-based botanic antioxidants. J Food Sci. 2021;86:284–92.
Patil P, Parit S, Waifalkar P, Patil S, Dongale T, Sahoo SC, Kollu P, Nimbalkar M, Patil P, Chougale A. pH triggered curcumin release and antioxidant activity of curcumin loaded γ-Fe2O3 magnetic nanoparticles. Mater Lett. 2018;223:178–81.
Mai T, Hilt JZ. Magnetic nanoparticles: reactive oxygen species generation and potential therapeutic applications. J Nanopart Res. 2017;19:1–10.
Latorre A, Couleaud P, Aires A, Cortajarena AL, Somoza Á. Multifunctionalization of magnetic nanoparticles for controlled drug release: a general approach. Eur J Med Chem. 2014;82:355–62.
Bilal M, Mehmood S, Rasheed T, Iqbal HM. Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry. 2019;5:42.
Srinivasan SY, Paknikar KM, Bodas D, Gajbhiye V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine. 2018;13:1221–38.
Ahmed Ali AM, Ahmed NM, Kabir NA, Algadri NA, AL-Diabat AM, Wadi I, Alsadig A, Aldaghri OA, Ibnaouf KH. Towards extended gate field effect transistor-based radiation sensors: impact of thicknesses and radiation doses on al-doped zinc oxide sensitivity. Crystals. 2023;13:314.
Ali AMA, Ahmed NM, Kabir NA, Al-Diabat AM, Algadri NA, Alsadig A, Aldaghri OA, Ibnaouf KH. Sensitivity of al-doped zinc-oxide extended gate field effect transistors to low-dose X-ray radiation. Materials. 2023;16:1868.
Es’haghi Z, Vafaeinezhad F, Hooshmand S. Green synthesis of magnetic iron nanoparticles coated by olive oil and verifying its efficiency in extraction of nickel from environmental samples via UV–vis spectrophotometry. Process Saf Environ Protect. 2016;102:403–9.
Roque AC, Bicho A, Batalha IL, Cardoso AS, Hussain A. Biocompatible and bioactive gum Arabic coated iron oxide magnetic nanoparticles. J Biotechnol. 2009;144:313–20.
Yew YP, Shameli K, Miyake M, Khairudin NBBA, Mohamad SEB, Naiki T, Lee KX. A review of green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system. Arab J Chem. 2020;13:2287–308.
Beg MS, Mohapatra J, Pradhan L, Patkar D, Bahadur D. Porous Fe3O4-SiO2 core–shell nanorods as high-performance MRI contrast agent and drug delivery vehicle. J Magn. 2017;428:340–7.
Salihov SV, Ivanenkov YA, Krechetov SP, Veselov MS, Sviridenkova NV, Savchenko AG, Klyachko NL, Golovin YI, Chufarova NV, Beloglazkina EK. Recent advances in the synthesis of Fe3O4@AU core/shell nanoparticles. J Magn. 2015;394:173–8.
Abdelhalim AO, Meshcheriakov AA, Maistrenko DN, Molchanov OE, Ageev SV, Ivanova DA, Iamalova NR, Luttsev MD, Vasina LV, Sharoyko VV. Graphene oxide enriched with oxygen-containing groups: on the way to an increase of antioxidant activity and biocompatibility. Colloids Surf B. 2022;210: 112232.
Feng L, Wu L, Qu X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv Mater. 2013;25:168–86.
Tiwari SK, Sahoo S, Wang N, Huczko A. Graphene research and their outputs: status and prospect. J Sci Adv Mater Devices. 2020;5:10–29.
Feger G, Angelov B, Angelova A. Prediction of amphiphilic cell-penetrating peptide building blocks from protein-derived amino acid sequences for engineering of drug delivery nanoassemblies. J Phys Chem B. 2020;124:4069–78.
Shams SS, Zhang R, Zhu J. Graphene synthesis: a review. Mater Sci. 2016;33:566–78.
Choi W, Lahiri I, Seelaboyina R, Kang YS. Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci. 2010;35:52–71.
Cooper DR, D’Anjou B, Ghattamaneni N, Harack B, Hilke M, Horth A, Majlis N, Massicotte M, Vandsburger L, Whiteway E. Experimental review of graphene. Int Sch Res Not. 2012;2012: 941814.
Alam SN, Sharma N, Kumar L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene. 2017;6:1–18.
Karthika V, AlSalhi MS, Devanesan S, Gopinath A, Arumugam A, Govindarajan MJSR. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. J Sci Rep. 2020;10:18912.
Guo F, Kim F, Han TH, Shenoy VB, Huang J. Hydration-responsive folding and unfolding in graphene oxide liquid crystal phases. J ACS Nano. 2011;5:8019–25.
Wang C, Ravi S, Garapati US, Das M, Howell M, Mallela J, Alwarappan S, Mohapatra SS. Multifunctional chitosan magnetic-graphene (CMG) nanoparticles: a theranostic platform for tumor-targeted co-delivery of drugs, genes and MRI contrast agents. J Mater Chem B. 2013;1:4396–405.
He F, Fan J, Ma D, Zhang L. The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. J Carbon. 2010;48:3139–44.
Liu YW, Guan MX, Feng L, Deng SL, Bao JF, Xie SY, Chen Z, Huang RB, Zheng LS. Facile and straightforward synthesis of superparamagnetic reduced graphene oxide–Fe3O4 hybrid composite by a solvothermal reaction. J Nanotechnol. 2012;24: 025604.
Mahdavi M, Ahmad MB, Haron MJ, Namvar F, Nadi B, Rahman MZA. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. J Mol. 2013;18:7533–48.
Ma X, Tao H, Yang K, Feng L, Cheng L, Shi X, Li Y, Guo L. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. J Mater Chem B. 2012;5:199–212.
Li S, Xiao L, Deng H, Shi X. Remote controlled drug release from multi-functional Fe3O4/GO/Chitosan microspheres fabricated by an electrospray method. J Colloids Surf B Biointerfaces. 2017;151:354–62.
Wang K, Xu X, Li Y, Rong M, Wang L, Lu L, Wang J, Zhao F, Sun B. Preparation Fe3O4@ chitosan-graphene quantum dots nanocomposites for fluorescence and magnetic resonance imaging. J Chem Phys Lett. 2021;783: 139060.
Daniyal M, Liu B. Comprehensive review on graphene oxide for use in drug delivery system. J Curr Med Chem. 2020;27:3665–85.
Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc. 2014;9:421–38.
Li H, Krstin S, Wink M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine. 2018;50:213–22.
Neves-Petersen MT, Gajula GP, Petersen SB. UV light effects on proteins: from photochemistry to nanomedicine. Methods Mol Biol. 2012;875:125–58.
Korchowiec B, Janikowska-Sagan M, Kwiecińska K, Stachowicz-Kuśnierz A, Korchowiec J. The role of cholesterol in membrane activity of digitonin: experimental and theoretical model studies. J Mol Liq. 2021;323: 114598.
Sudji IR, Subburaj Y, Frenkel N, García-Sáez AJ, Wink M. Membrane disintegration caused by the steroid saponin digitonin is related to the presence of cholesterol. Molecules. 2015;20:20146–60.
Geelen M. The use of digitonin-permeabilized mammalian cells for measuring enzyme activities in the course of studies on lipid metabolism. Anal Biochem. 2005;347:1–9.
Fiskum G. Intracellular levels and distribution of Ca2+ in digitonin-permeabilized cells. Cell Calcium. 1985;6:25–37.
Lee VT, Anderson DM, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601.
Temel RE, Brown JM, Ma Y, Tang W, Rudel LL, Ioannou YA, Davies JP, Yu L. Diosgenin stimulation of fecal cholesterol excretion in mice is not NPC1L1 dependent. J Lipid Res. 2009;50:915–23.
Chen T, Zeng B, Liu J, Dong J, Liu X, Wu Z, Yang X, Li Z. High throughput exfoliation of graphene oxide from expanded graphite with assistance of strong oxidant in modified Hummers method. J Phys Conf Ser. 2015;617: 012051.
Abraham S, Ciobota V, Srivastava S, Srivastava SK, Singh RK, Dellith J, Malhotra B, Schmitt M, Popp J, Srivastava A. Mesoporous silica particle embedded functional graphene oxide as an efficient platform for urea biosensing. Anal Methods. 2014;6:6711–20.
Mustafa M, Qasim Q, Mahdi A, Izzat S, Alnassar Y, Abood E, Alhakim Z, Mahmoud Z, Al-Salman HNK. Supercapacitor performance of Fe3O4 and Fe3O4@SiO2-bis(aminopyridine)-Cu hybrid nanocomposite. Int J Electrochem Sci. 2022;17: 221057.
Abskharoun SB, Shawakfeh KQ, Albiss BAA, Alsharaeh EH. Magnetic based graphene composites with steroidal diamine dimer as potential drug in hyperthermia cancer therapy. Mater Res Express. 2020;7: 095103.
Zhuang L, Zhang W, Zhao Y, Shen H, Lin H, Liang J. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci Rep. 2015;5:1–6.
Shahriary L, Athawale AA. Graphene oxide synthesized by using modified hummers approach. Int J Renew Energy Environ Eng. 2014;2:58–63.
Xue B, Zhu J, Liu N, Li Y. Facile functionalization of graphene oxide with ethylenediamine as a solid base catalyst for Knoevenagel condensation reaction. Catal Commun. 2015;64:105–9.
Waldron R. Infrared spectra of ferrites. Phys Rev. 1955;99:1727.
Bordbar A, Rastegari A, Amiri R, Ranjbakhsh E, Abbasi M, Khosropour A. Characterization of modified magnetite nanoparticles for albumin immobilization. Biotechnol Res Int. 2014;2014: 379349.
Nalbandian L, Patrikiadou E, Zaspalis V, Patrikidou A, Hatzidaki E, Papandreou CN. Magnetic nanoparticles in medical diagnostic applications: synthesis, characterization and proteins conjugation. Curr Nanosci. 2016;12:455–68.
Mathew MS, Sreenivasan K, Joseph K. Hydrogen-bond assisted, aggregation-induced emission of digitonin. RSC Adv. 2015;5:100176–83.
Yashaswini, Pandurangappa C, Dhananjaya N, Murugendrappa M. Photoluminescence, Raman and conductivity studies of CaSO4 nanoparticles. Int J Nanotechnol. 2017;14:845–58.
Qian S, Fang X, Dan D, Diao E, Lu Z. Ultrasonic-assisted enzymatic extraction of a water soluble polysaccharide from dragon fruit peel and its antioxidant activity. RSC Adv. 2018;8:42145–52.
Al-Qudah MA. Antioxidant activity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J Biol Act Prod Nat. 2016;6:101–11.
Loay A, Abu-Orabi ST, Hlail HM, Alkhatib RQ, Al-Dalahmeh Y, Al-Qudah MA. Anthemis cotula L. from Jordan: essential oil composition, LC-ESI-MS/MS profiling of phenolic acids-flavonoids and in vitro antioxidant activity. Arab J Chem. 2023;16:104470.
Al-Qudah MA, Al-Smadi ZM, Al-Jaber HI, Tashtoush HI, Alkhatib RQ, Bataineh TT, Al-Dalahmeh Y, Orabi STA. GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity. Arab J Chem. 2023;16: 104641.
Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37.
Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002;163:1161–8.
Wu Q, Li H, Wu L, Bo Z, Wang C, Cheng L, Wang C, Peng C, Li C, Hu X. Synergistic lubrication and antioxidation efficacies of graphene oxide and fullerenol as biological lubricant additives for artificial joints. Lubricants. 2022;11:11.
Acknowledgements
The authors thank Dr. Yusuf Selim Ocak for his valuable efforts. This study was supported by Jordan University of Science and Technology with Grand No. 148/2022
Author information
Authors and Affiliations
Contributions
BA: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft. KS: Conceptualization, Methodology, Resources, Funding acquisition, Writing—Review and Editing. BAA: Conceptualization, Resources, Funding acquisition, Writing—Review and Editing. AA: Methodology, Investigation, Formal analysis, Visualization. MA: Methodology, Investigation, Formal analysis, Visualization. TB: Methodology, Investigation, Formal analysis, Visualization. LS: Methodology, Investigation, Formal analysis, Visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aljawrneh, B., Shawakfeh, K., Albiss, B.A. et al. A novel digitonin/graphene oxide/iron oxide nanocomposite: synthesis, physiochemical characterization and antioxidant activity. Discover Nano 19, 15 (2024). https://doi.org/10.1186/s11671-024-03960-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-024-03960-7