Abstract
Prolonged inflammation can impede wound healing, which is regulated by several proteins and cytokines, including IL-4, IL-10, IL-13, and TGF-β. Concentration-dependent effects of these molecules at the target site have been investigated by researchers to develop them as wound-healing agents by regulating signaling strength. Nanotechnology has provided a promising approach to achieve tissue-targeted delivery and increased effective concentration by developing protein-functionalized nanoparticles with growth factors (EGF, IGF, FGF, PDGF, TGF-β, TNF-α, and VEGF), antidiabetic wound-healing agents (insulin), and extracellular proteins (keratin, heparin, and silk fibroin). These molecules play critical roles in promoting cell proliferation, migration, ECM production, angiogenesis, and inflammation regulation. Therefore, protein-functionalized nanoparticles have emerged as a potential strategy for improving wound healing in delayed or impaired healing cases. This review summarizes the preparation and applications of these nanoparticles for normal or diabetic wound healing and highlights their potential to enhance wound healing.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have high drug-loading efficacy, which can be attributed to a high surface area-to-volume ratio. They respond quickly to any minute alteration in the surrounding environment, like a magnetic field, pH, and temperature [1]. The bionanomaterials like peptides, biomolecules, enzymes, and protein-functionalized formulations have various biological applications ranging from bioimaging [2], catalysis [3], fluorescent biolabeling [4, 5] hyperthermia [6, 7] tissue engineering [8], gene and drug delivery [9, 10], and so on. Moreover, protein-functionalized and stabilized nanomaterials exhibit numerous features such as sensing, biocompatibility, plasmon-enhanced catalysis, targeted nanocarriers, and drug delivery [11, 12]. The constituting units of the proteins behave not only as reducing and chelating agents that help in developing nanoclusters but also allow crystalline [13, 14] and amorphous [15] growth of the nanostructures of different sizes and shapes [16]. The target-specific binding ability of proteins enhances their action efficiency and helps to cure the wound [15, 17]. However, the poor permeability through membranes, short half-life, and high enzymatic degradation risk pose a serious issue to the targeted delivery of potent therapeutic proteins to the site and thus require slight modifications for efficient delivery [18, 19]. Protein-functionalized nanoformulations, such as protein-capped metal nanoparticles, protein-encapsulated nanostructures, and protein nanocarriers, including hydrogels, scaffolds, liposomes, nanotubes, nanogels, nanoparticles, polymeric particles and poly(ester amide) PEA (synthesized using amino acids, diacids, and diols), were developed to effectively deliver the protein/drug at the effected/targeted site [20, 21].
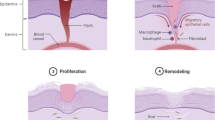
The skin is the largest human body organ that, by acting as a barrier, assists in preventing the entry of harmful microbes into the body. The skin consists mainly of epidermal, dermal, and hypodermal layer, which contains certain hormonal glands, hair, and nerve endings that make the skin a complex organ. It has the ability to self-healing to a certain extent which is impaired in case of extensive damage caused by chemical or physical shock, making the healing a challenging process [22]. Wound healing is a cumbersome process that activates a series of physiological and simultaneous phases such as hemostasis, inflammation, proliferation, and remodeling [23, 24], which have a significant effect on the effective wound or infected area treatment. The classical process of healing begins with the hemostasis, which includes clot formation due to the activation of platelets that release the chemokines and growth factors (including fibroblasts and keratinocytes) at the wound site and acts as key parameters of hemostasis and coagulation. They further assist in preventing the entry of bacteria at the affected site and regulate antimicrobial peptide production by expressing distinct toll-like receptors (TLR) [25, 26]. After this inflammatory phase begins, which is quite complex due to the extrinsic and intrinsic factors and the excessive and limited, both inflammation conditions delay the wound healing and promote injury. It acts as the basic defense system against pathogenic invasion and is started in response to signals induced by injury, the release of damage-associated molecular patterns, and pathogen-associated molecular patterns by necrotic cells and damaged tissue and bacterial components, respectively. Vasodilation is promoted by pro-inflammatory cytokines, and these proinflammatory signals and activated signaling pathways stimulate the secretion of cytokines by neutrophils. Neutrophils, by phagocytosis, remove the necrotic tissue and pathogens and cause the secretion of antimicrobial peptides, proteolytic enzymes, and eicosanoids [27, 28]. Following this, the proliferative phase begins, in which there is the accumulation of cells and connective tissues along with the activation of numerous factors, including fibroblasts, keratinocytes, macrophages, angiogenesis factors, and endothelial cells across the wound site. Keratinocytes are responsible for regenerating the epidermis by differentiation and basement membrane reformation. Fibroblasts play a role in developing the matrix consisting of granulation tissue which comprises fibronectin, proteoglycans, and immature collagen, which collectively acts as a scaffold essential for cell adhesion and migration across the wound [29, 30]. During angiogenesis, new blood vessels are generated in order to fulfill the demand for highly proliferative regenerative tissue, and macrophages assist in guiding vessel tips together and eradicating the superfluous vessels by phagocytosis for making new vasculature critical for transportation across the site [31, 32]. In the last phase of remodeling, there occurs the resynthesis of the extracellular matrix for maintaining the balance between the death of old cells and the formation of new cells. Fibroblasts are the major cells crucial for remodeling by replacing the fibrin clot with fibronectin, proteoglycan, and collagen fibril synthesis. Collagen III is finally replaced with collagen I, which enhances the tensile strength of wound scar and helps wound closure at a faster pace [33,34,35]. In the initial healing stages, there occur the release of cytokines and activation of leukocytes by macrophages in order to exhibit a pro-inflammatory environment [36]. The potent release of anti-inflammatory cytokines, insulin-like growth factor (IGF), and proteins such as insulin are crucial for aiding in efficient wound healing, which is initiated by inducing angiogenesis and re-epithelialization [37]. The proteins (insulin, collagen, keratin, gelatin), growth factors such as insulin-like growth factors (IGF), epidermal growth factors (EFG), fibroblast growth factors (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta 1 (TGF-β1), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF), etc., help in reducing inflammation, cell proliferation, and remodeling [38, 39]. The growth factors act as the endothelial signaling factors which assists in regulating the cellular processes during wound healing. There are numerous studies investigating the critical role played by them in modulating the healing effects in normal and diabetic conditions. The treatment of wounds at a faster pace is highly dependent upon the transition of cytokines from pro-inflammatory to anti-inflammatory ones [40]. In chronic wounds, a halt in the conversion of cytokines from one form to another causes a prolonged pro-inflammatory phase which eventually results in delayed wound healing [41].
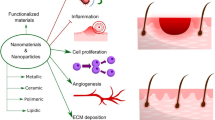
Depending upon these protein and growth factor features and their role in normal and diabetic wound healing, there is an increasing demand for the development of reliable and cost-effective wound dressings, and protein-functionalized nanoformulations are the ones that have high wound-healing potency, target specificity, and are easy to synthesize using green methods. The significance of this field is very diverse as the treatment of chronic wounds is becoming difficult day by day due to the development of antibiotic resistance against the available medications, degradation of formulations under unfavorable conditions, uncontrolled drug release, and less target specificity and efficiency. The cost of healing ranges fall between $28.1 billion and $96.8 billion for acute and chronic wounds, and the maximum amount is used for surgical wounds, followed by diabetic ulcers [42]. The increasing prevalence of chronic wounds, the growing geriatric population, and the rising number of surgeries are some factors driving the growth of the wound care market. The number of patients affected by chronic wounds is approximately 5.7 million people in the USA alone, and an estimated cost of USD 25 billion is spent per year [43]. Recent advancements in bionanomaterials have led researchers toward developing optimized protein-based nanoformulations for wound-healing purposes as they showed sustained drug release, reduced administration frequency, an adequate concentration of medicine for an extended period, high efficiency in wound healing compared to the free proteins, high protein stability, easy transport through the body and less denaturation under environmental conditions, all work together to form advanced formulations critical for wound healing. The high surface area to volume ratio, water solubility, stability, biocompatibility, target specificity, and biodegradability have given an upper hand to the use of nanoformulations over traditional therapeutics. All these factors have significantly contributed to gaining the enormous interest of researchers in this desirable field for developing and exploring better futuristic approaches in synthesizing protein-functionalized materials [44]. Figure 1 shows various proteins and growth factors primarily used in wound healing along with the major nanoformulations being developed using them.
This review article focuses on protein-functionalized nanomaterials for wound-healing applications. The key terms covered in this article include protein-functionalized nanoparticles, bionanomaterials, drug delivery, tissue engineering, gene delivery, cytokines, growth factors, inflammation, macrophages, angiogenesis, and skin regeneration. Other terms discussed include bioimaging, catalysis, fluorescent biolabeling, hyperthermia, plasmon-enhanced catalysis, and targeted nanocarriers. The article highlights the advantages of protein-functionalized nanomaterials over only protein, including their high drug-loading efficacy, quick response to environmental changes, and target-specific binding ability. This review demonstrates the enormous possibilities of developing green and biocompatible protein nanoformulations with high efficacy and specificity for wound-healing applications.
Structural details of major proteins and growth factors and their potential role in skin wound healing
Several proteins are involved in wound healing, including antidiabetic proteins such as insulin or extracellular matrix proteins such as heparin, keratin, silk fibroin, and collagen. We will discuss them briefly in the coming sections.
Insulin
It is derived from its inactive form, proinsulin, in which the N-terminal of chain A is connected with the C-terminal of chain B through a C-peptide which is removed during the maturation. Insulin is a peptide hormone composed of two polypeptide chains, chain A and chain B having a total of 51 amino acids, of which 17 are proteinogenic amino acids. The two chains are interconnected via three disulfide bridges, and its folding occurs with three alpha-helices (two in chain A and 1 in chain B) and a small β-sheet segment. The presence of zinc ions and phenolic ligands helps in the dimerization of insulin and further promotes the formation of hexamers (Fig. 2A) The molecular weight of insulin is around 5 kDa. Depending upon its structure and low molecular weight, it is widely employed for synthesizing nanoformulations that are biocompatible, non-toxic, and can be easily inserted into the body or topically applied over the wound and hence promote wound healing by following critical mechanisms. It regulates blood glucose levels by stimulating glucose uptake in cells. It also plays essential roles in cellular differentiation, lipid and protein biosynthesis, growth factor activity, and wound healing. It also acts as a growth factor and increases the migration ability of cells, thereby aiding in wound healing [45, 46]. The insulin receptor is related to receptor tyrosine kinase transmembrane signaling proteins present on the surface of cells. The Akt and Erk signaling pathways are activated upon binding, improving wound healing [47, 48]. Insulin induces the synthesis of proteins by the PI3K and Akt pathway, which helps form 4EBPI and ribosomal protein S6 essential for cell survival. It also binds to IGF receptors and exhibits anti-inflammatory activity through different signaling pathways, such as Akt and PI3K, which activate pro-inflammatory cytokine STAT-3, promoting cell growth and angiogenesis [39]. In addition, insulin inactivates the TNF-α-mediated inflammatory pathway, which inactivates the pro-inflammatory cytokines [39]. Insulin enhances the proliferation, migration, and secretion of different cells, including keratinocytes, fibroblasts, and endothelial cells [49]. Hence, it is used in integration with other wound dressings due to its low cost compared with other growth regulators [46]. Insulin signaling in wound healing plays a crucial role in cell proliferation, migration, and angiogenesis, making it an essential peptide hormone for wound healing as shown in Fig. 3.
The crystal structures of different proteins and growth factors along with their PDB IDs a insulin, b silk fibroin, c keratin, d collagen, and e heparin, f fibroblast growth factor (FGF), g keratinocyte growth factor (KGF), h vascular growth factor (VGF-B), i epidermal growth factor (EGF), and j platelets-derived growth factor (PDGF)
The figure shows the signaling pathway followed by insulin and other growth factors for wound recovery. The generation of IFN-γ and TNF-α activated the STAT-1, IRF-3, and NF-kβ, which are responsible for the secretion of IL-10, IL-12, and other interleukins for the transition of pro-inflammatory cytokines to anti-inflammatory ones and promotes healing
Silk fibroin
Silk fibroin, derived from Bombyx mori, is a natural fibrous protein that acts as an essential biomaterial for tissue engineering and regeneration [50]. The silk fibroin is the main structural protein of silk and consists of a polypeptide chain with molecular weight in the range of 200–350 kDa. The repetitive units of hydrophobic heavy chains (391.6 kDa) and hydrophilic light chains (27.7 kDa) with terminal N and C groups constitute the primary silk fibroin structure, and the disulfide bonds connect the two chains together. Glycoprotein P25 provides integrity to the above chains, and the molar ratio of heavy chain, light chain, and P25 is 6:6:1 (Fig. 2B). The amino acids present in the hydrophobic chain include 45.9% glycine (Gly), 30.3% alanine (Ala), 12.1% serine (Ser), 5.3% tyrosine (Tyr), and 1.8% valine (Val), while the hydrophilic chain consists of 14% alanine (Ala), 10% serine (Ser), 9% glycine (Gly), and acetylated N-terminal Ser residues [51]. The structure of silk fibroin is such that it acts as a tissue scaffold or mesh for the attachment of growing cells and promotes functional tissue regeneration crucial for healing. Further, the β-sheet pattern is high in silk fibroin which promotes cell adhesion and differentiation in mesenchymal stem cells. It supports the proliferation, differentiation, and adhesion of various cells, including keratinocytes, endothelial cells, epithelial cells, fibroblasts, and osteoblasts, promoting wound healing [52, 53]. In addition to its role as a biomaterial, silk fibroin also regulates different signaling pathways crucial for wound healing. The NF-ĸB pathway is activated by silk fibroin, resulting in the upregulation of genes involved in cell proliferation, migration, and angiogenesis. Silk fibroin also enhances the activation of the ERK1/2 and Akt signaling pathways, leading to increased cell proliferation, migration, and extracellular matrix synthesis [54]. Due to its biocompatibility, non-toxicity, non-carcinogenicity, and less immunogenicity, silk fibroin is extensively studied in various biomedical and biological areas, and its ability to regulate different signaling pathways and support various cell functions makes it a promising biomaterial for wound-healing applications [34, 55] (Fig. 4).
Keratin
Keratin is a natural fibrous protein found in both humans and animals [56]. It is a scleroprotein made up of different amino acid residues but mainly rich in cysteine, and these amino acids are connected by intra- and intermolecular hydrogen bonds, disulfide bonds, ionic bonds, and hydrophobic interactions. Generally, it is found in two forms: alpha keratins, in which polypeptide chains are arranged in the form of alpha helices, and filaments have a diameter of 7–10 nm, whereas the beta keratins comprise beta sheets which are of 3–4 nm diameter (Fig. 2C). The alpha form is dominant in hairs, nails, horns, wool, and stratum corneum, whereas the beta form is found in feathers, reptilian claws, and scales [57]. Based on the structure, keratin is used for making scaffolds as it contains both alpha and beta forms which help in cell adhesion to the scaffold and enhances their proliferation and differentiation. Further, the presence of cell binding motifs, including leucine-aspartic acid-valine and glutamic acid-aspartic acid-serine, promotes the ability to promote cellular attachment. In various forms, such as hydrogels, scaffolds, and films, it has been widely used for bone regeneration, nerve regeneration, cell culture, and wound healing [58]. Keratin supports wound healing by accelerating hemostasis, promoting cell growth, and upregulating the expression of proteins relevant to wound healing. Additionally, it enhances plasma coagulation and lateral growth of fibrils [59, 60]. Previous studies have shown that keratin can arrest hemorrhage in bleeding animals, increase fibroblast proliferation and attachment, and upregulate the expression of keratinocytes involved in migration and collagen deposition [61, 62]. The mechanism underlying keratin-mediated wound healing is complex. TNF-α activates the NFκβ/C/EBPβ, and IL-1 activates the C/EBPβ, which in turn activates the K6 keratin. IFN-γ upregulates STAT1, activating the k6 and k17 keratins. Similarly, the k16 and k6 get activated by EGF/TGF α. All three keratins, K6, K16, and K17, activate the keratinocytes and promote E-cadherin secretion or phosphorylation of EGFR, ERK1/2, and K6, which increases epidermal differentiation and ultimately enables wound healing [63, 64] as shown in Fig. 5.
Collagen
Collagen is a fibrous protein that provides structural support to the extracellular matrix, essential for tissue repair and regeneration. Skin mainly consists of collagen I. Collagen fiber has a coiled structure, and each fiber is up to 3 μm in diameter. Each fiber further consists of bundles of small fibrils, and the diameter of each fibril is 10–300 nm in diameter and several micrometers long. Fibrils are made up of triple-stranded collagen molecules. Based on this triple helix structure, the strands are interwoven together and allow the robust structure to maintain itself in tissues for years due to its stereo-dynamic stability as given in Fig. 2D [65]. Due to its fiber-like nature, it is widely employed for making scaffolds that have the potency of enhanced cellular attachment and thus promote wound-healing activity by providing space for cell growth and differentiation. Collagen-based biomaterials have been extensively studied for their wound-healing properties due to their ability to promote healing by enhancing cell proliferation, angiogenesis, and collagen deposition [66, 67]. Collagen stimulates the expression of growth factors such as TGF-β, activates the MAPK/ERK signaling pathway, and modulates the activity of enzymes involved in wound healing, such as MMPs [68]. Collagen-based biomaterials have shown great potential for wound-healing applications due to their biocompatibility, biodegradability, and ability to support cell growth and tissue regeneration [69].
Heparin
Heparin is a natural, branched, helical glycosaminoglycan (GAG) with high sulfation and anticoagulant properties. It is basically divided into two main types: unfractionated heparin and low molecular weight heparin. Among the naturally occurring GAGs, heparin is the most sulfated one and composed mainly of tri-sulfated disaccharides of 2-O-sulphated α-L-iduronic acid and N-,6-O-disulphated glucosamine repeating units (Fig. 2E). The molecular weight of natural heparin is 3000–30,000 Da, while that of unfractionated one is 12–16,000 Da [70]. It is effective in wound healing due to its ability to protect growth factors from proteolytic degradation, thus enhancing their bioactivity [71]. It promotes rapid and effective repair of endothelial cells, making it a useful agent in both in vitro and in vivo wound-healing applications [72]. Heparin has been employed in treating burn wounds and diabetic foot ulcers, where it has been shown to decrease wound recovery time and increase capillary circulation. The wound-healing capability of heparin-based formulations is due to their ability to enhance the secretion of various growth factors, such as FGF1 and FGF7. FGF1, after binding to its receptor FGF1-R, promotes cell proliferation and angiogenesis, while FGF-7 increases the proliferation of keratinocytes, which is essential for re-epithelialization [73]. Heparin also inhibits specific cytokines, including Elastase, Cathepsin G, and IL-8, as well as eosinophil peroxidase, eosinophil cationic protein, and stromal-derived factor-1, which are responsible for the augmentation of inflammation. Overall, heparin-based formulations have shown great potential for wound-healing applications due to their ability to promote cell proliferation, angiogenesis, and re-epithelialization, while inhibiting inflammation as shown in Fig. 6. Using heparin-based formulations in wound healing may lead to the development of novel therapies and biomaterials for improving wound-healing outcomes [71, 74].
Fibroblast growth factor (FGF)
FGF plays a crucial role in wound healing and includes 22 polypeptides which are responsible for activating the FGFR1-4 (four receptor-type tyrosine kinases) and promotes healing [75]. The granulation tissue formation and angiogenesis are mediated by FGF2. FGF2 also promotes cell proliferation, differentiation, and migration in different tissues, including skin, bone, cartilage, and muscles [76]. Further, the migration of keratinocytes in wounds, both in vitro and on skin samples, is promoted by FGFR-1 and FGFR-2 [77]. FGF7 and FGF10 are responsible for stimulating the endothelial cells and inducing the expression of VEGF, both of which are required for re-epithelialization and angiogenesis in its later stages. Further, hair follicles are generated with the help of FGF9, and its overexpression increases hair generation up to three times. Keratinocyte growth factors (KGF) also fall under this category and promote reepithelialization by affecting the morphogenesis, proliferation, and migration of keratinocytes. KGF1 regulates the inflammatory phase, and its expression is increased by the PDGF and proinflammatory cytokines released from macrophages and leukocytes. KGF2 acts on epithelial cells and is secreted by fibroblasts. It promotes angiogenesis, hair follicle growth, wound closure, scar formation, fibroblast migration, granulation tissue formation, and so on [78, 79] (Fig. 2F, G).
Vascular endothelial growth factor (VEGF)
Various cell types, including fibroblasts, keratinocytes, platelets, endothelial cells, macrophages, and neurocytes present in the wound site, express the growth factors of the VEGF family. VEGF plays a crucial role in initiating the scar formation and the early phases of angiogenesis after the tissue injury, and its expression is initiated by the release of hypoxia-inducible factor-1α in response to the blood capillary disruption and hypoxic conditions generated thereafter at the wound [79]. It also promotes vasculogenesis, re-epithelialization, collagen deposition, and enhanced vascular permeability, which allows the inflammatory cells to reach the wounded tissue and initiate proliferation and migration. It also assists in burn wound healing [80] (Fig. 2H).
Epidermal growth factor (EGF)
It is a polypeptide chain having 53 amino acid residues and three intramolecular disulfide bonds. The migration and proliferation of fibroblast, endothelial cells, and keratinocytes toward the wound site is initiated by EGF. It also activates the EGF receptor (EGFR), which initiates the signaling pathway involved in promoting cell survival, proliferation, and migration without causing any harm to stem cell pluripotency. EGF is found to enhance the healing rate in diabetic foot ulcers, venous ulcers, and skin grafts by increasing epithelialization [81, 82] (Fig. 2I).
Platelet-derived growth factor (PDGF)
PDGF is secreted in the form of five isoforms by fibroblasts, macrophages, smooth muscle cells, keratinocytes, and endothelial cells. PDGF proteins are present in monomeric forms, and to get themselves biologically active and bind to the PDGF receptors, they form homodimers or heterodimers. The differentiation and proliferation of fibroblasts to myofibroblasts is initiated by the PDGF. It plays a role in re-epithelialization, intraepithelial collagen deposition, inflammatory cell deposition, and stabilization of blood capillaries in granulation tissue. Further, it promotes the secretion of MMPs and thus is critical in the remodeling phase. The PDGF-BB (Becaplermin) is the only recombinant growth factor approved for chronic wound healing by FDA, and it acts as a profibrotic agent [79, 83] (Fig. 2J).
Synthesis of protein-based nanoformulations
Nanoparticles are promising drug delivery agents for early diagnosis and treatment of various diseases. Different methods have been employed for their synthesis, including colloidal, sonochemical, thermal decomposition, microemulsion, and hydrothermal processes [84, 85]. However, these methods have limitations due to the toxicity of drugs on normal cells and tissues and the difficulty in loading hydrophobic agents [86, 87]. To overcome these limitations, protein-based nanoformulations have been developed. Two main synthesis pathways are crosslinking with derivative groups modified on the surface of protein molecules or crosslinking with native proteins' functional groups [88, 89]. Recent techniques involve the electrospray method and desolvation or coacervation process, which provide better control over the size and loading of nucleic acids and therapeutic drugs [90,91,92,93]. UV illumination was used to induce the self-assembly of protein nanoparticles, and solvent extraction or emulsion processes were found to have high encapsulation rates [94]. The heat denaturation process is equipped with targeting moieties but lacks a large particle size. Hence, various synthesis methods with modifications can be employed to efficiently and effectively synthesize protein-functionalized nanoparticles [90]. Here we are going to discuss some of the most widely employed synthesis methods used for protein-functionalized nanomaterials as shown in Fig. 7 and their advantages and limitations are given in Table 1 [95]. The synthesis procedure to be followed will be highly dependent on the specific application and the characteristic features of the resulting nanostructures.
Emulsification technique
In 1972, the method was developed by Scheffel and co-workers for preparing the spheres of albumin protein. This technique involves two distinct phases, an aqueous phase is made up by dissolving the protein in distilled water, and an organic phase in which plant oils are used. The two phases are mixed mechanically by using a homogenizer in a large container until the oil–water or water–oil emulsion is obtained. This emulsion solution is then poured drop by drop into a preheated oil having a temperature of up to 120 °C. At this temperature, the water from the emulsion will evaporate, and the irreversible destruction of protein will begin, eventually leading to the synthesis of protein nanoparticles which will be suspended in an ice bath [96, 97].
Complex coacervation
For the entrapment of DNA, a complex coacervation technique is usually followed. By adjusting the pH, the proteins can be made cationic or anionic due to their amphoteric nature. In this technique, the protein is taken in an aqueous solution, and during its pH adjustment duration, the particles having a positive charge come upward toward the surface. In this solution, a freshly prepared solution of DNA mixed with salt is added. When the DNA and protein interact together, the complex coacervation occurs, followed by the addition of crosslinkers like EDC (1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide) to get the crosslinked protein particles loaded with the DNA. This entrapment of DNA into the protein matrix is completed in the last stage of synthesis. Cationized protein can also be used for making complexes with DNA [98, 99].
Desolvation
Desolvation, also known as the coacervation technique, was developed in 1978 by Martyr and his coworkers. In this technique, protein is taken in an aqueous solution into which a desolvating agent like natural salt or alcohol is added. Adding a desolvation agent starts slow structural changes in the protein after addition. After a specific time interval, the formation of protein clumps begins, followed by the crosslinking between clumps to yield protein nanoparticles which are separated from the solution by gradually increasing the turbidity of the solution [100, 101].
Electrospray technique
The electrospray technique is a newly developed one mainly used for elastin and gliadin-based protein nanoparticles. In this technique, a very high voltage is applied to the protein solution, which is supplied through the emitter. This emitter is responsible for emitting a liquid jet stream via the nozzle, which is crucial for forming aerosolized size liquid droplets. These droplets consist of drugs along with nucleic acid. Through this method, the monodispersity of synthesized nanoparticles is retained [102, 103].
Other techniques
In addition to the methods mentioned earlier, other techniques have been developed to synthesize protein-based nanoformulations. One such technique is the layer-by-layer (LbL) assembly method, which involves the sequential deposition of oppositely charged polyelectrolytes onto a charged surface, such as a protein or a nanoparticle. This method is highly versatile and can be used to create multilayered nanostructures with controlled size and surface charge, allowing for precise control over the release of drugs or other therapeutic agents [104, 105]. Another method that has gained attention in recent years is using genetically engineered proteins to synthesize protein-based nanoformulations. By engineering the amino acid sequence of proteins, it is possible to introduce specific functional groups that can be used for crosslinking or chemical modification, allowing for the precise control of the size and shape of the resulting nanostructures [106, 107]. Furthermore, there is a growing interest in using biocompatible and biodegradable polymers, such as chitosan, alginate, and poly(lactic-co-glycolic acid) (PLGA), for the synthesis of protein-based nanoformulations. These polymers offer several advantages over traditional protein-based formulations, including improved stability, enhanced biocompatibility, and controlled release properties [108,109,110].
Kinetic and thermodynamic study of protein-based nanoformulations
Thermodynamic and kinetic studies are crucial in thoroughly understanding the interactions between proteins and external agents such as drugs, metal ions, polymers. Thermodynamics critically affect the different parameters including stability, adsorption, nucleation, and interaction between proteins and the surface of nanoparticles and helps in the determination of optimal conditions for the development of protein-functionalized nanomaterials having desired characteristic features and applications. The stability of protein is very crucial for its bioactivity at the targeted site, and with the help of thermodynamics, one can study the structural alterations in proteins which can be due to various environmental or man-made parameters ultimately causing its misfolding or deactivation and eventually resulting in losing the stability. The surface adsorption of proteins on the molecular surface depends upon certain factors such as size, shape, composition, and the extent of binding interactions both at the surface and within the molecules. The formulations show wide variations in behavior when different proteins are used, which further depends upon the bulk solution constituents, the ratio of sizes between the proteins used, and protein–surface interactions. Additionally, the potential of different proteins to alter their structure after getting adsorbed greatly affects the kinetics of adsorption. Different adsorption patterns can be obtained based on the internal rates of structural modifications when compared with the protein diffusion, which can also be determined using intermolecular interactions.
Moreover, there is a strong relation between various relaxation times with the kinetics of adsorption and depends upon the morphology of particles [115]. A convex isotherm is formed when there is an association between protein and immobilized ligands. In contrast, when a protein molecule is adsorbed on another protein molecule, a sigmoid isotherm is formed, which is concave at low concentrations while convex at higher concentrations. For instance, insulin has concave isotherms at low concentrations [116]. One needs to study different parameters to understand the effect of all these factors. The thermodynamic parameters consist of Gibbs free energy (ΔG), enthalpy (H), and entropy (S), which can be calculated by using the following set of equations for all kinds of protein-based formulations.
where T is the temperature, and R represents the universal gas constant.
The other factors include Ka (association constant) and Kd (dissociation constant). Different protein-based formulations have different values for these parameters, which are briefly explained using some examples. The adsorption isotherms were plotted to determine the relationship between molecules that get absorbed on the surface and those remaining in solution to monitor the binding capacity between the insulin protein and different magnetic nanoparticles (MMIP and MNIP). The slope of the adsorption curve increases sharply when a low initial concentration of the sample is used, while at higher concentrations, the slope is almost constant. Additionally, the ∆G values (in kJ/mol) for MMIP and MNIP are − 39.2 and − 36, the H values (in kJ/mol) are − 39.3 and − 36, while the ∆S values (in J/mol K) are − 0.26 and − 0.27. The negative values of ∆G indicate that insulin adsorption is a spontaneous process.
Conversely, the negative values of ∆H and ∆S indicate that electrostatic forces, hydrogen bonds, and van der Waals forces are involved in insulin adsorption. Similarly, equilibrium results were evaluated using Langmuir and Freundlich isotherms to clarify the binding mechanisms of proteins with external agents. The adsorption kinetic model was used to study the kinetic mechanism of insulin adsorption, and it was observed that adsorption occurs via a pseudo-first-order mechanism during the initial 8 h due to the presence of empty binding sites and lower concentrations of molecules, while after 8 h, adsorption occurs via a pseudo-second-order reaction [117]. Similarly, novel nanoinsulin formulations were developed using silver nanoparticles. Their ∆G values were found to be − 6.72, − 6.98, and − 7.48 kcal/mol at 27 °C, 32 °C, and 37 °C, respectively, indicating the favorability of the forward reaction with the highest affinity at physiological conditions. Similarly, the ∆H value is 16.08 kcal/mol, indicating the endothermic nature of the reaction between insulin and AgNPs. Furthermore, it was found that the reaction followed a first-order kinetic model based on the fluorescence quenching of insulin [49]. In the case of regenerated silk fibroin, crystallization is exothermic and is accompanied by entropy reduction when the temperature is kept constant. As the draw ratio increases, thermal stability and crystallinity are observed [118]. For the adsorption of heparin molecules on different molecularly imprinted polymers, the ∆G values were found to be − 5.95, − 4.70, and − 2.73 kJ/mol at 299.15 K, 309.15 K, and 324.15 K, respectively, confirming the feasibility and spontaneity of the adsorption process. Moreover, the values of ∆H are negative, indicating electrostatic interactions between molecules, while the ∆S values are also negative, implying a decrease in entropy due to decreased randomness at the solution-solid binding point [119]. Further, the energy expenditure during the different wound-healing phases varies greatly by 50% and 20% in proliferative and remodeling phases. Also, the thermodynamics greatly influence the inflammatory phase by affecting the key features including redness, swelling, and heat production during inflammation of which swelling and redness are influenced by osmotic pressure and fluid movement. The fluid movement raises by 100 times in order to meet the wound requirements by supplying nutrients and blood to the tissue site. Gibb’s free energy plays an important role in wound healing as it affects the cell migration, collagen deposition, and angiogenesis. The healing wounds have a positive ∆G indicating the progress through phases of wound healing. Chronic wounds have negative ∆G which indicates that the wound-healing process is stuck in inflammatory phase and cannot be proceeded. In this way, kinetic and thermodynamic factors play a crucial role in understanding the stability of nanoparticles and the feasibility of synthesizing protein-based nanoformulations for further applications.
Protein-based nanoformulations in normal and diabetic wound healing
Insulin-based formulations
Insulin has anti-inflammatory, antidiabetic, and wound-healing properties by activating the cytokines, thus reducing inflammation. Insulin upregulates the NF-kβP50/P50 by suppressing the expression of NF-kβP50/P65 and TNF-α. P65 suppression downregulates IL-12, IL-1β, TNF-α, and IL-6 cytokines at the wound site [120,121,122]. Inflammatory cytokines inhibition shifts the equilibrium toward anti-inflammatory cytokines expression, like VEGF, IL-4, IL-10, etc., which further induces the proliferation of the cells [123, 124]. Numerous approaches for developing nanoformulations of insulin have been carried out. In 2017, Li and co-workers developed silk fibroin-based microparticles (insulin-SFPs) encapsulating insulin; the particles provide biostability to the insulin and help in its sustained release. The particles showed significant collagen deposition, and vascularization stimulated the migration of keratinocytes and endothelial cells and promoted wound healing compared to the free insulin [125, 126] (Fig. 8A). In 2018, Ehterami et al. followed ion gelation method for preparing insulin-loaded chitosan particles (insulin-CPs) followed by their coating on poly ε-caprolactone (PCL) (insulin-PCL-CNPs); the particles showed a reduction in inflammatory cytokines infiltration and 96.9 ± 1.11% wound healing in 14 days [127] (Fig. 8B).
SEM/TEM images of the different protein-loaded nanoformulations A Insulin-silk fibroin nanoparticles, B Insulin-PCL-Chitosan nanoparticles, C Insulin-PLGA nanoparticles, D Insulin-Ag nanoparticles, E Insulin-Chitosan nanoparticles, F Insulin-Cu Quantum clusters, G Insulin-Zinc quantum clusters, H Gelatin-AgNPs-PDGF-BB, I Keratin nanoparticles, J Collagen nanofibers, K Heparin nanofibers, L Fibroblast growth factor-CMCS nanoparticles, M bFGF-loaded chitosan nanoparticles, N VEGF-PLGA nanoparticles, O Vascular endothelial growth factor-loaded PLGA nanoparticles, P Keratin Growth factor-Au nanoparticles, Q KGF-loaded fibrin nanoparticles, R rh-EGF-loaded carboxymethyl chitosan nanoparticles, S rh-EGF-loaded solid lipid nanoparticles, T PDGF-BB nanoparticles [Adapted with permission from ACS, RSC, Elsevier, Taylor and Francis, Springer Nature, MDPI], all rights reserved
In 2018, DH Abdelkader et al. developed insulin-loaded polyvinyl alcohol borate nanoparticles (insulin-PLGA CNPs), significantly enhancing wound healing. With PLGANP-insulin, 29.15% more wound recovery occurs on the 10th day of the treatment. The insulin embedded in the particles is released by the diffusion method. The difference in wound closure up to 16 days in diabetic rat models treated with control, free insulin, and insulin-loaded PLGA particles showed faster wound healing and increased exudates formation and angiogenesis with insulin PLGA insulin particles [128] (Fig. 8C). In 2019, Kaur P et al. prepared insulin protein-coated silver nanoparticles (insulin-AgNPs) to deliver insulin at the place of injury (Fig. 8D). Along with anti-bacterial effect, AgNPs also have significant role in treating wounds and exhibit anti-inflammatory action. The inflammatory cytokine transition and wound-healing properties of AgNPs improved by encapsulating them with insulin. The wound-healing effect of IAgNPs was determined in vitro and in vivo in diabetic and non-diabetic conditions. Both free insulin and IAgNPs treatment showed a faster recovery of the wound. 20% and 12%, more rapid wound recovery in diabetic and normoglycemic rats, respectively, on the 5th day of IAgNPs treatment than free insulin 4.67% (diabetic model) and 7.27% (non-diabetic model). After 11 days of IAgNPs treatment, 73.33%, 60.0%, and free insulin, 40% and 33.33%, were observed in diabetic and normoglycemic models, respectively, compared to the respective controls. In addition to the wound contraction assay, the histological expression of the pro- and anti-inflammatory cytokines was also examined. Histological evaluations of rat models on the 5th and 11th days showed significant collagen deposition along with reepithelialization. A decrease in the leukocyte infiltration was observed with IAgNPs and insulin with respect to additional solutions. On the 5th day of treatment, IAgNPs, the pro-inflammatory cytokines expression level decreases by 50% in both diabetic and non-diabetic groups, which is higher than free insulin. On the 11th day, IL-6 and TNF-α decreased by 45% in diabetic and normoglycemic sets. The IL-10 anti-inflammatory cytokines expression level after 5 days of treatment with IAgNPs increases by 70% and 50% in non-diabetic and diabetic rat animals. On the 11th day, wound healing accelerated by 65% and 50% in non-diabetic and diabetic groups [49]. Ribeiro et al. [130] used chitosan to synthesize insulin-loaded nanoparticles (insulin-CNPs) and examined the effect on wound healing. Histological evaluation showed angiogenesis, reduction in inflammation like monocytes, macrophages, neutrophils, keratinocytes, endothelial cell proliferation, collagen deposition, and wound maturation after treatment compared to free insulin [129] (Fig. 8E). The particles of Poly (lactide-co-glycolide) (insulin-PLGA NPs) loaded with insulin were prepared by Lee and Blaber [131] by evaporating the solvent using the double-emulsion technique. The prepared particles are biodegradable and have viscoelastic properties—the insulin embedded in the particles is released by diffusion. The difference in wound closure in control, free insulin, and insulin-loaded PLGA particles was up to 16 days. With insulin PLGA particles, the healing rate is faster and increases the exudate formation and angiogenesis formation [130].
The nanoclusters (NCs) of insulin with metals have bioimaging properties and wound-healing activity. These NCs comprise several to a hundred metal atoms with an outer layer of protein to protect them from aggregation. Kaur et al. [14] prepared insulin copper quantum clusters (Insulin-CuQCs) that exhibited wound healing and bioimaging activity. Similar to zinc, copper also promotes cell growth and division. At a 5% concentration of ICuQCs in 24h, there was an almost double increase in HeLa cell growth compared to the control (insulin + Cu salt, insulin, and blank), indicating the ICuQCs' cell growth-promoting effect [14] (Fig. 8F). Kaur et al. [15] prepared insulin zinc quantum clusters (Insulin-ZnQCs) and tested their in vitro wound-healing efficiency and bioimaging activity using HEKa (normal human epidermal keratinocyte). The cells treated with IZnQCs at concentrations of 1.5, 7.5, 30, and 60 µM showed 39.49 ± 1.29%, 42.03 ± 3.04%, 45.25 ± 2.14%, and 52.88 ± 0.83% cell migration, respectively, after 6 h compared to control. After 12 h of incubation, cell migration of 27.58 ± 3.72%, 34.08 ± 1.57%, 36.32 ± 1.63%, and 46.86 ± 1.46% occurred with 1.5, 7.5, 30, and 60 µM IZnQCs treatment compared to control. After 24 h, the % of cell migration was 43.02 ± 1.62%, 46.51 ± 3.38%, 58.60 ± 0.72%, and 67.81 ± 0.83% after 1.5, 7.5, 30, and 60 µM IZnQCs concentrations treatment, respectively, in comparison with control. An increase in migration was observed with an increase in time, and at 24h, maximum wound recovery was observed [15] (Fig. 8G). Similarly, insulin-nickel quantum clusters (INiQCs) were prepared by Sharda et al. to test wound-healing efficiency in vitro, bioimaging, and detecting lead in silico and in vitro. They effectively healed wounds at different concentrations of 1.5, 7.5, 30, and 60 µM after different time intervals of 6, 12, and 24 h, respectively, and showed bioimaging effects at varying concentrations [16]. Also, insulin-cobalt core–shell nanoparticles were synthesized for the treatment of wounds and are found to be very effective against both normal and diabetic wound healing. The effect of particle concentration was studied, and it was found that with increasing concentrations of 1.5, 7.5, 30, and 60 µM, there is an increase in the rate of wound healing with time [131]. A hydrogel scaffold was developed using morin incorporated into polysaccharide protein, which decreased the re-epithelialization rate and increased the wound contraction rate by enhancing collagen deposition in diabetic rats [132]. Wan et al. [133] prepared Gelatine cryogel loaded on the surface of silver nanoparticles (Gelatin-AgNPs-PDGF-BB) at the PDGF-BB bottom layer, which exhibited better wound healing, re-epithelialization, angiogenesis, deposition of collagen, and granulation tissue formation in diabetic wounds (Fig. 8H).
Silk fibroin-based formulations
Curcumin-loaded silk fibroin conjugated with polycaprolactone or polyvinyl alcohol was used to make a nanofibrous mat for healing diabetic wounds faster in both in vitro and in vivo models [134]. Maity et al. [135] synthesized antioxidant silk fibroin composite hydrogel to heal diabetic wounds rapidly. They were biocompatible, stimulated fibroblast cell migration, and controlled oxidative stress in vitro. Silk fibroin and silk sericin-based formulations were developed in combination with aloe vera gel for wound healing in diabetic mice, and it was found that the wounds healed within 13 days of applying the formulation, with a wound contraction of 98.33 ± 0.80% [136]. Hydrogel films were created by combining boric acid-impregnated silk fibroin/gelatin and hyaluronic acid, resulting in an improved tissue healing process and increased strength [137]. Silk fibroin interlinked with glycyrrhizic acid and silver hydrogels was also prepared for the effective treatment of wounds having bacterial infection [138]. Additionally, a multitasked aerosolized nanopowder formula made from silk fibroin exhibited antioxidant, anti-bacterial, and enhanced cell proliferation effects, providing a promising approach to wound healing [139]. Wu et al. [140] found that adipose-derived stem cell-seeded silk fibroin-chitosan films improved wound healing in a diabetic rat model. Sen et al. [141] synthesized silk fibroin-immobilized polyurethane conjugated with epidermal growth factor, which enhanced the rate of burn wound healing of full-thickness burn and reduced pro-inflammatory cytokines IL-6,8,10 levels in diabetic rats. Silk fibroin-integrated biliverdin-based bioinspired green hydrogel-stimulated angiogenesis and wound healing in a full-thickness rat model by exhibiting anti-inflammatory effects in vitro and in vivo [142]. Silk fibroin-hyaluronic acid-based composite scaffolds were developed for monitoring the cellular growth at wound sites and were found to enhance the scarless reconstruction of skin in nude mice with skin tissue defects [143]. Silk fibroin and poly(lactide-co-glycolic acid) nanofiber were loaded with zinc oxide nanoparticles for their successful delivery at the wound site and to promote reepithelialization, collagen deposition, granulation tissue formation, and angiogenesis [144].
Keratin-based formulations
Veerasubramanian et al. [145] developed konjac-glucomannan-keratin hydrogel wound dressings having oat extract, which significantly enhanced the wound treating ability in diabetic conditions by promoting the synthesis and deposition of collagen in the wounded area. In another study, recombinant keratin particles (Keratin-NPs) were prepared by Gao et al. [146] to improve wound healing, vascularization, epithelization, and collagen deposition (Fig. 8I). Chicken feather proteins were utilized by Kumaran et al. [147] to synthesize keratin hydrogels for treating dermal injuries. Furthermore, keratin hydrogels loaded with ciprofloxacin were developed to promote the healing of full-thickness excision wounds and prevent infection caused by Pseudomonas aeruginosa bacteria [148]. Human platelet lysate-loaded keratin hydrogels were also synthesized to exhibit sustained release of pro-regenerative growth factors essential for healing and enhancing cell proliferation without toxicity in vitro models [149]. Robert et al. [150] utilized keratin-based wound dressing to treat a patient suffering from recessive dystrophic epidermolysis bullosa in 2012. Electro-spun fibers, loaded with keratin and hyaluronic acid, were also found to be potent wound-healing dressings due to their ability to increase cell viability and proliferation [151]. Keraderm is a matrix dressing obtained from keratin powder by freeze-drying which is utilized for healing exuding venous ulcers and healing the complete wound in 30 weeks. Similarly, kerafoam, an absorbent polyurethane foam dressing having a keratin film lamination, was developed for highly exuding venous ulcers, which get completely healed in 16 weeks. To treat partial-thickness wounds, an absorbable matrix called keramatrix was developed, which is found to enhance the epithelialization process of wounds [152].
Collagen-based formulations
Other extracellular proteins, such as amnion and collagen-based composite hydrogels, were developed to treat cutaneous burn wounds by enhancing the re-epithelialization abilities [153]. Curcumin-loaded fish scale collagen-hydroxypropyl methyl cellulose nanogel promoted healing with better percent contraction of the injury and prolonged drug release [154]. Chitosan/collagen scaffolds loaded with Norfloxacin were also developed to enhance skin reconstruction and improve adhesivity and mechanical strength for healing [155]. Sun et al. [156] prepared collagen nanofibers (Coll-NFs) in 2018, which were found to decrease inflammation, promote faster recovery of wounds, and increase angiogenesis (Fig. 8J). A matrix of Collagen-laminin having resveratrol loaded with hyaluronic acid-DPPC microparticles was used in treating the injury in diabetic rats, and a controlled drug release was achieved along with its antioxidant activity [157]. Collagen-chitosan scaffolds loaded with pioglitazone were synthesized, which were biocompatible, promoted cell growth, and exhibited enhanced wound contraction in diabetic wounds [158]. In yet another formulation, collagen dressing was loaded with neurotensin, which facilitated the controlled release of drugs at the site of injury, enhanced re-epithelialization, and decreased the secretion of inflammatory cytokines in diabetic foot ulcers [159].
Heparin-based formulations
Heparin microislands were developed by Pruett et al. [160] in microporous scaffolds, which promoted diabetic wound-healing abilities by epidermal regeneration and re-vascularization. The heparin-poloxamer hydrogel was also developed by Xu et al. [161] using polylysine to heal endometrial injury by controlling KGF release and enhancing adhesiveness. Double-emulsion nanoparticles were developed using sulfated alginate and polycaprolactone to improve the delivery of heparin-binding growth factors that promote healing due to connective tissue growth factor secretion [162]. Additionally, pro-angiogenic nanofibrous membranes based on chitosan and heparin were developed using an efficient and new electrospinning method for wound-healing applications, which enhanced tissue angiogenesis [163]. Senturk et al. [164] prepared heparin memetic particles (heparin-NFs) in 2016, which were found to enhance re-epithelialization, promote a fast transition of pro-inflammatory to anti-inflammatory cytokines, improve angiogenesis, and lead to high VEGF levels and wound recovery (Fig. 8K). Analyses of re-epithelialization, granulation tissue formation, blood vessel density, VEGF, and inflammatory response measurements quantified wound recovery [160]. The synthesis of heparin-based hydrogel incorporating Cu5.40 nanozymes was done for the inhibition of inflammation by decreasing the pro-inflammatory cytokines and ROS scavenging and eventually leading to wound healing [165]. Lu et al. [166] developed a thermoresponsive hydrogel involving heparin protein with Lactococcus incorporated in it, which is helpful in wound healing by promoting angiogenesis, reducing the inflammatory microenvironment across the wound area, and decreasing the risk of systemic toxicities by preventing the entry of bacteria at the infection site.
P311 peptide-based micelles were assembled with ROS-responsive polymer for the transformation of an oxidative wound environment to a regenerative environment for wound healing by promoting collagen deposition, re-epithelialization, cell migration, and granular tissue synthesis [167]. Recently Ge et al. developed a novel nanoarmor that mimics the earth’s defense mechanism for the transport of IL-4 by protecting its biological activity and enhancing circulation throughout the blood. The synthesized copolymer consists of two layers: outer polyethylene glycol layer and intermediate rosamirinic acid layer for protecting the innermost IL-4, which is helpful in ROS scavenging, decrease in the secretion of inflammatory cytokines, and M1 to M2 transition crucial for healing [168]. The different nanoformulations obtained by using different proteins along with their potential outcome are given in Table 2.
Growth factors and growth regulators in healing wounds
Growth factors, like proteins, play a crucial role in wound healing. These physiologically active proteins bind to a specific receptor and stimulate molecular mechanisms for various cell functions, supporting cell proliferation, differentiation, migration, metabolism, and wound healing [174]. FGF is known to upregulate the activation of MEK-P, which in turn activates the NF-kβP50/P50 and inhibits NF-kβP50/P65 expression responsible for the activation of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12, and TNF-α [38, 175]. FGF also upregulates the expression of Akt, which activates eNOS, STAT3, PI3K, and MMP, responsible for tissue growth and angiogenesis [176]. Nguyen et al. in 2017 prepared FGF-loaded carboxyl methyl chitosan (CMCS) nanoparticles (FGF-CMCS NPS) using an ionic gelation method for biological applications and FGF-2 remains protected from degradation of trypsin and thus act as an efficient way of FGF2 delivery at wound site for in vivo applications [177] (Fig. 8L). Butko et al. in 2016 loaded fibroblast growth factor (bFGF) into N-succinyl-chitosan and chitosan/TPP to prepare its nanoformulation with 60% encapsulating efficiency. The bFGF stimulates the transition from inflammation to cell proliferation, remodeling, and wound recovery [178]. Also, Cetin et al., in 2007, reported bFGF (basic fibroblast growth factor; belongs to the FGF family), and a pleiotropic growth factor-loaded chitosan particles (bFGF-CNPs) with 27.388% encapsulation efficiency and the particles were found to be unaffected in their structure by any changes in release parameters and encapsulation procedure, thus maintaining their stability [179] (Fig. 8M).
Losi et al. [180] prepared bFGF and vascular endothelial growth factor (VEGF)-loaded poly lactic-co-glycolic acid (PLGA) nanoparticles (VEGF-PLGA NPs), which had a significant effect on cell division, cell proliferation, collagen deposition, re-epithelization, and helped in wound closure in comparison with controls in addition to angiogenesis (Fig. 8N). VEGF activates PI3K, Akt, and eNOS to support cell growth and angiogenesis [181]. Further, it activates JAK/STAT3, which helps in the survival of the cells and blocks the signaling of pro-inflammatory cytokines STAT1, SOCS, etc. [182]. Chereddy et al. [183] treated wounds with VEGF-loaded PLGA nanoparticles, which showed an enhancement in collagen deposition that helps in re-epithelialization, angiogenesis, and complete recovery of the injury in 28 days in comparison with the free GFs. Murphy et al. [184] synthesized VEGF-encapsulated PLGA particles (VEGF-PLGA) through leaching and observed 70% recovery within 12 days and found to enhance the wound-healing capacity at a faster pace. Mohandas et al. [185] prepared fibrin nanoparticles for VEGF loading to help improve the in vitro and in vivo angiogenesis, ultimately leading to enhanced healing effect due to blood vessel formation (Fig. 8O). An in vitro transcription (IVT) method was used to develop VEGF-A mRNA which are encapsulated for delivery into ionizable lipid-mediated nanoparticles using microfluidic method and are found to be very effective in enhancing the proliferation of cells along with efficient delivery of mRNA, are found to be non-toxic in nature and used for diabetic wound healing [186]. A new chitosan-modified hydrogel having silver ions and epidermal growth factor encapsulated in nanoparticles is developed for healing the diabetic wounds and exhibited excellent collagen deposition and maturation ability, increased re-epithelialization, and optimized delivery of silver and growth factor at the wound site [187]. A polymeric path containing epidermal growth factor, GelMA hydrogel, and PHBV membranes is developed for treating the diabetic wounds. This path not only enhances the angiogenesis but also promotes proliferation and differentiation of fibroblasts, endothelial cells, and keratinocytes at the wound site [188]. Pan et al. [189] synthesized keratinocyte growth factor (KGF)-linked gold nanoparticles (KGF-AuNPs), which promotes keratinocyte proliferation; promotes wound healing through re-epithelization rather than granulation (Fig. 8P). KGF works similarly to VEGF through the KGF receptor (KGFR) [190]. The similar nanoparticles used for in vivo studies on diabetic rat models by Li et al. [191] showed the binding of KGF with KGF receptors. They enhanced the collagen-I level, TGF-β1, and alpha-smooth actin (α-SMA) that assists in wound healing. Muhamed et al., in 2019, synthesized KGF-loaded nanoparticles (KGF-F NPs) using fibrin which enhanced in vivo migration of cells and wound closure [192, 193] (Fig. 8Q). Like other growth factors, EGF also shifts the equilibrium toward the anti-inflammatory cytokines by activating PI3K/Akt, mTOR, MEK-P, and STAT 3, etc. [194]. Zhang et al. [195] prepared carboxymethyl chitosan (CC) nanoparticles by hydrophobic conjugation of linoleic acid with the carboxymethyl chitosan and loaded them with recombinant human epidermal growth factor (rh-EGF-CC NPs) (Fig. 8R). Controlled release of the loaded unstable growth factor showed the wound-healing effect when tested in vitro and in vivo. Even in chronic wounds, CC showed more inflammation recovery and healing efficiency than free rh-EGF. Gainza et al. [196] prepared a similar SLN particles nanocage by emulsification ultrasonication method for loading rh-EGF that showed significant wound healing compared to the free rh-EGF. In chronic wound, rh-EGF-loaded nanoparticles promote the proliferation of cells, reduce inflammation, and help in re-epithelization and wound healing. Chu et al. [197] used identical rh-EGF-loaded SLN particles (rh-EGF-SLNPs) prepared using the double-emulsion method (Fig. 8S). The growth factor-loaded particles enhance fibroblast proliferation and wound healing in diabetic rat models. Rajam et al. [198] prepared EGF and FGF encapsulated inside chitosan tpp nanoparticles having 83% and 84% release capacity, respectively, up to 35 days.
Xie et al. [199] used two growth factors, platelet-derived growth factor (PDGF-BB) (Fig. 2J) and VEGF; VEGF loaded within the nanofibers and PDGF-BB inside the PLGA nanoparticles. It accelerated wound regeneration, tissue remodeling, and collagen deposition with platelet-derived and VEGF-stimulated angiogenesis. Circolo et al. [200] showed that PDGF decreases the expression of TNF-α and IL-1 inflammatory cytokine. Piran et al. prepared electro-spun embedded chitosan nanoparticles for the controlled release of PDGF-BB (PDGF-BB CNPs) at the site of the wound (Fig. 8T). PDGF-BB showed significant changes in the chemotactic behavior of the cells, induced the proliferation of the fibroblast cells and neutrophils, caused the migration of the cells at the wound site, and helped in wound closure [201]. On the basis of all the instances mentioned above regarding the utilization of growth factors and growth regulators as potent stimulators of wound healing by regulating cellular proliferation, migration, and differentiation, more research needs to be done to enhance their stability, absorption rate, efficacy, target specificity, biocompatibility, and growth-promoting abilities to yield better outcomes in the near future. The mechanism of action of these growth factors is well known to the scientific community, but their application in preclinical and clinical trials is still lacking despite their wonderful features. Moreover, in recent years the number of patients suffering from intractable wounds, including diabetic ulcers, foot ulcers, etc., is increasing drastically, and to eliminate these ailments, research in the field of understanding the role of growth factors for optimizing the wound surroundings for treatment will be an exciting area of interest.
Conclusion and future directions
Wound healing is a complex process involving several phases that occur simultaneously to promote faster recovery and prevent infection. Nanoformulations involving inorganic, organic, or biological precursors have been developed to make the formulations for wound healing biocompatible, cost-effective, and efficient. Protein and growth factors are commonly used due to their unique properties. They control inflammation at the wound site through distinct signaling pathways, leading to fast recovery. Protein-based nanoformulations can be entrapped inside the particle, protein-embedded particles, or loaded on the surface of the particles. Further, the incorporation of proteins into nanoformulations promotes the stability and activity of the proteins by preventing their degradation under unfavorable conditions. These formulations control the release of proteins for a longer time, hence enhancing efficacy and effect at the wound site. They also assist in targeted drug delivery and enhance solubility and biocompatibility. The kinetic and thermodynamic behavior of proteins and growth factors based on particles plays a critical role in timely wound healing without complications such as infection. An appropriate protein-based healing system in the form of an ointment, dressing, scaffold, hydrogel, film, powder, or electro-spun fibers can be utilized depending on the wound's requirements. Protein-based particles offer many advantages, including biocompatibility, efficiency, size, structure, easy availability, low production cost, and high biological efficiency. They also exhibit bioactivity, biodegradability, non-toxicity, enhanced re-epithelialization, cell growth, wound contraction, better repair, infection control, and antioxidant abilities.
The demand for protein-functionalized nanomaterials is enormously increasing, and emphasis should be laid on making the synthesis process less cumbersome and more effective. The elaborative discussion of the future perspective of these formulations is very crucial as their development will revolutionize healthcare facilities in wound healing in the near future, and these should be developed by keeping in mind the specific target or the effective area in the body. These formulations have a bright future in therapeutic and theranostics, and they can be used as promising drug carriers making their delivery targeted and effective. The proteins that can be transformed into scaffolds or fibers have a wider potency to act as a carrier of different therapeutics, including drugs, dyes, and inorganic and small organic moieties, thus diversifying their potential applications. In the future, these nanoformulations are expected to play a significant role in developing personalized medicine for wound healing due to the advances in technology, which makes it possible to customize the formulations based on the individual patient's needs and the specific characteristics of the wound and ultimately lead to more effective and efficient wound healing by reducing the time and cost of treatment. Furthermore, the commercial potential of protein-based nanoformulations is enormous. With the increasing prevalence of chronic wounds, such as diabetic foot ulcers and pressure ulcers, the demand for effective and efficient wound-healing treatments is rising, and protein-functionalized nanoformulations have the potential to become a widely used treatment option due to their efficacy, safety, and cost-effectiveness. Several companies have already entered the protein-based nanoformulations market, and many more are expected to follow in the near future. The market is expected to grow rapidly, and the global wound care market is estimated to reach USD 24.8 billion by 2026. Developing new and innovative protein-based nanoformulations could help drive this growth further.
In conclusion, protein-based nanoformulations have shown immense potential in wound healing and have a bright future in both medical and commercial applications. Further research and development in this area are expected to lead to the development of more effective and efficient wound-healing treatments, improving the quality of life for millions of people worldwide.
Data availability
Not applicable.
References
Wang Z, Wang Z, Lu WW, et al. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017;9(10):e435–e435. https://doi.org/10.1038/am.2017.171.
Oliveira SF, Bisker G, Bakh NA, et al. Protein functionalized carbon nanomaterials for biomedical applications. Carbon N Y. 2015;95:767–79. https://doi.org/10.1016/J.CARBON.2015.08.076.
Liu K, Han L, Zhuang J, Yang DP. Protein-directed gold nanoparticles with excellent catalytic activity for 4-nitrophenol reduction. Mater Sci Eng C Mater Biol Appl. 2017;78:429–34. https://doi.org/10.1016/J.MSEC.2017.04.052.
Wang F, Tan WB, Zhang Y, et al. Luminescent nanomaterials for biological labelling. Nanotechnology. 2006. https://doi.org/10.1088/0957-4484/17/1/R01.
Swift JL, Cramb DT. Nanoparticles as fluorescence labels: is size all that matters? Biophys J. 2008;95:865–76. https://doi.org/10.1529/BIOPHYSJ.107.127688.
Gawali SL, Shelar SB, Gupta J, et al. Immobilization of protein on Fe3O4 nanoparticles for magnetic hyperthermia application. Int J Biol Macromol. 2021;166:851–60. https://doi.org/10.1016/J.IJBIOMAC.2020.10.241.
Liu X, Zhang H, Chang L, et al. Human-like collagen protein-coated magnetic nanoparticles with high magnetic hyperthermia performance and improved biocompatibility. Nanoscale Res Lett. 2015. https://doi.org/10.1186/S11671-015-0752-3.
Vieira S, Vial S, Reis RL, Oliveira JM. Nanoparticles for bone tissue engineering. Biotechnol Prog. 2017;33:590–611. https://doi.org/10.1002/BTPR.2469.
Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014. https://doi.org/10.1155/2014/180549.
Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater Med. 2020;1:10–9. https://doi.org/10.1016/J.SMAIM.2020.04.001.
Brito AMM, Belleti E, Menezes LR, et al. Proteins and peptides at the interfaces of nanostructures. An Acad Bras Cienc. 2019;91:e20181236–e20181236. https://doi.org/10.1590/0001-3765201920181236.
Siriwardana K, Wang A, Gadogbe M, et al. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J Phys Chem C Nanomater Interfaces. 2015;119:2910–6. https://doi.org/10.1021/JP512440Z.
Subbiah R, Veerapandian M, Yun KS. Nanoparticles: functionalization and multifunctional applications in biomedical sciences. Curr Med Chem. 2010;17:4559–77. https://doi.org/10.2174/092986710794183024.
Kaur P, Sharma S, Choudhury SD, et al. Insulin-copper quantum clusters preparation and receptor targeted bioimaging. Colloids Surf B Biointerfaces. 2020. https://doi.org/10.1016/J.COLSURFB.2020.110785.
Kaur P, Choudhury D. Functionality of receptor targeted zinc-insulin quantum clusters in skin tissue augmentation and bioimaging. J Drug Target. 2021;29:541–50. https://doi.org/10.1080/1061186X.2020.1864740.
Sharda D, Attri K, Kaur P, Choudhury D. Protection of lead-induced cytotoxicity using paramagnetic nickel-insulin quantum clusters. RSC Adv. 2021;11:24656–68. https://doi.org/10.1039/D1RA03597E.
Tofanello A, Miranda ÉGA, Dias IWR, et al. pH-dependent synthesis of anisotropic gold nanostructures by bioinspired cysteine-containing peptides. ACS Omega. 2016;1:424–34. https://doi.org/10.1021/ACSOMEGA.6B00140.
Yu M, Wu J, Shi J, Farokhzad OC. Nanotechnology for protein delivery: overview and perspectives. J Control Release. 2016;240:24–37.
Zhu X, Wu J, Shan W, et al. Polymeric nanoparticles amenable to simultaneous installation of exterior targeting and interior therapeutic proteins. Angew Chem. 2016;128:3370–3.
Wu J, Kamaly N, Shi J, et al. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew Chem Int Ed. 2014;53:8975–9.
Chen X, Ling X, Zhao L, et al. Biomimetic shells endow sub-50 nm nanoparticles with ultrahigh paclitaxel payloads for specific and robust chemotherapy. ACS Appl Mater Interfaces. 2018;10:33976–85.
Hama R, Reinhardt JW, Ulziibayar A, et al. Recent tissue engineering approaches to mimicking the extracellular matrix structure for skin regeneration. Biomimetics. 2023;8:130.
Ghosh G, Panicker L. Protein-nanoparticle interactions and a new insight. Soft Matter. 2021;17:3855–75. https://doi.org/10.1039/D0SM02050H.
Strodtbeck F. Physiology of wound healing. Newborn Infant Nurs Rev. 2001;1:43–52. https://doi.org/10.1053/NBIN.2001.23176.
Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–62.
Scully D, Sfyri P, Wilkinson HN, et al. Optimising platelet secretomes to deliver robust tissue-specific regeneration. J Tissue Eng Regen Med. 2020;14:82–98.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75.
Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40:635–47.
Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle). 2015;4:119–36.
Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–65.
Honnegowda TM, Kumar P, Udupa EGP, et al. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthet Res. 2015;2:243–9.
Poché RA, Hsu C-W, McElwee ML, et al. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Dev Biol. 2015;403:30–42.
Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–11.
Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering. 2021;8:63.
Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223.
Kahle B, Hermanns H-J, Gallenkemper G. Evidence-based treatment of chronic leg ulcers. Dtsch Arztebl Int. 2011. https://doi.org/10.3238/ARZTEBL.2011.0231.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. https://doi.org/10.1177/0022034509359125.
Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care (New Rochelle). 2018;7:209–31. https://doi.org/10.1089/WOUND.2017.0761.
Kaur P, Choudhury D. Insulin promotes wound healing by inactivating NFkβP50/P65 and activating protein and lipid biosynthesis and alternating pro/anti-inflammatory cytokines dynamics. Biomol Concepts. 2019;10:11–24. https://doi.org/10.1515/BMC-2019-0002.
McCormick SM, Heller NM. Regulation of macrophage, dendritic cell, and microglial phenotype and function by the SOCS proteins. Front Immunol. 2015. https://doi.org/10.3389/FIMMU.2015.00549.
Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76:169–72. https://doi.org/10.1016/J.JDERMSCI.2014.11.001.
Sharda D, Attri K, Choudhury D. Future research directions of antimicrobial wound dressings. Antimicrob Dress. 2023. https://doi.org/10.1016/B978-0-323-95074-9.00007-5.
Gupta SK. Impact of ulceration. Ulcers Lower Extrem. 2016. https://doi.org/10.1007/978-81-322-2635-2_2/COVER.
Martins LM, Oliveira ARS, Cruz KJC, et al. Obesity, inflammation, and insulin resistance. Braz J Pharm Sci. 2014;50:677–92. https://doi.org/10.1590/S1984-82502014000400003.
Apikoglu-Rabus S, Izzettin FV, Turan P, Ercan F. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol. 2010;35:180–5. https://doi.org/10.1111/J.1365-2230.2009.03419.X.
Watters C, Everett JA, Haley C, et al. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infect Immun. 2013;82:92–100. https://doi.org/10.1128/IAI.00651-13.
Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014;40:1433–46. https://doi.org/10.1016/J.BURNS.2014.03.020.
Oryan A, Alemzadeh E. Effects of insulin on wound healing: a review of animal and human evidences. Life Sci. 2017;174:59–67. https://doi.org/10.1016/J.LFS.2017.02.015.
Kaur P, Sharma AK, Nag D, et al. Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing. Nanomedicine. 2019;15:47–57. https://doi.org/10.1016/J.NANO.2018.08.013.
Ju HW, Lee OJ, Lee JM, et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int J Biol Macromol. 2016;85:29–39. https://doi.org/10.1016/J.IJBIOMAC.2015.12.055.
Nguyen TP, Nguyen QV, Nguyen V-H, et al. Silk fibroin-based biomaterials for biomedical applications: a review. Polymers (Basel). 2019;11:1933.
Martínez-Mora C, Mrowiec A, García-Vizcaíno EM, et al. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE. 2012. https://doi.org/10.1371/JOURNAL.PONE.0042271.
Kambe Y. Functionalization of silk fibroin-based biomaterials for tissue engineering. Polym J. 2021;53(12):1345–51. https://doi.org/10.1038/s41428-021-00536-5.
Park YR, Sultan MT, Park HJ, et al. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018;67:183–95. https://doi.org/10.1016/J.ACTBIO.2017.12.006.
Wu R, Li H, Yang Y, et al. Bioactive silk fibroin-based hybrid biomaterials for musculoskeletal engineering: recent progress and perspectives. ACS Appl Bio Mater. 2021;4:6630–46. https://doi.org/10.1021/ACSABM.1C00654.
Wang J, Hao S, Luo T, et al. Feather keratin hydrogel for wound repair: preparation, healing effect and biocompatibility evaluation. Colloids Surf B Biointerfaces. 2017;149:341–50. https://doi.org/10.1016/J.COLSURFB.2016.10.038.
Feroz S, Muhammad N, Ratnayake J, Dias G. Keratin-based materials for biomedical applications. Bioact Mater. 2020;5:496–509.
Chen X, Zhai D, Wang B, et al. Hair keratin promotes wound healing in rats with combined radiation-wound injury. J Mater Sci Mater Med. 2020. https://doi.org/10.1007/S10856-020-06365-X.
Burnett LR, Rahmany MB, Richter JR, et al. Hemostatic properties and the role of cell receptor recognition in human hair keratin protein hydrogels. Biomaterials. 2013;34:2632–40. https://doi.org/10.1016/J.BIOMATERIALS.2012.12.022.
Rahmany MB, Hantgan RR, Van Dyke M. A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials. 2013;34:2492–500. https://doi.org/10.1016/J.BIOMATERIALS.2012.12.008.
Xu S, Sang L, Zhang Y, et al. Biological evaluation of human hair keratin scaffolds for skin wound repair and regeneration. Mater Sci Eng C Mater Biol Appl. 2013;33:648–55. https://doi.org/10.1016/J.MSEC.2012.10.011.
Wang S, Wang Z, Foo SEM, et al. Culturing fibroblasts in 3D human hair keratin hydrogels. ACS Appl Mater Interfaces. 2015;7:5187–98. https://doi.org/10.1021/ACSAMI.5B00854.
Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–40. https://doi.org/10.1046/J.1523-1747.2001.01327.X.
Zhang X, Yin M, Zhang LJ. Keratin 6, 16 and 17—critical barrier alarmin molecules in skin wounds and psoriasis. Cells. 2019. https://doi.org/10.3390/CELLS8080807.
Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res. 2021;8:2.
Chattopadhyay S, Raines RT. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–33. https://doi.org/10.1002/BIP.22486.
Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering (Basel). 2021. https://doi.org/10.3390/BIOENGINEERING8050063.
Hatz RA, von Jan NCS, Schildberg F-W. Mechanisms of action of collagenase in wound repair. Wound Heal Skin Physiol. 1995. https://doi.org/10.1007/978-3-642-77882-7_20.
Sekine T, Nakamura T, Shimizu Y, Ueda H, Matsumoto K, Takimoto Y, Kiyotani T. A new type of surgical adhesive made from porcine collagen and polyglutamic acid. J Biomed Mat Res. 2001;54(2):305–10.
Hao C, Xu H, Yu L, Zhang L. Heparin: an essential drug for modern medicine. Prog Mol Biol Transl Sci. 2019;163:1–19.
Goh MC, Hwang Y, Tae G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr Polym. 2016;147:251–60. https://doi.org/10.1016/J.CARBPOL.2016.03.072.
Galvan L. Effects of heparin on wound healing. J WOCN. 1996;23:224–6. https://doi.org/10.1016/S1071-5754(96)90095-9.
Olczyk P, Mencner Ł, Komosinska-Vassev K. Diverse roles of heparan sulfate and heparin in wound repair. Biomed Res Int. 2015. https://doi.org/10.1155/2015/549417.
Nawaz A, Zaman Safi S, Sikandar S, et al. Heparin-loaded alginate hydrogels: characterization and molecular mechanisms of their angiogenic and anti-microbial potential. Materials (Basel). 2022. https://doi.org/10.3390/MA15196683.
Zheng J, Zhang W, Li L, et al. Signaling pathway and small-molecule drug discovery of FGFR: a comprehensive review. Front Chem. 2022;10:860985.
Benington L, Rajan G, Locher C, Lim LY. Fibroblast growth factor 2: a review of stabilisation approaches for clinical applications. Pharmaceutics. 2020;12:508.
Takaya K, Aramaki-Hattori N, Sakai S, et al. Fibroblast growth factor 7 suppresses fibrosis and promotes epithelialization during wound healing in mouse fetuses. Int J Mol Sci. 2022;23:7087.
Xiaojie W, Banda J, Qi H, et al. Scarless wound healing: current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022;66:26–37.
Monavarian M, Kader S, Moeinzadeh S, Jabbari E. Regenerative scar-free skin wound healing. Tissue Eng Part B Rev. 2019;25:294–311.
Goswami AG, Basu S, Huda F, et al. An appraisal of vascular endothelial growth factor (VEGF): the dynamic molecule of wound healing and its current clinical applications. Growth Factors. 2022;40:73–88.
Mohanty C, Pradhan J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater Sci Eng C. 2020;111:110751.
Xu F, Wang H, Zhang J, et al. A facile design of EGF conjugated PLA/gelatin electrospun nanofibers for nursing care of in vivo wound healing applications. J Ind Text. 2022;51:420S-440S.
Jian K, Yang C, Li T, et al. PDGF-BB-derived supramolecular hydrogel for promoting skin wound healing. J Nanobiotechnol. 2022;20:1–9.
Gong X. Self-assembly technique for biomedical applications. Nano Life. 2015;05:1542002. https://doi.org/10.1142/S1793984415420027.
Liu X, Gong X, Hu Q, Li Y. Ion-modulated flow behavior of layer-by-layer fabricated polymer thin films. RSC Adv. 2015;5:64192–5. https://doi.org/10.1039/C5RA11734H.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–95. https://doi.org/10.1208/S12248-012-9339-4.
Bawa R. Nanoparticle-based therapeutics in humans: a survey. Nanotech L Bus. 2008;5:135.
Goncalves G, Marques PAAP, Granadeiro CM, et al. Surface modification of graphene nanosheets with gold nanoparticles: the role of oxygen moieties at graphene surface on gold nucleation and growth. Chem Mater. 2009;21:4796–802. https://doi.org/10.1021/CM901052S.
Mahmoudi M, Sant S, Wang B, et al. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46. https://doi.org/10.1016/J.ADDR.2010.05.006.
Cheng D, Yong X, Zhu T, et al. Synthesis of protein nanoparticles for drug delivery. Eur J BioMed Res. 2016;2:8. https://doi.org/10.18088/EJBMR.2.2.2016.PP8-11.
Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–7. https://doi.org/10.1210/JC.2005-1093.
Jain SK, Gupta Y, Jain A, et al. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine. 2008;4:41–8. https://doi.org/10.1016/J.NANO.2007.11.004.
Balthasar S, Michaelis K, Dinauer N, et al. Preparation and characterisation of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials. 2005;26:2723–32. https://doi.org/10.1016/J.BIOMATERIALS.2004.07.047.
Neves-Petersen MT, Gryczynski Z, Lakowicz J, et al. High probability of disrupting a disulphide bridge mediated by an endogenous excited tryptophan residue. Protein Sci. 2002;11:588–600. https://doi.org/10.1110/PS.06002.
Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161:38–49. https://doi.org/10.1016/J.JCONREL.2012.04.036.
Cui S, Yang Z, McClements DJ, et al. Stability mechanism of Pickering emulsions co-stabilized by protein nanoparticles and small molecular emulsifiers by two-step emulsification with different adding sequences: from microscopic to macroscopic scales. Food Hydrocoll. 2023;137:108372.
Crisante F, Francolini I, Bellusci M, et al. Antibiotic delivery polyurethanes containing albumin and polyallylamine nanoparticles. Eur J Pharm Sci. 2009;36:555–64.
Meiguni MSM, Salami M, Rezaei K, et al. Fabrication and characterization of a succinyl mung bean protein and arabic gum complex coacervate for curcumin encapsulation. Int J Biol Macromol. 2023;224:170–80.
Yan C, Zhang W. Coacervation processes. In: Gaonkar AG, Vasisht N, Khare A, Sobel S, editors. Microencapsulation in the Food industry. Academic Press; 2014. pp 125–37. https://doi.org/10.1016/B978-0-12-404568-2.00012-1.
Habibi N, Mauser A, Ko Y, Lahann J. Protein nanoparticles: uniting the power of proteins with engineering design approaches. Adv Sci. 2022;9:2104012.
Estrada LPH, Champion JA. Protein nanoparticles for therapeutic protein delivery. Biomater Sci. 2015;3:787–99.
Zhao S, Huang C, Yue X, et al. Application advance of electrosprayed micro/nanoparticles based on natural or synthetic polymers for drug delivery system. Mater Des. 2022;220:110850.
Quevedo DF, Habibi N, Gregory JV, et al. Multifunctional synthetic protein nanoparticles via reactive electrojetting. Macromol Rapid Commun. 2020;41:2000425.
Hua F, Lvov YM. Layer-by-layer assembly. In: Erokhin V, Ram MK, Yavuz O, editors. The new frontiers of organic and composite nanotechnology. Elsevier Science; 2008. pp 1–44. https://doi.org/10.1016/B978-00804505
Zhang S, Xia F, Demoustier-Champagne S, Jonas AM. Layer-by-layer assembly in nanochannels: assembly mechanism and applications. Nanoscale. 2021;13:7471–97. https://doi.org/10.1039/D1NR01113H.
Bulaklak K, Gersbach CA. The once and future gene therapy. Nat Commun. 2020;11:1–4. https://doi.org/10.1038/s41467-020-19505-2.
Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5:1–23. https://doi.org/10.1038/s41392-019-0089-y.
Arif U, Haider S, Haider A, et al. Biocompatible polymers and their potential biomedical applications: a review. Curr Pharm Des. 2019;25:3608–19. https://doi.org/10.2174/1381612825999191011105148.
Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Investig. 2019;49(4):347–80. https://doi.org/10.1007/S40005-019-00439-X.
Kothale D, Verma U, Dewangan N, et al. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr Drug Deliv. 2020;17:755–75. https://doi.org/10.2174/1567201817666200810110226.
Parveen K, Banse V, Ledwani L (2016) Green synthesis of nanoparticles: their advantages and disadvantages. In: AIP conference proceedings. AIP Publishing
Bourganis V, Karamanidou T, Kammona O, Kiparissides C. Polyelectrolyte complexes as prospective carriers for the oral delivery of protein therapeutics. Eur J Pharm Biopharm. 2017;111:44–60.
Fathi M, Donsi F, McClements DJ. Protein-based delivery systems for the nanoencapsulation of food ingredients. Compr Rev Food Sci Food Saf. 2018;17:920–36.
Butterfield GL, Lajoie MJ, Gustafson HH, et al. Evolution of a designed protein assembly encapsulating its own RNA genome. Nature. 2017;552:415–20.
Fang F, Szleifer I. Kinetics and thermodynamics of protein adsorption: a generalized molecular theoretical approach. Biophys J. 2001;80:2568–89. https://doi.org/10.1016/S0006-3495(01)76228-5.
Mollerup JM. A review of the thermodynamics of protein association to ligands, protein adsorption, and adsorption isotherms. Chem Eng Technol. 2008;31:864–74. https://doi.org/10.1002/CEAT.200800082.
Goudarzi F, Hejazi P. Comprehensive study on the effects of total monomers’ content and polymerization temperature control on the formation of the polymer-layer in preparation of insulin-imprinted magnetic nanoparticles. Eur Polym J. 2020;126:109541. https://doi.org/10.1016/J.EURPOLYMJ.2020.109541.
Fan S, Zhang Y, Shao H, Hu X. Electrospun regenerated silk fibroin mats with enhanced mechanical properties. Int J Biol Macromol. 2013;56:83–8. https://doi.org/10.1016/J.IJBIOMAC.2013.01.033.
Zhang R, Zhang T, Lv Y, et al. Selective binding of heparin oligosaccharides in a magnetic thermoresponsive molecularly imprinted polymer. Talanta. 2019;201:441–9. https://doi.org/10.1016/J.TALANTA.2019.04.050.
Kianvash N, Bahador A, Pourhajibagher M, et al. Evaluation of propylene glycol nanoliposomes containing curcumin on burn wound model in rat: biocompatibility, wound healing, and anti-bacterial effects. Drug Deliv Transl Res. 2017;7:654–63. https://doi.org/10.1007/S13346-017-0405-4.
Zhu YP, Brown JR, Sag D, et al. Adenosine 5’-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194:584–94. https://doi.org/10.4049/JIMMUNOL.1401024.
Miller AM, Nolan MJ, Choi J, et al. Lactate treatment causes NF-kappaB activation and CD44 shedding in cultured trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:1615–21. https://doi.org/10.1167/IOVS.06-1086.
Hosoyama T, Aslam MI, Abraham J, et al. IL-4R drives dedifferentiation, mitogenesis, and metastasis in rhabdomyosarcoma. Clin Cancer Res. 2011;17:2757–66. https://doi.org/10.1158/1078-0432.CCR-10-3445.
Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013;946:225–39. https://doi.org/10.1007/978-1-62703-128-8_14.
Brundu SFA. Polarization and repolarization of macrophages. J Clin Cell Immunol. 2015. https://doi.org/10.4172/2155-9899.1000319.
Li X, Liu Y, Zhang J, et al. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater Sci Eng C Mater Biol Appl. 2016;72:394–404. https://doi.org/10.1016/J.MSEC.2016.11.085.
Ehterami A, Salehi M, Farzamfar S, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol. 2018;117:601–9. https://doi.org/10.1016/J.IJBIOMAC.2018.05.184.
Abdelkader DH, Tambuwala MM, Mitchell CA, et al. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly(vinyl alcohol)-borate hydrogels. Drug Deliv Transl Res. 2018;8:1053–65. https://doi.org/10.1007/S13346-018-0554-0.
Ribeiro MC, Correa VLR, da Silva FKL, et al. Wound healing treatment using insulin within polymeric nanoparticles in the diabetes animal model. Eur J Pharm Sci. 2020. https://doi.org/10.1016/J.EJPS.2020.105330.
Lee J, Blaber M. Increased functional half life of fibroblast growth factor-1 by recovering a vestigial disulphide bond. J Proteins Proteom. 2010;1:37–42.
Sharda D, Choudhury D. Insulin–cobalt core–shell nanoparticles for receptor-targeted bioimaging and diabetic wound healing. RSC Adv. 2023;13:20321–35.
Ponrasu T, Veerasubramanian PK, Kannan R, et al. Morin incorporated polysaccharide–protein (psyllium–keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv. 2018;8:2305–14. https://doi.org/10.1039/C7RA10334D.
Wan W, Cai F, Huang J, et al. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J Mater Chem B. 2019;7:2954–61. https://doi.org/10.1039/C8TB03341B.
Agarwal Y, Rajinikanth PS, Ranjan S, et al. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: an in-vitro and in-vivo studies. Int J Biol Macromol. 2021;176:376–86. https://doi.org/10.1016/J.IJBIOMAC.2021.02.025.
Maity B, Alam S, Samanta S, et al. Antioxidant silk fibroin composite hydrogel for rapid healing of diabetic wound. Macromol Biosci. 2022;22:2200097. https://doi.org/10.1002/MABI.202200097.
Tariq M, Tahir HM, Butt SA, et al. Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ. 2021;9:e10232. https://doi.org/10.7717/PEERJ.10232/SUPP-2.
Özen N, Özbaş Z, İzbudak B, et al. Boric acid-impregnated silk fibroin/gelatin/hyaluronic acid-based films for improving the wound healing process. J Appl Polym Sci. 2022;139:51715. https://doi.org/10.1002/APP.51715.
Zhang F, Yin C, Qi X, et al. Silk Fibroin crosslinked glycyrrhizic acid and silver hydrogels for accelerated bacteria-infected wound healing. Macromol Biosci. 2022;22:2100407. https://doi.org/10.1002/MABI.202100407.
Karaly AH, Sarhan WA, El-Sherbiny IM. Development of a silk fibroin-based multitask aerosolized nanopowder formula for efficient wound healing. Int J Biol Macromol. 2021;182:413–24. https://doi.org/10.1016/J.IJBIOMAC.2021.03.178.
Wu YY, Jiao YP, Xiao LL, et al. Experimental study on effects of adipose-derived stem cell-seeded silk fibroin chitosan film on wound healing of a diabetic rat model. Ann Plast Surg. 2018;80:572. https://doi.org/10.1097/SAP.0000000000001355.
Sen S, Basak P, Prasad Sinha B, et al. Anti-inflammatory effect of epidermal growth factor conjugated silk fibroin immobilized polyurethane ameliorates diabetic burn wound healing. Int J Biol Macromol. 2020;143:1009–32. https://doi.org/10.1016/J.IJBIOMAC.2019.09.219.
Yao Q, Lan QH, Jiang X, et al. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics. 2020;10:11719. https://doi.org/10.7150/THNO.47682.
Wang Q, Zhou S, Wang L, et al. Bioactive silk fibroin scaffold with nanoarchitecture for wound healing. Compos B Eng. 2021;224:109165.
Huang K, Jinzhong Z, Zhu T, et al. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J Mater Chem B. 2021;9:1452–65.
Veerasubramanian PK, Thangavel P, Kannan R, et al. An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf B Biointerfaces. 2018;165:92–102. https://doi.org/10.1016/J.COLSURFB.2018.02.022.
Gao F, Li W, Deng J, et al. Recombinant human hair keratin nanoparticles accelerate dermal wound healing. ACS Appl Mater Interfaces. 2019;11:18681–90. https://doi.org/10.1021/ACSAMI.9B01725.
Kumaran P, Gupta A, Sharma S. Synthesis of wound-healing keratin hydrogels using chicken feathers proteins and its properties. Int J Pharm Pharm Sci. 2016;9:171–8. https://doi.org/10.22159/ijpps.2017v9i2.15620.
Roy DC, Tomblyn S, Burmeister DM, et al. Ciprofloxacin-loaded keratin hydrogels prevent pseudomonas aeruginosa infection and support healing in a porcine full-thickness excisional wound. Adv Wound Care. 2015. https://doi.org/10.1089/WOUND.2014.0576.
Zuniga K, Isaac A, Christy S, et al. Characterization of a human platelet lysate-loaded keratin hydrogel for wound healing applications in vitro. Int J Mol Sci. 2022;23:4100. https://doi.org/10.3390/IJMS23084100.
Kirsner RS, Cassidy S, Marsh C, et al. Use of a keratin-based wound dressing in the management of wounds in a patient with recessive dystrophic epidermolysis bullosa. Adv Skin Wound Care. 2012;25:400–3. https://doi.org/10.1097/01.ASW.0000419404.44947.DE.
Su S, Bedir T, Kalkandelen C, et al. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur Polym J. 2021;142:110158. https://doi.org/10.1016/J.EURPOLYMJ.2020.110158.
Sharma S, Rostamabadi H, Gupta S, et al. Nano/micro-formulations of keratin in biocomposites, wound healing and drug delivery systems; recent advances in biomedical applications. Eur Polym J. 2022;180:111614.
Rana MM, Rahman MS, Ullah MA, et al. Amnion and collagen-based blended hydrogel improves burn healing efficacy on a rat skin wound model in the presence of wound dressing biomembrane. Biomed Mater Eng. 2020;31:1–17. https://doi.org/10.3233/BME-201076.
Pathan IB, Munde SJ, Shelke S, et al. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: ex-vivo and in-vivo evaluation. Int J Polym Mater Polym Biomater. 2018;68:165–74. https://doi.org/10.1080/00914037.2018.1429437.
Mahmoud AA, Salama AH. Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: preparation, evaluation and in-vivo wound healing assessment. Eur J Pharm Sci. 2016;83:155–65. https://doi.org/10.1016/J.EJPS.2015.12.026.
Sun L, Gao W, Fu X, et al. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater Sci. 2018;6:340–9. https://doi.org/10.1039/C7BM00545H.
Gokce EH, Tuncay Tanrıverdi S, Eroglu I, et al. Wound healing effects of collagen-laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur J Pharm Biopharm. 2017;119:17–27. https://doi.org/10.1016/J.EJPB.2017.04.027.
Natarajan J, Sanapalli BKR, Bano M, et al. Nanostructured lipid carriers of pioglitazone loaded collagen/chitosan composite scaffold for diabetic wound healing. Adv Wound Care. 2019;8:499–513. https://doi.org/10.1089/WOUND.2018.0831.
Moura LIF, Dias AMA, Suesca E, et al. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2014;1842:32–43. https://doi.org/10.1016/J.BBADIS.2013.10.009.
Pruett LJ, Jenkins CH, Singh NS, et al. Heparin microislands in microporous annealed particle scaffolds for accelerated diabetic wound healing. Adv Funct Mater. 2021;31:2104337. https://doi.org/10.1002/ADFM.202104337.
Xu HL, Xu J, Shen BX, et al. Dual regulations of thermosensitive heparin-poloxamer hydrogel using ϵ-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl Mater Interfaces. 2017;9:29580–94. https://doi.org/10.1021/ACSAMI.7B10211/ASSET/IMAGES/LARGE/AM-2017-10211P_0011.JPEG.
Maatouk B, Jaffa MA, Karam M, et al. Sulfated alginate/polycaprolactone double-emulsion nanoparticles for enhanced delivery of heparin-binding growth factors in wound healing applications. Colloids Surf B Biointerfaces. 2021;208:112105. https://doi.org/10.1016/J.COLSURFB.2021.112105.
Shahzadi L, Ramzan A, Anjum A, et al. An efficient new method for electrospinning chitosan and heparin for the preparation of pro-angiogenic nanofibrous membranes for wound healing applications. J Appl Polym Sci. 2022. https://doi.org/10.1002/APP.53212.
Senturk B, Mercan S, Delibasi T, et al. Angiogenic peptide nanofibers improve wound healing in STZ-induced diabetic rats. ACS Biomater Sci Eng. 2016;2:1180–9. https://doi.org/10.1021/ACSBIOMATERIALS.6B00238.
Peng Y, He D, Ge X, et al. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact Mater. 2021;6:3109–24.
Lu Y, Li H, Wang J, et al. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv Funct Mater. 2021;31:2105749.
Shi R, Li H, Jin X, et al. Promoting re-epithelialization in an oxidative diabetic wound microenvironment using self-assembly of a ROS-responsive polymer and P311 peptide micelles. Acta Biomater. 2022;152:425–39.
Ge X, Hu J, Peng Y, et al. Atmosphere-inspired multilayered nanoarmor with modulable protection and delivery of Interleukin-4 for inflammatory microenvironment modulation. Biomaterials. 2023;301:122254.
Shang Y, Wang P, Wan X, et al. Chlorhexidine-loaded polysulfobetaine/keratin hydrogels with antioxidant and antibacterial activity for infected wound healing. Int J Biol Macromol. 2023;242:124754.
Valentino C, Vigani B, Zucca G, et al. Formulation development of collagen/chitosan-based porous scaffolds for skin wounds repair and regeneration. Int J Biol Macromol. 2023;242:125000.
Budhiraja M, Zafar S, Akhter S, et al. Mupirocin-loaded chitosan microspheres embedded in Piper betle extract containing collagen scaffold accelerate wound healing activity. AAPS PharmSciTech. 2022;23:77.
Li M, Li X, Gao Y, et al. Composite nanofibrous dressing loaded with Prussian blue and heparin for anti-inflammation therapy and diabetic wound healing. Int J Biol Macromol. 2023;242:125144.
Du P, Diao L, Lu Y, et al. Heparin-based sericin hydrogel–encapsulated basic fibroblast growth factor for in vitro and in vivo skin repair. Heliyon. 2023;9:e13554.
Meng X, Zare I, Yan X, Fan K. Protein-protected metal nanoclusters: an emerging ultra-small nanozyme. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1602. https://doi.org/10.1002/WNAN.1602.
Fan L, Ding L, Lan J, et al. Fibroblast growth factor-1 improves insulin resistance via repression of JNK-mediated inflammation. Front Pharmacol. 2019. https://doi.org/10.3389/FPHAR.2019.01478.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–66. https://doi.org/10.1002/WDEV.176.
Nguyen CT, Nguyen TT, Nguyen TT, et al. Preparation and in vitro evaluation of FGF-2 incorporated carboxymethyl chitosan nanoparticles. Carbohydr Polym. 2017;173:114–20. https://doi.org/10.1016/J.CARBPOL.2017.05.080.
Butko A, Bonat Celli G, Paulson A, Ghanem A. Entrapment of basic fibroblast growth factor (bFGF) in a succinylated chitosan nanoparticle delivery system and release profile. J Biomater Sci Polym Ed. 2016;27:1045–57. https://doi.org/10.1080/09205063.2016.1178519.
Cetin M, Aktas Y, Vural I, et al. Preparation and in vitro evaluation of bFGF-loaded chitosan nanoparticles. Drug Deliv. 2007;14:525–9. https://doi.org/10.1080/10717540701606483.
Losi P, Briganti E, Errico C, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9:7814–21. https://doi.org/10.1016/J.ACTBIO.2013.04.019.
Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–4. https://doi.org/10.1042/BST0310020.
Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist. 2012;17:1014–26. https://doi.org/10.1634/THEONCOLOGIST.2012-0043.
Chereddy KK, Lopes A, Koussoroplis S, et al. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine. 2015;11:1975–84. https://doi.org/10.1016/J.NANO.2015.07.006.
Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–7. https://doi.org/10.1016/S0142-9612(00)00120-4.
Mohandas A, Anisha BS, Chennazhi KP, Jayakumar R. Chitosan-hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf B Biointerfaces. 2015;127:105–13. https://doi.org/10.1016/J.COLSURFB.2015.01.024.
Zha W, Wang J, Guo Z, et al. Efficient delivery of VEGF-A mRNA for promoting diabetic wound healing via ionizable lipid nanoparticles. Int J Pharm. 2023;632:122565.
Lee Y-H, Hong Y-L, Wu T-L. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385.
Augustine R, Hasan A, Dalvi YB, et al. Growth factor loaded in situ photocrosslinkable poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater Sci Eng, C. 2021;118:111519.
Pan A, Zhong M, Wu H, et al. Topical application of keratinocyte growth factor conjugated gold nanoparticles accelerate wound healing. Nanomedicine. 2018;14:1619–28. https://doi.org/10.1016/J.NANO.2018.04.007.
Xie Y, Su N, Yang J, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020. https://doi.org/10.1038/S41392-020-00222-7.
Li S, Tang Q, Xu H, et al. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine (Lond). 2019;14:2909–23. https://doi.org/10.2217/NNM-2018-0487.
Muhamed I, Sproul EP, Ligler FS, Brown AC. Fibrin nanoparticles coupled with keratinocyte growth factor enhance the dermal wound-healing rate. ACS Appl Mater Interfaces. 2019;11:3771–80. https://doi.org/10.1021/ACSAMI.8B21056.
Béduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–67. https://doi.org/10.1016/J.BIOMATERIALS.2007.06.011.
Mortaz E, Reza Masjedi M, Allameh A, Adcock IM. Inflammasome signaling in pathogenesis of lung diseases. Curr Pharm Des. 2012;18:2320–8. https://doi.org/10.2174/138161212800166077.
Zhang P, Liu C. Enhancement of skin wound healing by rhEGF-loaded carboxymethyl chitosan nanoparticles. Polymers (Basel). 2020. https://doi.org/10.3390/POLYM12071612.
Gainza G, Pastor M, Aguirre JJ, et al. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: in vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51–61. https://doi.org/10.1016/J.JCONREL.2014.04.032.
Chu Y, Yu D, Wang P, et al. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18:499–505. https://doi.org/10.1111/J.1524-475X.2010.00612.X.
Rajam M, Pulavendran S, Rose C, Mandal AB. Chitosan nanoparticles as a dual growth factor delivery system for tissue engineering applications. Int J Pharm. 2011;410:145–52. https://doi.org/10.1016/J.IJPHARM.2011.02.065.
Xie Z, Paras CB, Weng H, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9. https://doi.org/10.1016/J.ACTBIO.2013.07.030.
Circolo A, Pierce GF, Katz Y, Strunk RC. Antiinflammatory effects of polypeptide growth factors. Platelet-derived growth factor, epidermal growth factor, and fibroblast growth factor inhibit the cytokine-induced expression of the alternative complement pathway activator factor B in human fibroblasts. J Biol Chem. 1990;265:5066–71. https://doi.org/10.1016/S0021-9258(19)34085-2.
Piran M, Vakilian S, Piran M, et al. In vitro fibroblast migration by sustained release of PDGF-BB loaded in chitosan nanoparticles incorporated in electrospun nanofibers for wound dressing applications. Artif Cells Nanomed Biotechnol. 2018;46:511–20. https://doi.org/10.1080/21691401.2018.1430698.
Acknowledgements
The authors are thankful to Thapar Institute of Engineering and Technology for providing them with all the facilities. Authors are grateful to TIET, the library, for providing access to journals. DC is willing to thank TIET-VT CEEMS and ICMR -Govt of India (Project No. 17X(3)/Adhoc/63/2022-ITR) for funding.
Author information
Authors and Affiliations
Contributions
D.C., P.K., and D.S conceptualized the study. P.K, D.S drafted the manuscript . Figures. 1, 3 and graphical abstract were prepared by (P.K.), and Figs. 2, 4, 5, 6, 7, 8 were prepared by (D.S.) D.C critically reviewed the manuscript. D.S revised the manuscript. D.S., P.K., and D.C approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharda, D., Kaur, P. & Choudhury, D. Protein-modified nanomaterials: emerging trends in skin wound healing. Discover Nano 18, 127 (2023). https://doi.org/10.1186/s11671-023-03903-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-023-03903-8