Abstract
We reported a facile two-step electrochemical-chemical approach for in situ growth of nickel sulfide and graphene counter electrode (CE) decorated with silver nanoparticles (signed NiS/Gr-Ag) and served in dye-sensitized solar cells (DSSCs). Under optimum conditions, the DSSC achieved a remarkable power conversion efficiency of 8.36 % assembled with the NiS/Gr-Ag CE, much higher than that based on the Pt CE (7.76 %). The surface morphology of NiS/Gr-Ag CE exhibited a smooth surface with cross-growth of NiS, graphene, and Ag nanoparticles, which was beneficial to the fast mass transport of electrolytes; increased the contact area of electrolytes and active materials; and enabled to speed up the reduction of triiodide to iodide. The research on the electrochemical properties also showed that the NiS/Gr-Ag CE possessed lower charge transfer resistance and more excellent electrocatalytic activity in iodide/triiodide electrolyte compared to the Pt electrode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
With photovoltaic technology being realized as a suitable renewable power for the fulfillment of increasing world energy consumption, dye-sensitized solar cells (DSSCs) have attracted a great deal of attention because of their potential as next-generation photovoltaic devices [1–3]. Up to the present, the highest photoelectric conversion efficiencies (PCEs) of DSSCs have achieved over 13 % [4]. In general, one DSSC consists of a dye-adsorbed nano-sized TiO2 anode, an iodide electrolyte, and a platinum (Pt) counter electrode (CE). Though Pt for the functional role fulfills requirements of CE, such as good transparency, excellent electrical conductivity, and electrocatalytic ability, researchers are still struggling to seek for low-cost substitutes for its high cost and scarcity [5–7]. In recent years, many interesting materials [8–18] have emerged as earth-abundant Pt replacements for DSSC CE catalysts including carbon materials, conducting polymers, transition metal sulfides, nitrides, carbides, and alloy. Within the group, nickel sulfide (NiS), as an electron collector and electrocatalyst in DSSCs, has aroused particular interest because of its low cost, superior electrocatalytic activity, and conductivity [19, 20]. The DSSC with NiS CE electrodeposited by a potential reversal technique showed comparable PCE (6.82 %) to the Pt-based cell (7.00 %) [21]. Ku et al. prepared a highly transparent NiS CE and presented good PCE for thiolate/disulfide-mediated DSSCs [22].
Moreover, the electrocatalytic ability and chemical activity of graphene were highly desirable for DSSCs; therefore, graphene and its composites (graphene and conducting polymers, sulfides, nitrides, and so on) were believed as an efficient route in enhancing the electrical and electrochemical performances of DSSCs [23–25]. Also, it is well known that silver (Ag) nanoparticles have been studied intensively for their potential applications in catalysis, biosensors, and environmental filtration for their high surface energy, excellent catalytic activity, and unique optical and electrical properties [26, 27].
Therefore, taking into account all of these factors, to search for more robust Pt-free CEs and improve DSSC performance, we prepared the nickel sulfide/graphene CE decorated with Ag nanoparticles (signed NiS/Gr-Ag) by using a facile two-step electrochemical-chemical approach for in situ growth and served in DSSCs. The extensively characterized DSSC with NiS/Gr-Ag CE was carried out and exhibited a considerably improved performance in PCE of 8.10 % under irradiation of 100 mW cm−2 (AM 1.5 G). The electrocatalytic ability of the NiS/Gr-Ag CE was also systematically investigated. This type of electrode was anticipated with many advantages including good electrical conductivity, easy electrolyte penetration, and high electrocatalytic ability.
Methods
Preparation of NiS/Gr-Ag CE
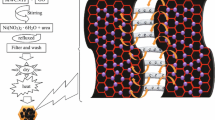
A NiS/Gr-Ag CE was prepared by a two-step electrochemical/chemical process and is outlined below. Firstly, a fluorine-doped tin oxide (FTO) glass (8 Ω cm−2, the thickness of 350 nm, Hartford Glass Co., USA) was modified by 4-aminothiophenol as we reported [28] and signed as FTO*. Secondly, the electrodeposition of NiS/Gr CE was carried out with an electrochemical analyzer system (CHI660E, Shanghai Chenhua Device Company, China). All experiments were implemented in a three-electrode cell, including a Pt foil as CE, an Ag/AgCl reference electrode, and FTO* (with an exposed area of 1 cm2) as the working electrode. The base electrodeposition solution consisted of 0.05 M nickel (II) chloride hexahydrate and 1.0 M thiourea in 50 ml deionized water and treated by ultrasonication for 30 min. The graphene flakes (UNI-ONWARD Corp., 99.9 %, Taiwan) were added into the base plating solution in weights ranging from 0, 5, 10, 15, and to 20 wt.%. The NiS/Gr CE sintered at 100 °C for 4 h in air, after being immersed in 50 ml ethanol solution, consisted of 2 ml thioglycolic acid and 0.2 g silver nitrate at 60 °C for 12 h and then sintered at 250 °C for another 30 min. The schematic of NiS/Gr-Ag CE is shown in Fig. 1. For comparison, the NiS, NiS/Gr, and NiS/Ag electrodes were prepared with a similar approach with NiS/Gr-Ag CE by using a three-electrode system; the Pt CE was prepared by soaking the FTO* substrates in 0.01 M H2PtCl6 ethanol solution; the graphene powder was mixed with a 1 wt.% solution of polyvinylidene fluoride in terpineol by a three-roll miller, and then the mixture was coated on the FTO* glass substrate by a doctor blade and dried at 200 °C for 30 min to get a Gr CE.
Fabrication of DSSC
The multilayer TiO2 photoanode was prepared as described previously [29, 30]. The dye was loaded by soaking the TiO2 anode in 0.3 mM of dye Z-907 (Switzerland) ethanol solution for 12 h. Thus, the dye-sensitized TiO2 anode with thickness of 8–10 μm was obtained. The liquid electrolyte consisted of 0.05 M of I2, 0.1 M of LiI, 0.6 M of tetrabutylammonium iodide, and 0.5 M of 4-tert-butyl-pyridine in acetonitrile. The active area of the dye-coated TiO2 film was 0.4 × 0.7 cm2.
Characterization
The surface morphology of the samples was observed by using a JSM-7001F field emission scanning electron microscope (SEM). Electrochemical impedance spectroscopy (EIS) measurements of the DSSC-based EIS were carried out under the simulating open-circuit conditions at ambient atmosphere, sealing with thermoplastic hot-melt Surlyn and leaving an exposed area of 1.0 cm2. The frequency of the applied sinusoidal AC voltage signal was varied from 0.1 to 105 Hz, and the corresponding amplitude was kept at 5 mV in all cases. The incident photon-to-current conversion efficiency (IPCE) was conducted using a Newport lamp (Oriel 300 W xenon arc lamp) and light filters as a monochromatic light source and Si photovoltaic cell as reference. The photovoltaic test of DSSC was carried out by measuring the photocurrent-photovoltage (J-V) character curve under white light irradiation of 100 mW cm−2 (AM 1.5 G) from a solar simulator (XQ-500 W, Shanghai Photoelectricity Device Company, China) in ambient atmosphere.
Results and Discussion
Figure 2a shows the SEM image of NiS CE, which exhibited the NiS nanoparticles uniformly coated on the FTO* substrate and a few of pores formed on its surface. Figure 2b, c, d, and e presented the images of the NiS/Gr-Ag CE at low and high magnifications. From the three images, it is clear that the NiS/Gr-Ag CE possessed a uniform and smooth surface with graphene, NiS, and Ag nanoparticles distributed on the FTO* surface, which featured a nanowall network-like shape. We attributed the formation of the NiS/Gr-Ag nanowall to the high surface energy of Ag nanoparticles which made the Ag nanoparticles easily clustered and coated on the NiS/Gr surface. Such a distinctive nanowall network structure was conducive to storage and penetration of electrolytes, provided a large contact area for the electrolytes and active materials, and enabled to speed up the reduction of triiodide to iodide.
To check the composition of NiS/Gr-Ag CE, energy-dispersive spectrometry (EDS) was carried out. Figure 2f revealed that the presence of C, S, Ag, and Ni elements and strong signals were found in NiS/Gr-Ag CE. This illustrated that NiS was successfully deposited on the FTO* substrate. The strong signals for the Ag element as shown in Fig. 2f indicated that the Ag nanoparticles were filled in the pores and surface of NiS/Gr CE. The large amount of C element was responsible for the introduction of graphene flakes into the NiS/Gr-Ag CE. Consequently, the results could prove effectively that the NiS/Gr-Ag CE was successfully prepared.
To further confirm the composition of the NiS/Gr-Ag CE, XRD and Raman analyses were carried out as shown in Fig. 3 and Additional file 1: Figure S1. According to the Joint Committee on Powder Diffraction Standards (JCPDS card no. 3-1149), five very strong diffraction peaks at around 26.6°, 37.8°, 51.6°, 61.6° ,and 65.6° were corresponding to the signals of FTO and the diffraction peaks marked ※, ✰, and * were the contribution of graphene flakes (26.4° and 44.3°), Ag nanoparticles (44.1° and 64.1°), and NiS (35.5°, 45.8, and 69.4°), respectively. The Raman spectroscopy also revealed that the strong characteristic peaks of graphene were observed at 1350, 1579, and 2684 cm−1, respectively. Consequently, considering the results of EDS, XRD, and Raman spectra, the NiS/Gr-Ag CE has been synthesized successfully.
Figure 4 presents the Nyquist plots of Pt, graphene, NiS, NiS/Gr, NiS/Ag, and NiS/Gr-Ag CEs, and their corresponding parameters are listed in Table 1. R s was the resistance value at the onset point of the first semicircle, and the R ct was the radius of the first semicircle, and the semicircle at a low frequency represented the Nernst diffusion impedance (Z w ) corresponding to the diffusion resistance of the I −/I 3 − redox species. To our knowledge, R s and R ct were two crucial parameters for comparing the electrocatalytic abilities of various CEs, which were inversely correlated with the electrocatalytic ability of the CEs [31]. It is clear from Fig. 4 and Table 1 that the R s for the Pt, graphene, NiS, NiS/Gr, NiS/Ag, and NiS/Gr-Ag CEs were 5.50 ± 0.02, 8.10 ± 0.02, 7.33 ± 0.02, 6.83 ± 0.02, 6.19 ± 0.02, and 4.05 ± 0.02 Ω cm2, respectively. The NiS/Gr-Ag CE possessed much lower R s than the others, indicating a better electrical conductivity and electrocatalytic ability. The R ct of Pt, graphene, NiS, NiS/Gr, and NiS/Ag CEs were 2.78 ± 0.02, 6.59 ± 0.02, 5.45 ± 0.02, 3.65 ± 0.02, and 3.18 ± 0.02 Ω cm2, respectively, following the orders of graphene CE > NiS CE > NiS/Gr CE > NiS/Ag CE > Pt CE. The NiS/Gr-Ag CE showed the smallest R ct than that of the abovementioned CEs, but very close to that of the Pt CE. This meant that the NiS/Gr-Ag CE can rapidly reduce I 3 − to I − to speed up the diffusion of I 3 − as effectively as Pt CE. The Z w values obeyed the similar orders of NiS CE > graphene CE > NiS/Gr CE > NiS/Ag CE > Pt CE > NiS/Gr-Ag CE. However, in comparison with the NiS electrode, the graphene CE had a little smaller Z w for its high conductivity. In a word, the NiS/Gr-Ag CE was provided with the least overpotential and most excellent electrochemical catalytic ability for an electron transferring in I −/I 3 − redox species among the six CEs.
Figure 5a depicted the cyclic voltammograms of the various electrodes under the I −/I 3 − electrolyte system at a scan rate of 50 mV s−1. The peak to peak separation (E pp), the cathodic current density (I pc), and potential (V pc) were crucial parameters for comparing the electrocatalytic abilities of CEs [32, 33]. Among them, the |I pc| was positively correlated with the catalytic ability of CEs and the |V pc| and |E pp| were inversely correlated with the electrocatalytic activity of the CEs. From Fig. 5a, the NiS/Gr-Ag CE showed much higher |I pc| (3.43 ± 0.02 mA cm−2) than the Gr, NiS, NiS/Gr and NiS/Ag electrodes, a little higher than the Pt CE (3.18 ± 0.02 mA cm−2), indicating that the NiS/Gr-Ag CE effectively acted as a catalyst in the reaction of the I −/I 3 − electrolyte, which attributed to the large active surface area and their synergistic catalytic effect of NiS (with superior electrocatalytic activity) and graphene (with high conductivity). The |V pc| of the CEs increased in the order of Pt CE (0.16 ± 0.01 V) < NiS/Gr-Ag CE (0.17 ± 0.01 V) < NiS/Ag CE (0.19 ± 0.01 V) < NiS/Gr CE (0.20 ± 0.01 V) < NiS CE (0.24 ± 0.01 V) < graphene CE (0.25 ± 0.01 V), while the |E pp| obeyed the order of NiS/Gr-Ag CE (0.25 ± 0.01 V) < Pt CE (0.27 ± 0.01 V) < NiS/Ag CE (0.32 ± 0.01 V) < NiS/Gr CE (0.33 ± 0.01 V) < NiS CE (0.39 ± 0.01 V) < graphene CE (0.42 ± 0.01 V). Interestingly, the NiS/Gr-Ag CE showed larger |V pc| but much higher |I pc| and smaller |E pp| compared to the Pt CE. This was responsible for the incorporated Ag nanoparticles with good electrical conductivity. In the case of NiS/Gr-Ag CE, Ag nanoparticles evenly coated on the NiS/Gr surface and still basically followed the morphology of the conductive graphene network, which would effectively facilitate transport of the electron and diffusion of redox electrolyte.
Furthermore, the nearly linear relationship can be found for the NiS/Gr-Ag CE for the relationship between the anodic current density (I pa), I pc, and the square root of the scan rate (v) as exhibited in Fig. 5b. This can be attributed to the introduction of Ag nanoparticles and graphene flakes, which was advantageous to the electron transport effectively for the I 3 − reduction at the electrolyte|NiS/Gr-Ag CE interface, even at high scan rates. The results revealed that the reaction of I −/I 3 − redox couple at CE was dominated by the diffusion-controlled transport and there was no specific interaction between I −/I 3 − redox couple and NiS/Gr-Ag CE [34, 35].
The diffusion coefficients (D n ) of I 3 − in electrolytes of NiS/Gr-Ag and Pt CEs were also estimated of 3.30 × 10−6 and 2.4 × 10−6 cm−2 s−1 in the light of the Randles-Sevcik equation [36]. The D n of NiS/Gr-Ag CE was much larger than that of the Pt CE, which can be attributed to the high conductivity for the graphene flakes, excellent electrocatalytic ability for the Ag nanoclusters, and the abundant pores in the NiS surface. Therefore, that was able to shorten the ion diffusion path and promote ion diffusion flux.
where K was the constant of 2.69 × 105; n meant the number of electrodes contributing the charge transfer; A was the area of the CE; and C and v represented the concentration of I 3 − species and the scan rate, respectively.
The long-term electrochemical stability for the CE was very important to the performance of the device. As presented in Fig. 5c, the cyclic voltammetry (CVs) and the normalized cathodic and anodic peak current densities remained scarcely changed after being tested for 50 consecutive cycles, suggesting that the NiS/Gr-Ag electrode was provided with the characteristics of reversible redox activity, strong adhesiveness on the FTO* substrate, excellent electrochemical properties, and chemical stability [37].
To further verify the results of the EIS, Fig. 6 displays the Tafel polarization curves of the Pt, graphene, NiS, NiS/Gr, NiS/Ag, and NiS/Gr-Ag CEs. The tangent slope of Tafel curves provided the information regarding the exchange current density (J 0) [38]. The J 0 for the different CEs were also estimated and summarized in Table 1. Compared to the graphene, NiS, NiS/Gr, and NiS/Ag CEs, the NiS/Gr-Ag CE possessed a remarkably enhanced J 0, which was even slightly higher than that of the Pt CE. This meant that the NiS/Gr-Ag CE can trigger the reduction of I 3 − to I − more effectively than the others. The R ct was inversely dependent on J 0 and can be calculated based on Eqn. (2) [39]. The variance tendencies of the R ct values calculated from the J 0 values for the Pt, graphene, NiS, NiS/Gr, NiS/Ag, and NiS/Gr-Ag CEs were in accordance with those obtained in the EIS measurements. It may be concluded that NiS/Gr-Ag CE was more effective than the Pt CE in electrocatalyzing the I 3 − reduction for DSSC. Furthermore, the limiting diffusion current density (J lim), obtained from the intersection of the cathodic branch with the Y-axis, was associated with the diffusion coefficient (D f ) on the basis of Eqn. (3) [40]. For the different CEs, the changed tendencies of estimated D f were generally in agreement with the variation of D n obtained from the CV measurements. This result well confirmed that the NiS/Gr-Ag CE possessed excellent ion diffusivity.
where R was the gas constant, T was the temperature, F was the Faraday constant, and l was the spacer thickness.
To further research the electrocatalytic ability and the effect of graphene contents on NiS/Gr-Ag CE, Fig. 7 gives the EIS, Tafel, and CVs of the samples with various graphene contents. With the weight ratio of graphene increasing in the base plating solution from 0 to 5 wt.%, the R s, R ct, |E pp|, and |V pc| values decreased and the J 0 and |I pc| values shifted higher. The NiS/Gr-Ag CE achieved the most excellent electrochemical performance with 10 % of graphene. The possible reasons behind the performance with significant enhancements for the NiS/Gr-Ag CE were due to its large catalytic surface area and excellent conductivity of graphene and the synergetic effect of NiS, Ag, and graphene. As we know, the perfectly crystalline basal plane of a carbon material had very little catalytic effect [41]. Thus, the aggressive chemical treatments during the process of the electrodeposition also can possibly introduce significant oxygen-containing functional groups and provide the increased active sites for I 3 − reduction to further boost the electrocatalytic ability of the samples [42]. While further increasing the graphene content to 15 or 20 wt.%, it resulted in a negative effect on the electrochemical performance of the samples made from the EIS, Tafel, and CVs analyses, which may be responsible for the poor electrocatalytic activity of graphene. Thus, the results indicated that the NiS/Gr-Ag CE with the suitable graphene can remarkably improve conductivity and electrocatalytic ability for I 3 − reduction.
The photovoltaic performance of DSSCs assembled with various CEs including Pt, graphene, NiS, NiS/Gr, NiS/Ag, and NiS/Gr-Ag CEs (signed DSSC 1, DSSC 2, DSSC 3, DSSC 4, DSSC 5, DSSC 6) were evaluated under the illumination of 100 mW cm−2. According to Table 2 and Fig. 8a, the photovoltaic parameters of the DSSCs with various CEs followed the order of DSSC 6 > DSSC 1 > DSSC 5 > DSSC 4 > DSSC 3 > DSSC 2 except for the fill factor (FF). Among the DSSCs, the ones assembled with the composite CEs including NiS/Gr, NiS/Ag, and NiS/Gr-Ag all exhibited better PCEs compared to those of the DSSC based on the graphene or NiS CE. When the NiS or NiS/Gr CE was decorated with Ag nanoparticles, the FF, the short-circuit current density (J sc), and PCE all showed remarkable improvement. Thus, the DSSC using NiS/Gr-Ag CE generated an enhanced PCE of 8.36 %, which was much higher than that of the Pt-based (7.76 %) and NiS/Ag-based (7.17 %) DSSCs. The reasons of the enhancement on PCEs for the DSSC with NiS/Gr-Ag CE can be ascribed to the improved FF and J sc values, which was derived from the increased total current of I −/I 3 − redox reaction, and have been proven by its large cathodic current density and enhanced diffusivity of I −/I 3 − redox species, as indicated in the aforementioned CV tests. The improvement of FF possibly resulted from the decrease in the sum of R s and R ct.
To better understand the dynamics of electron transport and recombination, EIS were carried out for the DSSCs with various CEs under an illumination of 100 mW cm−2. The R ct1 meant the interfacial charge transfer resistance at the CE and electrolyte; the R ct2 indicated the interfacial charge transfer resistance at the dye-sensitized photoanode and the electrolyte, and Cμ was the constant phase element resulting from the capacitor components in DSSC [43]. Figure 8b shows the EIS made from DSSCs 1 to 6. The series resistance (R s) were 5.72 ± 0.02, 15.73 ± 0.02, 14.88 ± 0.02, 7.10 ± 0.02, 6.46 ± 0.02, and 5.24 ± 0.02 Ω cm2, and the R ct1 were 4.30 ± 0.02, 7.05 ± 0.02, 6.26 ± 0.02, 5.90 ± 0.02, 4.51 ± 0.02, and 3.24 ± 0.02 Ω cm2 for the DSSCs from 1 to 6. The lowest R ct1 was noted at 3.24 ± 0.02 Ω cm2 for DSSC 6 due to its high active surface area and good catalytic property certified by CVs, Tafel, and EIS of the NiS/Gr-Ag CE. The tendency of R s and R ct1 well agreed with the J sc and open-circuit voltage (V oc) in Table 2, which reflected in the improvement of V oc and PCE in DSSCs. In comparison with DSSCs from 1 to 5, DSSC 6 showed the smallest R ct2 of 5.24 ± 0.02 Ω cm2, suggesting that it had the lowest recombination rate. According to the equation τ n(EIS) = R ct2 × C μ , the electron lifetimes (τ n ) were calculated to be 7.165, 5.296, 5.548, 6.222, and 7.274 ms for DSSCs 1, 2, 3, 4, and 6, respectively. The longest electron lifetime for DSSC 6 indicated more effective suppression of the back reaction between a photoanode in the conduction band of TiO2 and I 3 − in the electrolyte and also reflected in the enhancement of J sc, V oc, and PCE for DSSC 6. The reasons for the lowest recombination rate and longest electron lifetime of DSSC 6 perhaps ascribed to the excellent electrocatalytic ability and large contact areas between CEs and TiO2 photoanode that there would be less recombination for the generated electrons occurring at the interface of TiO2 nanocrystals|I −/I 3 − electrolyte. However, despite the larger τ n for the DSSC 5 than for the DSSC 6, DSSC 5 exhibited lower J sc, V oc, and PCE than DSSC 6 for its lager R ct1 and R ct2, which did not facilitate the charge transport interior of DSSC. As a consequence, compared with DSSCs 1 to 5, DSSC 6 showed lower recombination rate and longer electron lifetime than the others. Thus, DSSC 6 obtained the highest values of J sc and PCE.
Figure 9 shows the IPCE of DSSCs assembled with the NiS/Gr-Ag and Pt CEs, which displayed similar photoelectric responses at the range of 343 nm for the direct band gap photoelectron excitation of TiO2 [44]. Both DSSCs exhibited the highest quantum efficiency of 79.2 and 70.1 %, respectively, at 520 nm. The remarkable improvement for the photoelectric performance of DSSC with NiS/Gr-Ag CE attributed to the synergistic effect of NiS, graphene, and Ag nanoparticles, and this promoted the light capture of dye in this region and perhaps resulted in a higher J sc and lower R ct than that of DSSC with the Pt CE. This result was also in good agreement with diffuse reflection results analyzed above.
Conclusions
In summary, NiS/Gr-Ag CE has been successful in situ grown on the surface of conductive glass through a facial two-step electrochemical-chemical approach and acted as Pt-free CE in DSSCs without any post-treatments. Under optimum conditions, the NiS/Gr-Ag CE exhibited amazing electrocatalytic activity and low charge transfer resistance for the reduction of I 3 − and the DSSC with it produced a higher short-circuit photocurrent and power conversion efficiency (16.205 mA cm−2 and 8.36 %, respectively) than the DSSC with a Pt CE (15.289 mA cm−2 and 7.76 %, respectively). In consideration of this facile approach, efficient and low-cost NiS/Gr-Ag CE has a vast potential in scalable production of DSSCs.
References
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Burschka J, Pellet N, Moon SJ, Humphry-Baker R, Gao P, Nazeeruddin MK et al (2013) Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499:316–319
Yella A, Lee HW, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK et al (2011) Porphyrin-sensitized solar cells with cobalt (ii/iii)–based redox electrolyte exceed 12 percent efficiency. Science 334:629–634
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BFE, Ashari-Astani N et al (2014) Dye-sensitized solar cells with 13 % efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242–247
Duan YY, Tang QW, He BL, Li R, Yu LM (2014) Transparent nickel selenide alloy counter electrodes for bifacial dye-sensitized solar cells exceeding 10 % efficiency. Nanoscale 6:12601–12608
He BL, Meng X, Tang QW (2014) Low-cost counter electrodes from CoPt alloys for efficient dye-sensitized solar cells. ACS Appl Mater Inter 6:4812–4818
Hao F, Dong P, Luo Q, Li JB, Lou J, Lin H (2013) Recent advances in alternative cathode materials for iodine-free dye-sensitized solar cells. Energy Environ Sci 6:2003–2019
Hao F, Wang Z, Luo Q, Lou J, Li J, Wang J et al (2012) Highly catalytic cross-stacked superaligned carbon nanotube sheets for iodine-free dye-sensitized solar cells. J Mater Chem 22:22756–22762
Kavan L, Yum J, Grätzel M (2011) Graphene nanoplatelets outperforming platinum as the electrocatalyst in co-bipyridine-mediated dye-sensitized solar cells. Nano Lett 11:5501–5506
Yue G, Wu J, Xiao Y, Lin J, Huang M, Lan Z (2012) Application of poly (3, 4-ethylenedioxythiophene):polystyrenesulfonate/polypyrrole counter electrode for dye-sensitized solar cells. J Phys Chem C 116:18057–18063
Li Q, Wu J, Tang Q, Lan Z, Li P, Lin J (2008) Application of microporous polyaniline counter electrode for dye-sensitized solar cells. Electrochem Commun 10:1299–1302
Yue G, Wu J, Xiao Y, Huang M, Lin J, Lin J (2013) High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J Mater Chem A 1:1495–1501
Song J, Li GR, Wu CY, Gao XP (2014) Metal sulfide counter electrodes for dye-sensitized solar cells: a balanced strategy for optical transparency and electrochemical activity. J Power Sources 266:464–470
Li G, Song J, Pan G, Gao X (2011) Highly Pt-like electrocatalytic activity of transition metal nitrides for dye-sensitized solar cells. Energy Environ Sci 4:1680–1683
Wu M, Bai J, Wang Y, Wang A, Lin X, Wang L et al (2012) High-performance phosphide/carbon counter electrode for both iodide and organic redox couples in dye-sensitized solar cells. J Mater Chem 22:11121–11127
Reece S, Hamel JA, Sung K, Jarvi TD, Esswein AJ, Pijpers JJH et al (2011) Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334:645–648
Chen XX, Tang QW, He BL, Lin L, Yu LM (2014) Platinum-free binary Co-Ni alloy counter electrodes for efficient dye-sensitized solar cells. Angew Chem Int Ed 53:10799–10803
Wan JW, Fang GJ, Yin HJ, Liu XF, Liu D, Zhao MT et al (2014) Pt–Ni alloy nanoparticles as superior counter electrodes for dye-sensitized solar cells: experimental and theoretical understanding. Adv Mater 26:8101–8106
Sun X, Dou J, Xie FY, Li YF, Wei MD (2014) One-step preparation of mirror-like NiS nanosheets on ITO for the efficient counter electrode of dye-sensitized solar cells. Chem Commun 50:9869–9871
Jiang N, Bogoev L, Popova M, Gul S, Yano J, Sun YJ (2014) Electrodeposited nickel-sulfide films as competent hydrogen evolution catalysts in neutral water. J Mater Chem A 2:19407–19414
Sun HC, Qin D, Huang SQ, Guo XZ, Li DM, Luo YH et al (2011) Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ Sci 4:2630–2637
Ku ZL, Li X, Liu GH, Wang H, Rong YG, Xu M et al (2013) Transparent NiS counter electrodes for thiolate/disulfide mediated dye-sensitized solar cells. J Mater Chem A 1:237–240
Yue GT, Wu JH, Xiao YM, Huang ML, Lin JM, Fan LQ et al (2013) Platinum/graphene hybrid film as a novel counter electrode for dye-sensitized solar cells. Electrochim Acta 92:64–70
Wang MK, Anghel AM, Marsan B, Ha NC, Pootrakulchote N, Zakeeruddin SM et al (2009) CoS supersedes Pt as efficient electrocatalyst for triiodide reduction in dye-sensitized solar cells. J Am Chem Soc 131:15976–15977
Tang QW, Zhang HH, Meng YY, He BL, Yu LM (2015) Dissolution engineering of platinum alloy counter electrodes in dye-sensitized solar cells. Angew Chem Int Ed. doi:10.1002/anie.201505339.
Baruah B, Gabriel GJ, Akbashev MJ, Booher ME (2013) Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 29:4225–4234
Tang B, Li JL, Hou XL, Afrin T, Sun L, Wang XA (2013) Colorful and antibacterial silk fiber from anisotropic silver nanoparticles. Ind Eng Chem Res 52:4556–4563
Yue GT, Li FT, Tan FR, Li GQ, Chen C, Wu JH (2014) Nickel sulfide films with significantly enhanced electrochemical performance induced by self-assembly of 4-aminothiophenol and their application in dye-sensitized solar cells. RSC Adv 4:64068–64074
Li Z-Q, Chen W-C, Guo F-L, Mo L-E, Hu L-H, Dai S-Y (2015) Mesoporous TiO2 yolk-shell microspheres for dye-sensitized solar cells with a high efficiency exceeding 11 %. Sci Rep 5:14178
Wu JH, Lan Z, Lin JM, Huang ML, Hao SC, Sato T et al (2007) A novel thermosetting gel electrolyte for stable quasi-solid-state dye-sensitized solar cells. Adv Mater 19:4006–4011
Yue GT, Wu JH, Lin J-Y, Xiao YM, Lin JM, Huang ML et al (2013) A counter electrode of multi-wall carbon nanotubes decorated with tungsten sulfide used in dye-sensitized solar cells. Carbon 55:1–9
Duan YY, Tang QW, Liu J, He BL, Yu LM (2014) Transparent metal selenide alloy counter electrodes for high-efficiency bifacial dye-sensitized solar cells. Angew Chem Int Ed 53:14569–14574
Lin JY, Wang WY, Chou SW (2015) Flexible carbon nanotube/polypropylene composite plate decorated with poly (3,4-ethylenedioxythiophene) as efficient counter electrodes for dye-sensitized solar cells. J Power Sources 282:348–357
Yue GT, Zhang WF, Wu JH, Jiang QW (2013) Glucose aided synthesis of molybdenum sulfide/carbon nanotubes composites as counter electrode for high performance dye-sensitized solar cells. Electrochim Acta 112:655–662
Tai SY, Liu CJ, Chou SW, Chien FSS, Lin JY, Lin TW (2012) Facile synthesis of MoS2/graphene nanocomposite with high catalytic activity toward triiodide reduction in dye-sensitized solar cells. J Mater Chem 22:24753–24759
Hauch A, Georg A (2001) Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim Acta 46:3457–3466
Wu JH, Li Y, Tang QW, Yue GT, Lin JM, Huang ML et al (2014) Bifacial dye-sensitized solar cells: a strategy to enhance overall efficiency based on transparent polyaniline electrode. Sci Rep 4:4028
Papageorgiou N, Maier WF, Grätzel M (1997) An iodine/triiodide reduction electrocatalyst for aqueous and organic media. J Electrochem Soc 144:876–884
Yue G, Wu J, Xiao Y, Huang M, Lin J, Lan Z (2013) Functionalized graphene/poly (3, 4-ethylenedioxythiophene):polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy 54:315–321
Gong F, Wang H, Xu X, Zhou G, Wang Z-S (2012) In situ growth of Co0.85Se and Ni0.85Se on conductive substrates as high-performance counter electrodes for dye-sensitized solar cells. J Am Chem Soc 134:10953–10958
Banks CE, Davies TJ, Wildgoose GG, Compton RG (2005) Electrocatalysis at graphite and carbon nanotube modified electrodes: edge-plane sites and tube ends are the reactive sites. Chem Commun 18:829–841
Bajpai R, Roy S, Kumar P, Bajpai P, Kulshrestha N, Rafiee J et al (2011) Graphene supported platinum nanoparticle counter-electrode for enhanced performance of dye-sensitized solar cells. ACS Appl Mater Interfaces 3:3884–3889
Li Z-Q, Ding Y, Mo L-E, Hu L-H, Wu JH, Dai S-Y (2015) Fine tuning of nanocrystal and pore sizes of TiO2 submicrospheres toward high performance dye-sensitized solar cells. ACS Appl Mater Inter 7:22277–22283
Li Z-Q, Que Y-P, Mo L-E, Chen W-C, Ding Y, Ma Y-M et al (2015) One-pot synthesis of mesoporous TiO2 microspheres and its application for high-efficiency dye-sensitized solar cells. ACS Appl Mater Inter 7:10928–10934
Acknowledgements
This work is also supported by China Postdoctoral Science Foundation Funded Project (No. 2015M572102) and the Scientific Research Found of Henan Provincial Department of Science and Technology (No. 122300410107).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
YG carried out the experiments, participated in the sequence alignment, and drafted the manuscript. LF and YG were involved in the electrochemical characterization of the devices. ZW helped to draft the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
Raman spectra of the NiS/Gr-Ag CE.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yue, G., Li, F., Yang, G. et al. Efficient Nickel Sulfide and Graphene Counter Electrodes Decorated with Silver Nanoparticles and Application in Dye-Sensitized Solar Cells. Nanoscale Res Lett 11, 239 (2016). https://doi.org/10.1186/s11671-016-1456-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1456-z