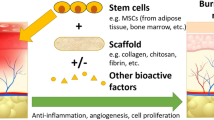
Abstract
Background
Burn injuries can be associated with prolonged healing, infection, a substantial inflammatory response, extensive scarring, and eventually death. In recent decades, both the mortality rates and long-term survival of severe burn victims have improved significantly, and burn care research has increasingly focused on a better quality of life post-trauma. However, delayed healing, infection, pain and extensive scar formation remain a major challenge in the treatment of burns. ADSCs, a distinct type of mesenchymal stem cells, have been shown to improve the healing process. The aim of this review is to evaluate the efficacy of ADSCs in the treatment of burn injuries.
Methods
A systematic review of the literature was conducted using the electronic databases PubMed, Web of Science and Embase. The basic research question was formulated with the PICO framework, whereby the usage of ADSCs in the treatment of burns in vivo was determined as the fundamental inclusion criterion. Additionally, pertinent journals focusing on burns and their treatment were screened manually for eligible studies. The review was registered in PROSPERO and reported according to the PRISMA statement.
Results
Of the 599 publications screened, 21 were considered relevant to the key question and were included in the present review. The included studies were almost all conducted on rodents, with one exception, where pigs were investigated. 13 of the studies examined the treatment of full-thickness and eight of deep partial-thickness burn injuries. 57,1 percent of the relevant studies have demonstrated that ADSCs exhibit immunomodulatory effects during the inflammatory response. 16 studies have shown improved neovascularisation with the use of ADSCs. 14 studies report positive influences of ADSCs on granulation tissue formation, while 11 studies highlight their efficacy in promoting re-epithelialisation. 11 trials demonstrated an improvement in outcomes during the remodelling phase.
Conclusion
In conclusion, it appears that adipose-derived stem cells demonstrate remarkable efficacy in the field of regenerative medicine. However, the usage of ADSCs in the treatment of burns is still at an early experimental stage, and further investigations are required in order to examine the potential usage of ADSCs in future clinical burn care.
Similar content being viewed by others
Introduction
Burn injuries are unpredictable traumas by their nature, and have varying degrees of severity. As with all wounds, the healing process of burns involves dynamic and overlapping phases including inflammation, proliferation and remodelling [1]. While partial thickness wounds can heal within 14 days with less scarring, deep partial and full-thickness burns are associated with prolonged healing, infection, an extensive inflammatory response and pathological scarring [1, 2]. Over the past decades, good progress has been made in the acute treatment of burn injuries. The mortality rate as well as the long-term survival of severely burned patients have improved significantly [3]. In recent years, burn care research has shifted to a better quality of survival by focusing on improvement wound healing, scar quality and contracture prevention [4]. However, delayed healing, infection, pain and pathological scar formation remain major challenges in burn care [1, 2]. The ultimate goal is to develop novel therapies that support the healing process and enable improved treatment outcomes.
Mesenchymal stem cells (MSCs) have emerged as a novel therapeutic approach in wound care and tissue regeneration [5, 6]. A distinct type of MSCs was discovered in large quantities within adipose tissue, namely adipose-derived stem cells (ADSCs) [7, 8].The effectiveness of ADSCs application in wound healing, including an improved immunoregulation, neovascularisation, granulation tissue formation, re-epithelialisation and remodelling, as well as their differentiation potential in various cell types was proven in several in vitro and in vivo studies [9,10,11,12,13,14,15]. The aim of this review is to evaluate the efficacy of adipose-derived stem cells in the treatment of burn injuries.
Methods
The present systematic review was registered in the PROSPERO database (CRD42022364221) and conducted following a protocol guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [16].
Identify the research question
The fundamental research question was formulated with the PICO framework as follows: How effective are adipose-derived stem cells in the treatment of burn injuries in vivo? The creation process is illustrated in Table 1.
Search strategy
A systematic review of the literature was performed, in order to detect concerns from in vivo studies published up to 30th September 2022 on the efficacy of adipose-derived stem cells in the treatment of burn injuries. To identify appropriate studies, the following online databases were searched: PubMed, Web of Science and Embase. The key terms of the applied search strategy for each online databank are displayed in Table 2. Additionally, pertinent journals that focus on burn care research were searched manually.
Study selection
The usage of adipose-derived stem cells in the treatment of burns was determined as the fundamental inclusion criteria. Firstly, all search results were exported into Mendeley Desktop (Version 1.19.8) and duplicates were eliminated. In the next step, titles, abstracts, and later, full-text articles, were analysed in relation to the inclusion criteria. Only full-text original articles published in the English language were eligible. Case reports, review articles, letter and comments, but also non in vivo studies were excluded. Furthermore, publications in which outcomes of ADSC therapy had inadequate focus or were not declared as primarily responsible for the treatment outcome were excluded. To ensure no inequity by wrongful exclusion, the whole analysis was performed by two investigators. In the event of a consensus between the two researchers being was found, a publication was included in the review process. With regard to missing or unclear information, the corresponding authors were contacted once by-email.
Study inclusion criteria:
-
Randomized and non-randomized controlled in-vivo studies
-
Only full-text original articles published in the English language will be eligible
-
Studies must focus on adipose-derived stem cells in the therapy of burns in vivo.
Study exclusion criteria:
-
Clinical trials
-
Ex vivo studies
-
Case reports
-
Reviews
-
Letters and comments
-
Paper not available as full-text
-
Paper not published in English language
-
Wounds not classified as burns
-
Depth extent of the injury is less than deep partial
-
The exact depth of the wound is not specified
-
ADSCs were not declared as primarily responsible for the treatment outcome
Data extraction
The following data were extracted from the text, tables, and graphs of the eligible studies by two independent study associates: (1) Study design; (2) Animal model; (3) Conditions of the wound; (4) Origin of ADSCs; (5) Dosage of ADSCs; (6) Carrier medium; (7) Method of application; (8) Comparison group; (9) Study duration; (10) Measured outcomes. Table 3 encompasses the key data extracted from the included studies.
Results
A total of 599 publications were identified from searches of electronic databases by using the specified search strategy. After the elimination of duplicates (n = 274), 325 publications were manually screened for relevant publications. Based on the title and the abstract, 281 were excluded due to the wrong topic or because they were not considered to be original quantitative research (e.g.; review articles, comments etc.), with 44 full text articles being retrieved and assessed for eligibility. Of these, 23 articles were excluded for the following reasons: 11 studies had inadequate focus on the efficacy of ADSCs or they were not declared as primarily responsible for the treatment outcome, one was unavailable in the English language, four were irretrievable, three had no burn injury model, and four other studies did not give clarity on wound depth. Consequently, 21 publications fulfilled the inclusion criteria. The study's inclusion process is displayed in Fig. 1.
Flow diagram (Preferred Reporting Items for Systematic Reviews and Meta-Analyses—PRISMA) of the study inclusion process. *Reports excluded: Reason 1: had no or inadequate focus on inadequate focus on the efficacy of ADSCs in the treatment of burns; Reason 2: unavailable in the English language; Reason 3: irretrievable, Reason 4: no burn injury model, Reason 5: the depth extent of the burn was not defined
Bias assessment
Upon applying the SYRCLE's Risk of Bias tool to the included 21 in vivo studies, the following observations were made (Fig. 2):
In the domain of sequence generation, only seven study indicated a low risk of bias, leaving 14 studies with an unclear risk. For allocation concealment, the risk remained unclear for all included studies. All included studies showed a low risk of bias in the domain of baseline characteristics. The absence of clarity in sequence generation and allocation concealment could potentially result in selection bias.
According to the publications, all 21 studies had an unclear risk in the domain of random housing and outcome assessment. In addition, seven studies had an unclear risk of blinding bias, both in the performance and detection bias section. This could potentially affect the reliability of the results and introduce performance and detection bias.
All of the included studies demonstrated a low risk of bias in the domain of incomplete outcome data and selective outcome reporting, indicating a low risk of attrition and reporting bias.
Moreover, all of 21 included studies demonstrated a low risk of bias in the domain of other sources of bias.
In conclusion, the application of the SYRCLE's Risk of Bias tool to these 21 animal studies has provided valuable insights into the methodological strengths and weaknesses present. While the low risk of bias in areas such as baseline characteristics, incomplete outcome data, selective outcome reporting, and other sources of bias is commendable, the high level of uncertainty in key domains, notably in selection, performance, and detection bias, is a cause for concern.
Study characteristics
Animal models
Of 21 studies included, 10 were based on a rat model [17,18,19, 21, 23, 26, 29, 31, 33, 35], 10 on a mouse model [20, 22, 24, 25, 27, 28, 30, 34, 36, 37], and a pig model [32] was employed in another instance. A total of 517 animals were examined in the studies. In 13 studies, full-thickness burns were inflicted on the laboratory animals, and in the remaining eight trials, deep partial-thickness burns were induced. The injuries were established by using specific heated devices on the animals' dorsa in 17 trials. In two studies [22, 33] were the wounds created by exposure to hot liquid and in another [31] by hydrochloric acid. 12 h after the burns, P. aeruginosa infection was induced in the treatment groups in the study by Banerjee et al. [21].
Intervention
ADSCs were injected in nine studies [17,18,19, 22, 25, 29, 31, 35, 36] or applied topically as wound dressings in 10 [20, 21, 23, 24, 27, 28, 32,33,34, 37]. In two studies, both variants were applied [26, 30]. Injections were either intradermal [17, 19, 26, 29, 35], subcutaneous [18, 22], or sub-escharal [25]. In six studies hydrogel [17, 21, 26, 27, 30, 37] was used as carriers for ADSCs. In another four the carrier was phosphate buffered saline (PBS) [18, 22, 25, 29]. Other carriers included medical honey [19], Dulbecco’s modified eagle medium (DMEM) [31, 35], human amniotic membrane [23, 24, 28], artificial dermis [20, 32], pig skin [28], and bio-printed gel scaffold [33, 34].
Please refer to Table 3 for the applied dose of ADSCs. In six studies, ADSCs were administered on the day of the burn [18, 20, 28, 29, 35, 36], in further five studies, on the day after the burn [22, 23, 25, 31, 33], in six other studies, two days [17, 19, 26, 32, 34, 37] and in one study, 9 days [21] after the burn respectively. In the study conducted by Barrera et al., the animals were treated with ADSCs five and 10 days after burning [30]. Zhou et al. compared a group that was injected with ADSCs on the day of the burn with a group in which the application was repeated on the fourth and eighth day post-burn [18]. One study did not specify the time-point of application [24].
In seven of the studies, the wounds were covered with transparent film dressings [19, 20, 27, 28, 33, 34, 37]. One study used hydrocolloid bandages [21] and another used Vaseline gauze as a secondary dressing [23]. Oryan et al. reported on the use of demineralized bone matrix to cover wounds [17]. In the study by Alemzadeh et al., acellular dermal matrix was prepared from sheep skin as a wound covering [26]. A self-adhesive absorbent dressing (Mepore) was used by Azam et al. [31]. Daily dressing with silver sulfadiazine impregnated sterile gauze was performed in the study by Karimi et al. [36]. In all other studies, no additional dressing was described or the authors did not respond to the e-mail enquiry.
Country of study-origin
In terms of the number of studies conducted in each country, Iran [17, 19, 23, 24, 26, 33, 36] ranked highest with seven, followed by USA [21, 22, 25, 27, 30, 32] with six. China [18, 34] had two, while Taiwan [29], Japan [20], Mexico [28], Pakistan [31], Brazil [35] and Singapore [37] each had one. Accordingly, 13 studies originate from Asia [17,18,19,20, 23, 24, 26, 29, 31, 33, 34, 36, 37], seven studies from North America [21, 22, 25, 27, 28, 30, 32] and one from South America [35]. None of the studies were conducted in Europe, Africa, Oceania or Antarctica.
Outcome
18 out of 21 studies demonstrated accelerated wound healing in the groups treated with ADSCs [17,18,19,20, 22,23,24, 26, 27, 29,30,31,32,33,34,35,36,37], with the remaining three studies reporting no differences in comparison with the control groups [21, 25, 28]. 16 studies compared the different closure rates in wounds [17,18,19,20, 23, 24, 26, 27, 29,30,31, 33,34,35,36,37], while two studies focused on the reduction in wound depth [22, 32].
13 studies have investigated the immunomodulation abilities of ADSCs during the inflammatory response [17,18,19, 23, 24, 26, 27, 30, 31, 33, 35,36,37], one of which found no difference from the control group [27]. A reduction in inflammatory cells through the use of ADSCs was demonstrated histologically in eight studies [17, 19, 23, 31, 33, 35,36,37]. The studies by Roshangar et al. and Karimi et al. showed a reduction in the number of cells including polymorphonuclear leukocytes and macrophages due to ADSCs application [33, 36]. In turn, in the study by Dong et al., no significant difference in macrophage or T-cell infiltration was detected in the groups treated either with or without ADSCs [27]. A decrease in pro-inflammatory cytokine interleukin 1-beta (IL-1β) after ADSCs application was shown in four studies [17, 19, 26, 31]. According to Gholipourmalekabadi et al., after administration of ADSCs, there was a significant increase in the pro-inflammatory cytokines macrophage inflammatory protein 2 (MIP-2) and tumor necrosis factor alpha 1 (TNF-α1), which returned to their physiological levels during wound healing, whereas the control group remained in the inflammatory response [24]. Further studies have confirmed the decrease of tumor necrosis factor alpha (TNF-α) [30, 31], along with other pro-inflammatory cytokines such as interleukin 6 (IL-6) [31], through the usage of ADSCs. Zhou et al. have shown an increase in the anti-inflammatory cytokine interleukin-1 receptor antagonist protein (IL-1ra) by ADSCs [18].
18 out of 21 studies involved the investigation of ADSCs in neovascularisation; 16 of these 18 demonstrated that the usage of ADSCs can support neovascularisation in vivo [17,18,19,20,21,22, 24,25,26,27, 29,30,31,32,33,34]. Only one study revealed a diminished neovascularisation due to the use of ADSCs [36], while another showed no effect [35]. For the research, tissue biopsies were harvested from within the wound areas. Evidence for the formation of the new vascular network was obtained either by haematoxylin and eosin [17, 19, 22,23,24, 26, 27, 32, 33] and Masson’s trichrome [18, 20, 24, 25, 27, 30, 32] staining, or by using specific antibodies including CD31 [18, 22, 24, 25, 27, 29, 30, 32, 34], CD34 [33], isolectinB4 (ILB4) [20], neural/glial antigen 2 (NG2) and von Willebrand factor [21] as well as vascular endothelial growth factor a1 (VEGFa1) and vascular endothelial growth factor receptor 2 (VEGFR2) [24]. In addition, several studies have demonstrated increased secretion of various proangiogenic growth factors including vascular endothelial growth factor (VEGF) [18, 24, 30, 31], basic fibroblast growth factor (bFGF) [17, 19, 24, 26, 31], hepatocyte growth factor (HGF) [31], hypoxia-inducible factor 1-alpha (HIF-1α) [31] and IL-1β [24] after the application of ADSCs.
15 studies have investigated the utility of ADSCs for granulation tissue formation [17,18,19, 21, 22, 24,25,26, 28, 31,32,33,34,35,36]. Enhanced granulation tissue formation in the ADSC groups compared to the control groups was demonstrated histologically in four studies [19, 21, 24, 31]. Cabello-Arista et al. reported an increase in granulation tissue in animals treated with human amnion and ADSCs, whereas the addition of ADSCs to porcine skin reduced granulation tissue formation [28]. An increase in fibroblast quantities by ADSCs was also demonstrated in four studies [17, 19, 26, 36]. Using green fluorescent protein (GFP) labelling, Zhou et al. demonstrated that ADSCs differentiate into fibroblast-like cells in vivo [18]. Improved collagen synthesis and deposition were reported in eight studies [21, 25, 26, 28, 31,32,33,34,35]. Immunohistochemically, Zhou et al. found a higher level of ki67-positive cells in the dermis of ADSCs-treated animals [18], whereas no difference was found between the various groups in the studies by Loder et al.[22].
The supportive role of ADSCs in re-epithelialisation has been investigated in 15 publications [17,18,19, 21,22,23,24,25,26, 28, 30, 31, 33, 34, 36]. Of these, 11 authors reported improved re-epithelialisation induced by ADSCs [17, 19, 21, 23, 24, 26, 30, 31, 33, 34], while the other four detected no difference compared with the control groups [18, 22, 25, 28]. The re-epithelialisation was investigated by histology [17, 19, 21, 23, 26, 28, 30, 34], immunohistochemistry [22], fluorescence microscopy [18] and comparison of 2-D photos [25, 28, 34]. An enhanced transforming growth factor beta (TGF-β) level 14 days after treatment with ADSCs, which returned to normal after 28 days, was observed in four studies [17, 19, 24, 26].
11 studies have reported on the influence of ADSCs during the remodelling phase [17, 19, 21, 24,25,26,27, 29, 30, 33, 37]. Barrera et al. reported significant smaller scars in ADSCs-treated animals compared to control groups [30]. Gholipourmalekabadi et. al found an approximate scar elevation index (SEI) in ADSCs treated wounds as in healthy skin. The authors assume that ADSCs are able to significantly reduce collagen expression and thus scar formation [24]. A significantly higher collagen type I to type III ratio in ADSCs treated animals was demonstrated in three studies [21, 27, 28]. Five studies document a more organized mature collagen in ADSCs treated groups compared with controls [17, 19, 21, 26, 33]. Furthermore, an increased collagen density by ADSCs was observed in four studies [19, 24, 26, 33], while a separate study found no difference between ADSCs and control groups [30].
Dong et al. found a significant reduction in myofibroblasts by using alpha-smooth muscle actin (α-SMA) staining [27]. No differences in α-SMA levels were observed between the ADSC and control groups in the study by Bliley and colleagues. Therefore, a significant increase in peroxisome proliferator-activated receptor gamma (PPARg) gene expression was observed in the ADSCS group at all test time points in this study [25]. In one study, elevated levels of matrix metalloproteinases 1 (MMP-1) and 2 (MMP-2) were detected [24]. Barrera et al. found that the expression of profibrotic tissue inhibitor of metalloproteinase 1 (TIMP-1) was significantly downregulated by ADSCs. Inhibition of excessive scarring by down-regulation of TGF-β1 and bFGF genes on day 28 after wounding was addressed by Alemzadeh et al. [26]. ADSC-associated hair follicle regeneration was observed in five studies [19, 24, 25, 29, 37]. The impact of ADSCs on wound healing and its respective phases is delineated in Fig. 3, while Fig. 4 is dedicated to the presentation of the findings from the included studies across these phases.
Studies examining the influence of ADSCs on wound healing and its respective phases. Green: ADSCs had a positive effect during this phase, grey: ADSCs had no effect on this phase, red: ADSCs had a negative effect during this phase, orange: The effect was positive or negative depending on the carrier substance
Analysis of the ADSCs associated improvement according to the wound healing phases. Green: ADSCs had a positive effect during this phase, grey: ADSCs had no effect on this phase, red: ADSCs had a negative effect during this phase, orange: The effect was positive or negative depending on the carrier substance, no colour: this phase was not investigated by the authors
Discussion
The result of our systematic review indicates a significant positive impact on different aspects of the wound healing process, including the initial inflammatory response, neovascularisation, granulation tissue formation, re-epithelialisation, and the remodelling phase. However, because of the remarkable variability among the studies, the possibility of conducting a meta-analysis was precluded.
The inflammatory response plays a fundamental role in wound healing and serves as the primary defence mechanism against microorganisms [38]. In severe burns, this response can be extensive and uncontrolled, leading to an augmented inflammation, which results in delayed wound healing [1, 39], and hypertrophic scar formation [1, 40,41,42].
The study conducted by Gholipourmalekabadi et al. demonstrated that the application of ADSCs promotes the initial inflammatory phase by stimulating the production of pro-inflammatory cytokines. This response subsequently diminishes over time, with the control group maintaining a sustained inflammatory state [24]. Based on this finding, it can be concluded that ADSCs first facilitate the immune response by promoting the inflammatory process, and then attenuate the extensive inflammatory response usually associated with severe burns to ensure a smooth transition to the proliferative phase.
Following a severe burn injury, the systemic inflammatory response encompasses the release of large quantities of pro-inflammatory cytokines such as IL-1β, MIP-2, IL-6 or TNF-α [43, 44]. Increased IL-1β delays wound healing by stimulating inflammasome activity in macrophages and inducing inflammation in other cells, hindering the polarization into the anti-inflammatory M2 phenotype [45]. MIP-2 acts as a chemokine and is secreted in response to infection or injury by cells including macrophages and monocytes. It exhibits pro-inflammatory effects by promoting the recruitment and activation of neutrophils, supporting inflammatory reactions, thus leading to tissue damage [46]. IL-6 is instrumental in triggering the acute inflammatory response. It is also essential for the transition into chronic inflammation by being the key stimulator for most acute-phase proteins, and by modifying leukocyte infiltration [47, 48]. Elevated levels of TNF-α are associated with decreased neovascularisation, cell migration and proliferation, and increased apoptosis [49]. Several of the studies included in this review, showed that the effects of ADSCs in reducing the levels of the pro-inflammatory cytokines IL-1β, MIP-2, IL-6 and TNF-α1 in animals with burn injuries [17, 19, 24, 26, 30, 31].
Furthermore, the majority of the included studies investigating the immunomodulatory capabilities of ADSCs during the inflammatory response have shown that ADSCs reduce the number of inflammatory cells [17, 19, 23, 24, 26, 27, 30, 31, 35].
Severe burn injuries with a large-scale surface area significantly heighten the risk of infection due to compromised immune response and disrupted skin barriers [1]. It would be interesting to analyse the effects of ADSCs on inflammation in the study conducted by Banerjee et al., in which the burns of experimental animals were infected with Pseudomonas aeruginosa [21]. However, the impact of ADSCs on infection-induced inflammation was not taken into account in their analysis. Instead, their focus was on examining the antimicrobial effect of chitosan microspheres loaded with silver sulfadiazine.
However, even if there are no results regarding the intentional bacterial infection of burns, one can summarily state that ADSCs appear to initially promote immunomodulation by enhancing the initial inflammatory response. Subsequently, they ensure that inflammation remains regulated, which is crucial for the transition to the proliferative phase and important for the progression of the physiological healing process, thus preventing the development of chronic wounds and pathological scars [42, 50,51,52].
The proliferative phase is distinguished by neovascularisation, the formation of granulation tissue, and re-epithelialisation. The majority of studies have indicated that ADSCs promote the development and formation of new blood vessels, resulting in enhanced neovascularisation. Furthermore, multiple studies have highlighted the incidence of the elevated secretion of diverse proangiogenic growth factors, such as VEGF [18, 24, 30, 31], bFGF [17, 19, 24, 26, 31], HGF and HIF-1α [31]. VEGF has a dual impact on endothelial cells, both stimulating their differentiation from endothelial progenitor cells and enhancing their migratory capacity, proliferation, and ability to organise into functional vascular tubules [12, 53,54,55]. HGF has the ability to induce the production of VEGF, and acts as a potent mitogen for endothelial cells, by interacting synergistically with VEGF [56, 57]. bFGF also supports the migration and proliferation of endothelial cells [58, 59]. HIF-1 activation serves as a primary stimulus for neovascularisation through blood vessel growth and remodelling, inducing important pro-angiogenic factors such as VEGF, angiopoietin 2 (Ang-2), and stromal cell-derived factor 1 (SDF-1). Furthermore, HIF-1 plays a contributory role in oxygen and nutrient delivery to hypoxic tissues, and thus enhancing cell survival [60, 61].
Neovascularisation plays a pivotal role in wound healing, by supplying oxygen and essential nutrients to developing tissues [54, 62, 63], while decreased local neovascularisation leads to impaired wound healing [63, 64]. The result of our review demonstrated that ADSCs support the neovascularisation process in burns.
Another important step in the wound healing process is the formation of granulation tissue. The creation of this new tissue is facilitated by fibroblasts which deposit extracellular matrix (ECM) components into the wound. These latter then become main components of the new granulation tissue, alongside the new blood vessels and fibroblasts themselves [38, 50, 51, 65]. The resulting newly formed tissue fills the wound gap and provides a scaffold for cell adhesion, migration, growth and differentiation during wound healing, thus enabling re-epithelialisation [50, 66, 67].
Several of the studies included in our review demonstrate that ADSCs result in an elevation of TGF-β1 levels on the 14th day after the initial injury, followed by a significant reduction by day 28 of the healing process [17, 19, 24, 26]. TGF- β1 plays a crucial role in various aspects of wound healing. It is instrumental in cellular migration, particularly for cell types like fibroblasts and keratinocytes, facilitating their movement towards the wound site. Furthermore, TGF-β1 contributes to the deposition of the ECM, which is essential for the structural support and organization of the newly formed tissue [68,69,70]. A multitude of included studies demonstrated enhanced granulation tissue formation [19, 21, 24, 31] and re-epithelialisation [17, 19, 21, 23, 24, 26, 30, 31, 33, 36] through the utilisation of ADSCs in burns. Interestingly, Cabello-Arista et al. revealed contrasting effects of ADSCs on granulation tissue formation depending on their carrier. The treatment with human amnion and ADSCs resulted in an increase in granulation tissue. However, when ADSCs were added to porcine skin, a reduction in granulation formation ensued [28]. These findings suggest that the interplay between ADSCs and their carrier may have varying effects depending on the material, and further research is warranted to optimize their therapeutic potential.
The application of ADSCs has been demonstrated to enhance the number of fibroblasts, according to several studies [17, 19, 26, 36]. bFGF is known to stimulate the proliferation of fibroblasts and induce the formation of granulation tissue [71, 72], and its levels have been reported to increase through the application of ADSCs [17, 19, 24, 26]. Another potential mechanism for the rise in fibroblasts is the differentiation of ADSCs into these cells, as mentioned by Zhou et al. [18]. This thesis is supported by several in vitro [10, 73,74,75,76] and other in vivo studies [74, 75]. According to Gersch et al., ADSC-differentiated fibroblasts surpass the performance of primary fibroblasts by exhibiting accelerated wound infiltration, heightened expression of ECM markers such as elastin and fibronectin, while reducing levels of scar tissue markers including α-SMA and MMP-1 [76].
One of the primary ECM components synthesized by fibroblasts is collagen, which provides structural support and strength to tissues. It plays a crucial role in wound healing by promoting tissue repair, wound closure, and eventually scar formation [38, 51, 65]. Numerous included studies have evidenced that ADSCs elicit an augmentation in collagen synthesis [21, 25, 26, 28, 34]. It is noteworthy that the accurate balancing of collagen synthesis is of paramount importance in attainment of wound healing. Insufficient collagen synthesis may inhibit wound closure and tissue repair, while excessive collagen production expedites pathological scar formation [41, 42, 77, 78]. Thus, sufficient collagen synthesis assists in the minimisation of scar formation and promotes more physiological tissue regeneration.
Furthermore, in the process of physiological healing process, a balance between deposition and degradation of the synthesised collagen is crucial [42]. MMPs play a primary role in ensuring this balance is achieved [79]. It has been observed that hypertrophic scars are associated with a decrease in the expression of MMP-1, along with elevated levels of TIMP-1 [79, 80]. The latter of which functions as an inhibitor of specific MMPs. It is noteworthy that the expression of TIMP-1 is stimulated by MMP activity [80,81,82]. Barrera et al. reported a decrease in TIMP-1 expression by ADSCs in burn injuries, which could have implications for hypertrophic scar formation [30]. The decreased expression of TIMP-1 by ADSCs suggests a potential mechanism by which the balance between MMPs and their inhibitors could be modulated by these cells. Through the reduction in TIMP-1 levels, a more favourable environment for MMP activity may be assisted by ADSCs. This result aligns with those reported by Gholipourmalekabadi et al., who observed elevated level of MMP-1 and MMP-2, which is associated with the degradation of various ECM components [24]. Thus its upregulation supports tissue remodelling, but can also foster extensive scar formation in the event of excessive levels. The presence of elevated MMP-2 levels in conjunction with increased MMP-1 and decreased TIMP-1 expression suggests a complex interplay between these factors and ADSCs in the regulation of scar formation after burns. These findings provide further evidence for the potential role of ADSCs in the modulation of MMP expression and their involvement in scar formation. While ADSCs may have beneficial effects on certain aspects of wound healing, further investigation is required to assess their potential influence on myofibroblasts, the expression of MMPs, and subsequent impact on scar formation.
If this interplay fails to operate effectively, an imbalance occurs, resulting in excessive or disorganised collagen deposition may result in hypertrophic or keloid scars [41, 42, 77, 83, 84], which can be aesthetically undesirable and functionally limiting. In several included studies, it was observed that ADSCs-treated groups exhibited well-organised and mature collagen bundles compared to the control groups [17, 19, 21, 26]. In physiological wound healing, the initial type III collagen is converted into mature type I collagen during the remodelling phase, resulting in strengthened wound integrity [83, 85]. Conversely, the progression of hypertrophic scars is characterized by a downregulation in collagen I expression alongside an excessive upregulation in collagen III [86]. Multiple studies included in our review consistently indicated an elevated collagen type I to type III ratio [21, 27, 28].
Moreover, hypertrophic scars are characterised by an elevated abundance of myofibroblasts, which express α-SMA and undergo apoptosis during the physiological wound healing but persist in hypertrophic scar formation [42, 84, 87, 88]. Dong et al. demonstrated a significant decrease in the population of myofibroblasts through the reduction of α-SMA [27]. Various further in vivo studies have exhibited the suppression of α-SMA levels and diminished scarring resulting from the administration of ADSCs [89,90,91,92,93,94,95].
Several studies have demonstrated that ADSCs treatment leads to a decrease in TGF-β1 levels concomitant with an elevation of bFGF, during remodelling [17, 19, 26]. This fact is of great interest, since TGF-β1 promotes the differentiation of fibroblasts into myofibroblasts [96, 97], while bFGF is known to inhibit extensive scar formation [98, 99].
Currently, hair follicle regeneration in full-thickness wounds continues to present a challenge in regenerative medicine [100, 101]. Whilst the body has the innate ability to repair certain tissues, such as the skin, hair follicles have a limited capacity for regeneration, especially in deep wounds involving the dermis [102]. In full-thickness burns, the destruction extends to the whole dermis [1] involving its appendages including hair follicles, the loss or damage of which can inhibit their regrowth [103,104,105]. Interestingly, five studies reported hair follicle regeneration in ADSCs-treated burns [19, 24, 25, 29, 37]. In four of these studies, the regeneration of hair follicles, which are usually damaged beyond repair, was observed in full-thickness burn wounds [19, 24, 25, 37]. This process namely wound-induced hair neogenesis (WIHN) is of particular interest in the field of regenerative medicine, as the restoration of hair growth in such wounds can significantly improve the aesthetic outcome and functional recovery. WIHN was first described in the middle of the twentieth century in various mammals [106,107,108,109] and was rediscovered by Ito et al. [110] in 2007, who demonstrated the development of completely new hair follicles in wounded mice. Several recent studies focusing on WIHN subsequently emerged [111,112,113,114,115,116,117]. According to several studies, full-thickness wounds with a diameter of at least 1 cm lead to neogenesis of hair follicles, while smaller full-thickness wounds heal with a hairless and adipose-free scar [110,111,112,113,114]. This largely aligns with our research, as, in three of the included studies, the full-thickness wound diameter was at least 1 cm [19, 24, 25]. Due to contraction in rodent wound healing, the edges of the hair-bearing areas are frequently distorted, giving the simulation of pre-existing hair follicles being encircled by scar tissue, thereby creating a false impression of WIHN [102]. Therefore, a detailed examination is of utmost importance to determine whether it is indeed WIHN. Recent insights suggest that adipocytes and their precursors are involved in hair follicle regeneration [118]. However, this insight necessitates comprehensive research, and further studies are imperative to understand the role of adipocyte lineage cells in hair follicle regeneration.
Despite their favorable properties in wound healing, ADSCs are presently used in burn care for experimental purposes only. Autologous skin grafting is still considered the gold standard for the treatment of severe burns [4]. Several studies have demonstrated that ADSCs support the therapeutic efficacy of split-thickness skin grafts in the treatment of burns [119,120,121]. Both Gao et al., and Foubert et al., have found that ADSCs can significantly enhance the elasticity of the split-thickness skin grafts, resulting in an improvement of skin texture and functionality [120, 121]. According to the research conducted by Osamu et al., the application of ADSCs significantly enhances skin graft take and inhibits transplant shrinkage throughout the healing process [119]. In addition, the studies indicate that ADSCs foster skin neovascularisation, enhance skin thickness, and expedite wound epithelialisation [119,120,121].
In summary, ADSCs are a promising candidate for future therapeutic approaches in the treatment of burns. All of these experiments demonstrated aspects of ADSCs that positively influence the inflammatory response, cell proliferation and migration, neovascularisation, granulation tissue formation and re-epithelialisation, as well as remodelling. However, the validity of all these results must be critically scrutinized, since most of the included studies are conducted in mice and rats. Rodent wound models are often considered limited because of the perception that rodents have a loose skin and heal primarily by contraction, offering a fast wound closure, while humans heal by re-epithelialisation [50, 122]. Nevertheless, rodents are the most extensively investigated animals in the field of burn research, primarily due to their ease of handling, rapid reproduction, and standardisation options, offering the significant benefit of accelerated healing process, which enhances research efficiency and reduces mortality [123, 124]. Rodent burn models are particularly suited for local phenomena investigations such as wound inflammation and application of various dressings [123]. Furthermore, rodents offer the opportunity to investigate the cellular architecture and interaction on wound healing, acknowledging differences from human biology [125, 126]. Whilst Chen et al. argue that re-epithelialisation in rodents is measurable [127], the predominant approach for examining re-epithelialisation involves manipulation through splinting, which minimises contraction to emulate human wound healing [122, 123, 125]. However, with the exception of two articles [25, 27], in which the use of splinting was negated, none of the included studies in our review reporting on its usage. Consequently, the effect of ADSCs on the re-epithelisation process remains unclear. Additionally, the reliability of comparing rodents to humans in the research of hair follicle regeneration remains questionable due to the significant differences in dermal cell biology [117, 128]. However, the investigation of ADSCs in the treatment of burns is at a very early experimental stage and the mechanism of their action is currently not completely understood. Further studies in species with skin structures and healing physiology similar to humans, such as pigs [122, 129], are essential to determine the efficiency of ADSCs in burn wound healing. It is crucial to comprehend the precise processes involved in the interplay between ADSCs and the different phases in wound healing in order to develop targeted therapeutic strategies for optimizing burn care. Additional research is needed to elucidate the specific signalling pathways and cellular interactions influenced by ADSCs in the context of wound healing and scar formation.
Limitations
Our systematic review has some inherent limitations. For one, only articles written in English language were taken in account within this review. As a result, some papers that are not available in English have not been considered. Only items discovered through our search strategy retrieved from PubMed, Web of Science and Embase, or manual search in relevant journals were considered, with the possibility of missed publications. Another addition to the limitations is that our review is limited to articles published before 30th September 2022. Since science is a dynamic process leading to constant developments, papers published after this date have not been considered within our review. in spite of literature screening by two investigators, a possible wrongful exclusion cannot be ruled out. A major limitation is that most of the studies were conducted on rodents, which makes reproducibility and transfer in a clinical context challenging. Finally, despite the usage of the SYRCLE's Risk of Bias tool and independent assessment by two reviewers, it's important to acknowledge that bias assessment can be inherently subjective, and so the results should be interpreted with this in mind.
Conclusion
In conclusion, it appears that adipose-derived stem cells demonstrate remarkable efficacy in the field of regenerative medicine, offering positive support throughout wound healing. However, the usage of ADSCs in the treatment of burns is still in the early experimental stage and the majority of the studies were conducted in rodents. The included studies have revealed varied approaches when considering cell count, administration protocol, and carrier selection. Given the foundational insights, it is imperative to elucidate the optimal administration protocol for ADSCs and to discern the most appropriate carrier, considering the specific state of the wound. Hence, further investigations are necessary to investigate the efficacy of ADSCs in the treatments of burns and its potential adoption in clinical settings.
Availability of data and materials
For data requests please contact the corresponding author.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- ADSCs:
-
Adipose-derived stem cells
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PBS:
-
Phosphate buffered saline
- DMEM:
-
Dulbecco’s modified eagle medium
- IL-1β:
-
Interleukin 1-beta
- MIP-2:
-
Macrophage inflammatory protein 2
- TNF-α1:
-
Tumor necrosis factor alpha 1
- TNF-α:
-
Tumor necrosis factor alpha
- IL-6:
-
Interleukin 6
- IL-1ra:
-
Interleukin-1 receptor antagonist protein
- ILB4:
-
Isolectin B4
- NG2:
-
Neural/glial antigen 2
- VEGFa1:
-
Vascular endothelial growth factor a1
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- VEGF:
-
Vascular endothelial growth factor
- bFGF:
-
Basic fibroblast growth factor
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- GFP:
-
Green fluorescent protein
- TGF-β:
-
Transforming growth factor beta
- SEI:
-
Scar elevation index
- α-SMA:
-
Alpha-smooth muscle actin
- PPARg:
-
Peroxisome proliferator-activated receptor gamma
- MMP-1:
-
Matrix metalloproteinases 1
- MMP-2:
-
Matrix metalloproteinases 2
- TIMP-1:
-
Profibrotic tissue inhibitor of metalloproteinase 1
- ANG-2:
-
Angiopoietin 2
- SDF-1:
-
Stromal cell-derived factor 1
- ECM:
-
Extracellular matrix
- WIHN:
-
Wound-induced hair neogenesis
References
Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Prim. 2020. https://doi.org/10.1038/s41572-020-0145-5.
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3–17. https://doi.org/10.1016/j.addr.2017.09.018.
Jeschke MG, Shahrokhi S, Finnerty CC, Branski LK, Dibildox M. Wound coverage technologies in burn care: established techniques. J Burn Care Res. 2018;39:313–8. https://doi.org/10.1097/BCR.0b013e3182920d29.
Kohlhauser M, Luze H, Nischwitz SP. Historical evolution of skin grafting—a journey through time. Medicina (B Aires). 2021;57:1–14.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. https://doi.org/10.1634/stemcells.2007-0226.
Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, LeRoux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–9. https://doi.org/10.5966/sctm.2011-0018.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. https://doi.org/10.1089/107632701300062859.
Zuk PA, Zhu M, Ashjian P, de Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. https://doi.org/10.1091/mbc.E02-02-0105.
Kim EK, Li G, Lee TJ, Hong JP. The effect of human adipose-derived stem cells on healing of ischemic wounds in a diabetic nude mouse model. Plast Reconstr Surg. 2011;128:387–94. https://doi.org/10.1097/PRS.0b013e31821e6de2.
An R, Zhang Y, Qiao Y, Song L, Wang H, Dong X. Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Res Ther. 2020;11:120. https://doi.org/10.1186/s13287-020-01621-x.
Kuo Y-R, Wang C-T, Cheng J-T, Kao G-S, Chiang Y-C, Wang C-J. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant. 2016;25:71–81. https://doi.org/10.3727/096368915X687921.
Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–16. https://doi.org/10.3727/096368910X520065.
Seo E, Lim JS, Jun J-B, Choi W, Hong I-S, Jun H-S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J Transl Med. 2017;15:35. https://doi.org/10.1186/s12967-017-1145-4.
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. https://doi.org/10.1161/01.CIR.0000121425.42966.F1.
Rongfeng S, Jin Y, Cao C, Han S, Shao X, Meng L, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016. https://doi.org/10.1186/s13287-016-0412-2.
Page MJ, McKenzie JE, Bossuyt P, Boutron I, Hoffmann TC, Mulrow CD, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. Med Flum. 2021;57:444–65. https://doi.org/10.21860/medflum2021_264903.
Oryan A, Alemzadeh E, Mohammadi AA, Moshiri A. Healing potential of injectable aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019;377:215–27. https://doi.org/10.1007/s00441-019-03015-9.
Zhou X, Ning K, Ling B, Chen X, Cheng H, Lu B, et al. Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev. 2019;28:1463–72. https://doi.org/10.1089/scd.2019.0113.
Oryan A, Alemzadeh E, Mohammadi AA. Application of honey as a protective material in maintaining the viability of adipose stem cells in burn wound healing: a histological, molecular and biochemical study. Tissue Cell. 2019;61:89–97. https://doi.org/10.1016/j.tice.2019.09.007.
Kaita Y, Tarui T, Yoshino H, Matsuda T, Yamaguchi Y, Nakagawa T, et al. Sufficient therapeutic effect of cryopreserved frozen adipose-derived regenerative cells on burn wounds. Regen Ther. 2019;10:92–103. https://doi.org/10.1016/j.reth.2019.01.001.
Banerjee IJ, Seetharaman S, Wrice NL, Christy RJ, Id SN. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration 2019:1–22.
Loder S, Peterson JR, Agarwal S, Eboda O, Brownley C, Delarosa S, et al. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. J Burn Care Res. 2014. https://doi.org/10.1097/BCR.0000000000000160.
Motamed S, Taghiabadi E, Molaei H, Sodeifi N, Hassanpour SE, Shafieyan S, et al. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am J Surg. 2017;214:762–9. https://doi.org/10.1016/j.amjsurg.2017.04.010.
Gholipourmalekabadi M, Seifalian AM, Urbanska AM, Omrani MD, Hardy JG, Madjd Z, et al. 3D protein-based bilayer artificial skin for the guided scarless healing of third-degree burn wounds in vivo. Biomacromol. 2018;19:2409–22. https://doi.org/10.1021/acs.biomac.7b01807.
Bliley JM, Argenta A, Satish L, McLaughlin MM, Dees A, Tompkins-Rhoades C, et al. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns. 2016;42:1212–22. https://doi.org/10.1016/j.burns.2015.12.007.
Alemzadeh E, Oryan A, Mohammadi AA. Hyaluronic acid hydrogel loaded by adipose stem cells enhances wound healing by modulating IL-1β, TGF-β1, and bFGF in burn wound model in rat. J Biomed Mater Res B Appl Biomater. 2020;108:555–67. https://doi.org/10.1002/jbm.b.34411.
Dong Y, Cui M, Qu J, Wang X, Kwon SH, Barrera J, et al. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020;108:56–66. https://doi.org/10.1016/j.actbio.2020.03.040.
Cabello-Arista B, Melgarejo-Ramírez Y, Retana-Flores A, Martínez-López V, Márquez-Gutiérrez E, Almanza-Pérez J, et al. Effects of mesenchymal stem cell culture on radio sterilized human amnion or radio sterilized pig skin in burn wound healing. Cell Tissue Bank. 2022. https://doi.org/10.1007/s10561-021-09976-y.
Feng C-J, Lin C-H, Tsai C-H, Yang I-C, Ma H. Adipose-derived stem cells-induced burn wound healing and regeneration of skin appendages in a novel skin island rat model. J Chin Med Assoc. 2019;82:635–42. https://doi.org/10.1097/JCMA.0000000000000134.
Barrera JA, Trotsyuk AA, Maan ZN, Bonham CA, Larson MR, Mittermiller PA, et al. Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns. Tissue Eng Part A. 2021;27:844–56. https://doi.org/10.1089/ten.TEA.2020.0320.
Azam M, Ghufran H, Butt H, Mehmood A, Ashfaq R, Ilyas AM, et al. Curcumin preconditioning enhances the efficacy of adipose-derived mesenchymal stem cells to accelerate healing of burn wounds. Burn Trauma. 2021. https://doi.org/10.1093/burnst/tkab021.
Foubert P, Barillas S, Gonzalez AD, Alfonso Z, Zhao S, Hakim I, et al. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns. 2015;41:1504–16. https://doi.org/10.1016/j.burns.2015.05.004.
Roshangar L, Rad JS, Kheirjou R, Khosroshahi AF. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med. 2021;15:546–55. https://doi.org/10.1002/term.3194.
Wu Y, Liang T, Hu Y, Jiang S, Luo Y, Liu C, et al. 3D bioprinting of integral ADSCs-NO hydrogel scaffolds to promote severe burn wound healing. Regen Biomater. 2021;8:rbab014. https://doi.org/10.1093/rb/rbab014.
Franck CL, Senegaglia AC, Leite LMB, de Moura SAB, Francisco NF, Ribas Filho JM. Influence of adipose tissue-derived stem cells on the burn wound healing process. Stem Cells Int. 2019;2019:2340725. https://doi.org/10.1155/2019/2340725.
Karimi H, Soudmand A, Orouji Z, Taghiabadi E, Mousavi SJ. Burn wound healing with injection of adipose-derived stem cells: a mouse model study. Ann Burns Fire Disasters. 2014;27:44–9.
Ng JY, Zhu X, Mukherjee D, Zhang C, Hong S, Kumar Y, et al. Pristine gellan gum-collagen interpenetrating network hydrogels as mechanically enhanced anti-inflammatory biologic wound dressings for burn wound therapy. ACS Appl Bio Mater. 2021;4:1470–82. https://doi.org/10.1021/acsabm.0c01363.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. https://doi.org/10.1177/147323000903700531.
Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0021245.
Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40:1255–66. https://doi.org/10.1016/j.burns.2014.02.011.
Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427–36. https://doi.org/10.1016/S0140-6736(16)31406-4.
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25. https://doi.org/10.2119/molmed.2009.00153.
Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DMM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. https://doi.org/10.1097/01.shk.0000223120.26394.7d.
Sio SWS, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol. 2008;180:8333–41. https://doi.org/10.4049/jimmunol.180.12.8333.
Mirza RE, Fang MM, Ennis WJ, Kohl TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–87. https://doi.org/10.2337/db12-1450.
Qin CC, Liu YN, Hu Y, Yang Y, Chen Z. Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury. World J Gastroenterol. 2017;23:3043–52. https://doi.org/10.3748/wjg.v23.i17.3043.
Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8:1–6. https://doi.org/10.1186/ar1917.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. https://doi.org/10.1101/cshperspect.a016295.
Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF- α in impaired diabetic wound healing. Biomed Res Int. 2013. https://doi.org/10.1155/2013/754802.
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94. https://doi.org/10.1159/000454919.
Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–85. https://doi.org/10.1007/s00018-016-2268-0.
Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. https://doi.org/10.1038/sj.jid.5700701.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–64. https://doi.org/10.1016/j.cell.2019.01.021.
Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3:647–61. https://doi.org/10.1089/wound.2013.0517.
Wu Y-Y, Jiao Y-P, Xiao L-L, Li M-M, Liu H-W, Li S-H, et al. Experimental study on effects of adipose-derived stem cell-seeded silk fibroin chitosan film on wound healing of a diabetic rat model. Ann Plast Surg. 2018;80:572–80. https://doi.org/10.1097/SAP.0000000000001355.
Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Matsumoto K, et al. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and Akt in endothelial cells. Hypertension. 2001;37:581–6. https://doi.org/10.1161/01.hyp.37.2.581.
Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, et al. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: The case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–90. https://doi.org/10.1161/01.CIR.97.4.381.
Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–9. https://doi.org/10.1038/325257a0.
Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297–301. https://doi.org/10.1073/pnas.83.19.7297.
Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care. 2014;3:390–9. https://doi.org/10.1089/wound.2013.0520.
Madanecki P, Kapoor N, Bebok Z, Ochocka R, Collawn JF, Bartoszewski R. Regulation of angiogenesis by hypoxia: the role of microRNA. Cell Mol Biol Lett. 2013;18:47–57. https://doi.org/10.2478/s11658-012-0037-0.
Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–14. https://doi.org/10.1002/jemt.10249.
Kumar P, Kumar S, Udupa EP, Kumar U, Rao P, Honnegowda T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthetic Res. 2015;2:243. https://doi.org/10.4103/2347-9264.165438.
Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv Ski Wound Care. 2012;25:304–14. https://doi.org/10.1097/01.ASW.0000416006.55218.d0.
Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22:407–12. https://doi.org/10.12968/jowc.2013.22.8.407.
Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75–76:12–26. https://doi.org/10.1016/j.matbio.2018.01.002.
Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–65. https://doi.org/10.1016/j.addr.2018.06.019.
Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care. 2013;2:215–24. https://doi.org/10.1089/wound.2012.0406.
Yang M, Yang Z, Pan X, Huang X, Yang L, Xue Y. miR-506-3p regulates TGF-1 and affects dermal fibroblast proliferation, migration and collagen formation after thermal injury. Tissue Cell. 2021;72: 101548. https://doi.org/10.1016/j.tice.2021.101548.
Gailit J, Welch MP, Clark RAF. TGF-β1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103:221–7. https://doi.org/10.1111/1523-1747.ep12393176.
Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med. 1990;172:245–51. https://doi.org/10.1084/jem.172.1.245.
Makino T, Jinnin M, Muchemwa FC, Fukushima S, Kogushi-Nishi H, Moriya C, et al. Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK1/2 and JNK pathways. Br J Dermatol. 2010;162:717–23. https://doi.org/10.1111/j.1365-2133.2009.09581.x.
Hu R, Ling W, Xu W, Han D. Fibroblast-like cells differentiated from adipose-derived mesenchymal stem cells for vocal fold wound healing. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0092676.
Zhou ZQ, Chen Y, Chai M, Tao R, Lei YH, Jia YQ, et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int J Mol Med. 2019;43:890–900. https://doi.org/10.3892/ijmm.2018.4006.
Hur W, Lee HY, Min HS, Wufuer M, Won LC, Hur JA, et al. Regeneration of full-thickness skin defects by differentiated adipose-derived stem cells into fibroblast-like cells by fibroblast-conditioned medium. Stem Cell Res Ther. 2017;8:1–13. https://doi.org/10.1186/s13287-017-0520-7.
Gersch RP, Raum JC, Calvert C, Percec I. Fibroblasts derived from human adipose stem cells produce more effective extracellular matrix and migrate faster compared to primary dermal fibroblasts. Aesthetic Surg J. 2020;40:108–17. https://doi.org/10.1093/asj/sjz071.
Tejiram S, Zhang J, Travis TE, Carney BC, Alkhalil A, Moffatt LT, Johnson LS, Shupp JW. Compression therapy affects collagen type balance in hypertrophic scar. Physiol Behav. 2018;176:139–48. https://doi.org/10.4049/jimmunol.1801473.The.
Karppinen S-M, Heljasvaara R, Gullberg D, Tasanen K, Pihlajaniemi T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research. 2019;8:787. https://doi.org/10.12688/f1000research.18293.1.
Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–21. https://doi.org/10.1016/j.matbio.2015.03.002.
Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119–36. https://doi.org/10.1089/wound.2013.0485.
Simon F, Bergeron D, Larochelle S, Lopez-Vallé CA, Genest H, Armour A, et al. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns. 2012;38:421–7. https://doi.org/10.1016/j.burns.2011.09.001.
Cabral-Pacheco GA, Garza-Veloz I, La RCCD, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21:1–53. https://doi.org/10.3390/ijms21249739.
Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18:396–402. https://doi.org/10.1097/01.mop.0000236389.41462.ef.
Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care. 2018;7:29–45. https://doi.org/10.1089/wound.2016.0696.
Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81:94–101. https://doi.org/10.1016/j.jcma.2017.11.002.
Oliveira GV, Hawkins HK, Chinkes D, Burke A, Tavares ALP, Ramos-E-Silva M, et al. Hypertrophic versus non hypertrophic scars compared by immunohistochemistry and laser confocal microscopy: Type i and III collagens. Int Wound J. 2009;6:445–52. https://doi.org/10.1111/j.1742-481X.2009.00638.x.
Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Research. 2016;5:1–8. https://doi.org/10.12688/f1000research.8190.1.
Hinz B, Lagares D, Diseases I, Hospital MG, Hospital G, Hospital MG. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2021;16:11–31. https://doi.org/10.1038/s41584-019-0324-5.
Zhu YZ, Hu X, Zhang J, Wang ZH, Wu S, Yi YY. Extracellular vesicles derived from human adipose-derived stem cell prevent the formation of hypertrophic scar in a rabbit model. Ann Plast Surg. 2020;84:602–7. https://doi.org/10.1097/SAP.0000000000002357.
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7:1–16. https://doi.org/10.1186/s13287-016-0356-6.
Chai C-Y, Song J, Tan Z, Tai I-C, Zhang C, Sun S. Adipose tissue-derived stem cells inhibit hypertrophic scar (HS) fibrosis via p38/MAPK pathway. J Cell Biochem. 2018. https://doi.org/10.1002/jcb.27689.
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:1–11. https://doi.org/10.1186/s13287-015-0133-y.
Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:1–12. https://doi.org/10.1038/s41598-017-12919-x.
Zhang C, Wang T, Zhang L, Chen P, Tang S, Chen A, et al. Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res Ther. 2021;12:1–13. https://doi.org/10.1186/s13287-020-02061-3.
Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12:1–16. https://doi.org/10.1186/s13287-021-02290-0.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. https://doi.org/10.1038/sj.jid.5700613.
Vallée A, Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019;9:1–15. https://doi.org/10.1186/s13578-019-0362-3.
Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z, et al. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res Ther. 2017;8:1–13. https://doi.org/10.1186/s13287-017-0549-7.
Spyrou GE, Naylor IL. The effect of basic fibroblast growth factor on scarring. Br J Plast Surg. 2002;55:275–82. https://doi.org/10.1054/bjps.2002.3831.
Farjo B, Farjo N, Williams G. Hair transplantation in burn scar alopecia. Scars Burn Heal. 2015;1:205951311560776. https://doi.org/10.1177/2059513115607764.
Mahjour SB, Ghaffarpasand F, Wang H. Hair follicle regeneration in skin grafts: current concepts and future perspectives. Tissue Eng Part B Rev. 2012;18:15–23. https://doi.org/10.1089/ten.teb.2011.0064.
Xue Y, Lim CH, Plikus MV, Ito M, Cotsarelis G, Garza LA. Wound-induced hair neogenesis model. J Invest Dermatol. 2022;142:2565–9. https://doi.org/10.1016/j.jid.2022.07.013.
Martin P. Wound healing—aiming for perfect skin regeneration. Science (80-). 1997;276:75–81. https://doi.org/10.1126/science.276.5309.75.
Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, et al. Skin tissue regeneration for burn injury. Stem Cell Res Ther. 2019;10:1–16. https://doi.org/10.1186/s13287-019-1203-3.
Blais M, Parenteau-Bareil R, Cadau S, Berthod F. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2013;2:545–51. https://doi.org/10.5966/sctm.2012-0181.
Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–9.
Billingham RE, Russell PS. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature. 1956;177:791–2. https://doi.org/10.1038/177791b0.
Mikhail GR. Hair neogenesis in rat skin. Arch Dermatol. 1963;88:713–28. https://doi.org/10.1001/archderm.1963.01590240037008.
Brook AH, Short BF, Lyne AG. Formation of new wool follicles in the adult sheep. Nature. 1960;185:51. https://doi.org/10.1038/185051a0.
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. https://doi.org/10.1038/nature05766.
Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, et al. dsRNA released by tissue damage activates TLR3 to drive skin regeneration. Cell Stem Cell. 2015;17:139–51. https://doi.org/10.1016/j.stem.2015.07.008.dsRNA.
Wang G, Sweren E, Liu H, Wier E, Alphonse MP, Chen R, et al. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe. 2021;29:777-791.e6. https://doi.org/10.1016/j.chom.2021.03.003.
Harn HIC, Wang SP, Lai YC, Van Handel B, Liang YC, Tsai S, et al. Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat Commun. 2021;12:1–16. https://doi.org/10.1038/s41467-021-22822-9.
Lim CH, Sun Q, Ratti K, Lee SH, Zheng Y, Takeo M, et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-07142-9.
Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489:561–5. https://doi.org/10.1038/nature11499.
Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J Invest Dermatol. 2013;133:881–9. https://doi.org/10.1038/jid.2012.398.
Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–23. https://doi.org/10.1038/nm.3181.
Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. https://doi.org/10.1016/j.cell.2011.07.019.
Fujiwara O, Prasai A, Perez-Bello D, El Ayadi A, Petrov IY, Esenaliev RO, et al. Adipose-derived stem cells improve grafted burn wound healing by promoting wound bed blood flow. Burn Trauma. 2020;8:tkaa009. https://doi.org/10.1093/burnst/tkaa009.
Gao Y, Gao B, Zhu H, Yu Q, Xie F, Chen C, et al. Adipose-derived stem cells embedded in platelet-rich plasma scaffolds improve the texture of skin grafts in a rat full-thickness wound model. Burns. 2020;46:377–85. https://doi.org/10.1016/j.burns.2019.07.041.
Foubert P, Liu M, Anderson S, Rajoria R, Gutierrez D, Zafra D, et al. Preclinical assessment of safety and efficacy of intravenous delivery of autologous adipose-derived regenerative cells (ADRCs) in the treatment of severe thermal burns using a porcine model. Burns. 2018;44:1531–42. https://doi.org/10.1016/j.burns.2018.05.006.
Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018;138:2095-2105.e1. https://doi.org/10.1016/j.jid.2018.08.005.
Vinaik R, Aijaz A, Jeschke MG. Small animal models of thermal injury. Methods Cell Biol. 2022;168:161–89. https://doi.org/10.1016/bs.mcb.2021.12.014.
Abdullahi A, Amini-Nik S, Jescheke M. Animal models in burn research. NIH Public Access. Cell Mol Life Sci. 2009;49:1841–50. https://doi.org/10.1007/s00018-014-1612-5.Animal.
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3:445–64. https://doi.org/10.1089/wound.2013.0473.
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. https://doi.org/10.1152/physrev.00067.2017.
Chen L, Mirza R, Kwon Y, DiPietro LA, Koh TJ. The murine excisional wound model: contraction revisited. Wound Repair Regen. 2015;23:874–7. https://doi.org/10.1111/wrr.12338.
Rahmani W, Sinha S, Biernaskie J. Immune modulation of hair follicle regeneration. NPJ Regen Med. 2020;5:1–13. https://doi.org/10.1038/s41536-020-0095-2.
Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. https://doi.org/10.1046/j.1524-475X.2001.00066.x.
Acknowledgements
We extend our gratitude to all authors who, upon request, provided us with unpublished information regarding their research works.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualisation and Methodology (K. M.), Data Acquisition and Analysis (K. M., T. A.), Commencement of Authorial Interaction (K. M.), Composing the original Draft (K. M.), Tabular Data Structuration (K. M.), Judicious Examination (T. A., K. L.-P.), Supervision (K. L.-P.). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, as this systematic review analysis published studies and does not involve new data collection or experimental procedures on human participants or animals.
Consent of publication
Not applicable, as this systematic review does not contain any individual person’s data in any form.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohlhauser, M., Tuca, A. & Kamolz, LP. The efficacy of adipose-derived stem cells in burn injuries: a systematic review. Cell Mol Biol Lett 29, 10 (2024). https://doi.org/10.1186/s11658-023-00526-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11658-023-00526-w