Abstract
Background
The initiation of migraine headaches and the involvement of neuroinflammatory signaling between parenchymal and meningeal cells remain unclear. Experimental evidence suggests that a cascade of inflammatory signaling originating from neurons may extend to the meninges, thereby inducing neurogenic inflammation and headache. This review explores the role of parenchymal inflammatory signaling in migraine headaches, drawing upon recent advancements.
Body
Studies in rodents have demonstrated that sterile meningeal inflammation can stimulate and sensitize meningeal nociceptors, culminating in headaches. The efficacy of relatively blood-brain barrier-impermeable anti-calcitonin gene-related peptide antibodies and triptans in treating migraine attacks, both with and without aura, supports the concept of migraine pain originating in meninges. Additionally, PET studies utilizing inflammation markers have revealed meningeal inflammatory activity in patients experiencing migraine with aura, particularly over the occipital cortex generating visual auras. The parenchymal neuroinflammatory signaling involving neurons, astrocytes, and microglia, which eventually extends to the meninges, can link non-homeostatic perturbations in the insensate brain to pain-sensitive meninges. Recent experimental research has brought deeper insight into parenchymal signaling mechanisms: Neuronal pannexin-1 channels act as stress sensors, initiating the inflammatory signaling by inflammasome formation and high-mobility group box-1 release in response to transient perturbations such as cortical spreading depolarization (CSD) or synaptic metabolic insufficiency caused by transcriptional changes induced by migraine triggers like sleep deprivation and stress. After a single CSD, astrocytes respond by upregulating the transcription of proinflammatory enzymes and mediators, while microglia are involved in restoring neuronal structural integrity; however, repeated CSDs may prompt microglia to adopt a pro-inflammatory state. Transcriptional changes from pro- to anti-inflammatory within 24 h may serve to dampen the inflammatory signaling. The extensive coverage of brain surface and perivascular areas by astrocyte endfeet suggests their role as an interface for transporting inflammatory mediators to the cerebrospinal fluid to contribute to meningeal nociception.
Conclusion
We propose that neuronal stress induced by CSD or synaptic activity-energy mismatch may initiate a parenchymal inflammatory signaling cascade, transmitted to the meninges, thereby triggering lasting headaches characteristic of migraine, with or without aura. This neuroinflammatory interplay between parenchymal and meningeal cells points to the potential for novel targets for migraine treatment and prophylaxis.
Similar content being viewed by others
Background
Pain typically accompanies an inflammatory response of varying intensity at the site where the nociceptive fibers are activated. Consistent with this observation, migraine headache is proposed to arise from the activation of nociceptive trigeminocervical afferents due to a sterile meningeal inflammatory process [1]. Meningeal headaches triggered by meningeal infections share similarities with migraine headaches, such as the throbbing nature of the headache, photophobia, and phonophobia. However, they are significantly more intense and are associated with evident inflammatory cell reactions in the cerebrospinal fluid (CSF) and gadolinium contrast enhancement in MRI scans [2]. The confined and mild sterile inflammatory process thought to cause migraine headaches either directly originates within the meninges such as in the case of volatile irritants like umbellulone of the headache tree (Umbellularia californica) or be triggered by stressful brain perturbations such as aura [3, 4]. However, the mechanism by which benign but stressful brain events underlying migraine prodrome or aura can activate meningeal nociceptors remains unclear. This has led to the hypothesis of episodic central dysregulation in pain pathways as the driving force behind migraine headaches, despite limited evidence [5]. The absence of aura or prodrome in a considerable proportion of migraine attacks has been proposed to support this perspective. Conversely, the effectiveness of anti-calcitonin gene-related peptide (CGRP) antibodies and sumatriptan, which primarily target meningeal nociceptors and trigeminal ganglia located outside the blood-brain barrier (BBB), in treating migraine attacks with and without aura has bolstered the idea that migraine pain originates in the meninges [1, 6]. This is because these agents have limited access to central pathways but effectively target the meningeal nociceptors, despite ongoing controversy [1, 6,7,8]. Central pathways, however, do play a crucial role in modulating peripheral nociceptive input, as evidenced by significant variability in pain threshold and perception depending on individual’s mental and mood status, notable placebo effect, pain suppression by stimulating the periaqueductal gray, and non-painful auras [5, 6, 9]. Evidence from animal studies indicates that parenchymal neuroinflammatory signaling involving neurons, astrocytes, and microglia, which eventually extends to the meninges [10,11,12,13,14,15,16,17,18], could potentially link non-homeostatic perturbations in the insensate brain to pain-sensitive meninges. Recent clinical imaging studies have provided further supporting evidence for the presence of parenchymal as well as meningeal inflammation in migraine patients [19, 20].
Cortical spreading depolarization (CSD), the neurophysiological event underlying migraine aura [21], is suggested to potentially trigger headaches, although this remains a topic of debate, particularly due to the challenges associated with observing and directly linking it with headache occurrences in humans [1, 10, 22,23,24]. Notably, aura manifests contralaterally to the side of the headache, supporting the notion that CSD-induced parenchymal algesic signals may propagate to the overlying meninges, initiating the headache. In other words, disturbances caused by CSD in the occipital cortex result in visual aura primarily on the lateral aspect of the contralateral visual field. Meanwhile, trigeminal nociceptive signals originating from the overlying dura mater enter the brainstem, cross over, and ascend on the contralateral side of the brain, leading to the perception of pain on the contralateral side of the head where the CSD occurred. Thus, a direct spread of CSD-evoked electrophysiological changes (a brief excitatory phase succeeded by several minutes of inhibition) from the cortex to ipsilateral thalamus is challenging to reconcile with the contralateral headache and the temporal gap of 10–60 min between aura and headache onset, as well as the transient nature of these electrophysiological changes. CSD occurring in the visual (V1) or insular cortex has been shown to elicit an early inhibition followed by a delayed facilitation of dura-evoked responses of Sp5C (nucleus caudalis) 2nd order neurons in the rat [25]. This indicates that corticotrigeminal projections have the capacity to modulate dural nociception. However, the clinical results with relatively BBB-impermeable anti-CGRP antibodies and triptans reinforce the idea that the sustained nociceptive activity in migraine is primarily driven by dural neurogenic inflammation [1, 6, 8], which can be modulated by various central mechanisms. These clinical findings also contradict extrapolations suggesting that central facilitatory mechanisms can convert spontaneous non-noxious activity in these areas to the headache of migraine without aura. Conversely, the proposition that “CSD can induce migraine headaches via parenchymal inflammatory signaling, subsequently culminating in sterile meningeal inflammation” is garnering increasing experimental and clinical support [12,13,14,15,16,17,18,19,20, 26,27,28,29,30,31,32] (Table 1). CSD appears to hold promise not only in unraveling the mechanisms behind aura and headache but also in studying the parenchymal neuroinflammatory response to transient brain perturbations that do not result in overt pathology. This form of “neuroinflammation” presents a challenge because much of the existing literature on brain inflammation is centered around disorders characterized by obvious inflammatory reactions, such as those seen in multiple sclerosis (MS) or around amyloid plaques and tumors. In this review, our emphasis will be on delineating the unique characteristics of parenchymal neuroinflammatory signaling induced by CSD, which serves as a model for benign yet impactful brain perturbation capable of precipitating headaches. This focus is a relative departure from our previous review 3 years ago [24], which highlighted meningeal neurogenic inflammation to a comparable extent. Additionally, we aim to present recent updates and underscore advancements in the signaling cascade since its discovery 11 years ago. Critically, we will discuss its potential relevance in understanding migraine headaches.
CSD and headache
CSD is accompanied by the spread of algesic mediators like H+, K+, ATP, and nitric oxide (NO) from the interstitium into the perivascular and subarachnoid spaces [4, 6, 33]. Although precise molecular mechanisms remain incompletely understood, CSD has been shown to activate perivascular pial nociceptors, as evidenced by the firing of a group of neurons in the trigeminal ganglion and nucleus caudalis concurrently with CSD in the rat, potentially explaining auras coinciding with headaches [34,35,36,37,38,39, 56]. However, headaches typically start 15–20 min after most migraine auras and, consistent with this clinical observation, the majority of nociceptive units begin firing 15 min after a CSD wave in the rat. The delayed firing of dural nociceptors corresponds with a gradual increase in meningeal artery blood flow, driven by a trigeminoparasympathetic reflex that can be non-invasively recorded through the intact skull, following CSD in rats and mice [10, 33, 40]. Since tissue homeostasis is quickly restored after CSD, this delay has been attributed to the time required for sensitization of trigeminocervical nociceptors [37, 57, 58] and the induction and synthesis of pro-inflammatory enzymes such as cyclooxygenase (COX) 2 and inducible nitric oxide synthase (iNOS) [10] as well as a delayed activation of dural macrophages and dendritic cells, occurring subsequent to the early activation of pial macrophages [39]. Supporting a role for astrocyte endfeet (e.g., for synthesis and release of pro-inflammatory mediators), inactivation of astrocytes abutting pia by fluoroacetate or L-a-aminoadipate has been shown to prevent CSD-induced nociceptive sensitization in the rat [49]. After the initial brief activity of constitutively expressed neuronal nitric oxide synthase (nNOS) and COX1 in cortical interneurons and astrocyte endfeet, the inducible isoforms, iNOS and COX2, can provide high throughput and longer-lasting NO and prostaglandin output [4].
Behavioral tests and electrophysiological recordings from dural afferents have unequivocally demonstrated that a single CSD is sufficient to activate trigeminocervical system and trigger headache-like symptoms in rodents [34,35,36, 38, 39, 53, 56, 59] (Table 1). Notably, the inflammatory response is intensified following multiple CSDs, leading to the emergence of M1-type inflammatory phenotype in microglia after 24 h [50, 51] (unlike a single CSD exhibiting no M1 phenotype [41, 52]) and perhaps facilitating the detection of headache-related behavior in rodents [10, 53, 60]. While multiple CSDs may serve as an experimental tool to reveal subtle CSD-induced changes, it is essential to recognize that typically, a single CSD precipitates most auras in humans, and multiple CSDs exhibiting a more complex expression profile could be a more suitable model of the inflammatory reaction observed in patients experiencing frequent migraine with aura attacks [13, 14, 50, 51]. Therefore, caution is advised when comparing expression results because not only transcripts but also the cell type that the transcription is altered can vary with the number of CSDs elicited. Accordingly, we will prioritize describing and discussing the neuroinflammatory signaling and transcriptional changes after a single CSD in this review.
Studies have shown that a single CSD induces the opening of neuronal pannexin-1 (Panx1) channels, formation of the inflammasome complex, activation of caspase-1, and subsequent release of interleukin-1 beta (IL-1β) and high mobility group box 1 (HMGB1), which initiate pro-inflammatory NF-ĸB activation in astrocytes [10, 26, 41]. The pro-inflammatory transcription (possibly not limited to the NF-ĸB pathway though not thoroughly explored), leads to the induction of enzymes such as COX2 and iNOS or cytokines such as CCL2, which are normally not appreciably expressed by astrocytes [10, 13, 16, 30, 43, 44, 46, 48, 61,62,63]. The subsequent release of prostaglandins, NO, and cytokines from the astrocyte endfeet along the glia limitans can participate in activation/sensitization of the pial nociceptors (directly and/or via resident inflammatory cells), thereby contributing to headache generation although precise mechanisms yet to be determined [4, 6, 49]. Supporting these hypotheses with experimental data from rodents, recent positron emission tomography (PET) studies conducted on patients with migraine aura, following the injection of [11C]PBR28 (a molecule taken up by glial cells during inflammation), revealed tracer uptake in both parenchymal and meningeal regions [19, 20]. Intriguingly, tracer uptake was simultaneously registered in the affected occipital (aura) cortex and the overlying dura in some patients. This finding supports the concept that CSD-induced parenchymal inflammatory signaling can propagate to the meninges, inducing meningeal inflammation and consequently, headache in patients as suggested by experimental studies [20]. The enhanced tracer uptake in the visual cortex overlying meninges was also found to extend to the adjacent bone marrow [20, 55]. As elucidated recently, skull channels provide direct communication between the meninges and the skull bone marrow [64]. In case of overt inflammation such as bacterial meningitis, bone marrow presents myeloid cells that migrate through these channels and initiate local inflammatory response [65]. The involvement of bone marrow in various neurological disorders such as MS or Alzheimer’s disease is increasingly being recognized in both experimental models and patients [66, 67]. The surprising finding of tracer uptake extending to the bone marrow in migraine with aura patients suggests that myeloid cells may contribute to inflammation and reinforces the significance of sustaining dural inflammation for headache generation in migraine.
Neuronal stress sensors – Pannexin1 channels
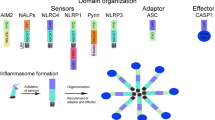
Pannexins are heptameric transmembrane proteins that host a large-pore ion channel [68] (Fig. 1). Within the nervous system, both Panx1 and Panx2 are identified. Panx1 exhibits broad expression across excitatory and inhibitory neurons, as well as oligodendrocytes, astrocytes, and microglia [69]. In neurons, its primary localization is at the postsynaptic membrane [70]. Panx1 serves as a modulator of glutamatergic transmission and acts as a sensor for stressful pro-inflammatory conditions in the brain by triggering inflammasome formation and downstream inflammatory signaling [71,72,73].
(A) Pannexins are heptameric transmembrane proteins that form large-pore ion channels. Subunits undergo post-translational modifications; for instance, Src-family kinases phosphorylate Y308, promoting pore opening, while caspase cleavage at the 378th amino acid leads to permanent channel opening and cell death under pathological conditions. During CSD, Panx1 channels in neurons can be activated by high extracellular K+, glutamate, and intracellular Ca2+ concentration, depolarization and NMDA receptor stimulation as well as by Src-family kinases. (B) CSD-induced NLRP3 inflammasome complex formation is a downstream event triggered by Panx1 channel activation. Inflammasome assembly serves as an initial step in inflammatory conditions, facilitating the processing of pro-inflammatory mediators into their active forms. This assembly involves the clustering of node-like receptors around a central hub which is facilitated by the recruitment of an adapter molecule containing a caspase recruitment domain (ASC). Pro-caspase-1 binding to this complex dimerizes and undergoes self-cleavage, releasing active caspase-1. Reproduced from [82] and [83] with permission.
Panx1 channels can be activated by various signals present during CSD such as high extracellular K+, glutamate, and intracellular Ca2+ concentration [74], depolarization and N-methyl-D-aspartate (NMDA) receptor stimulation [75], and plasma membrane stretch (e.g. spine swelling). They may also be permanently opened by cleavage of the C-terminal region during apoptosis, contributing to cell death under pathological conditions [76]. When Panx1 opens in a large-conductance state, its nonselective ion channel becomes permeable to molecules up to 900 Da, allowing considerable K+ and ATP efflux [73, 77]. This unique property enables the detection of Panx1 opening using membrane-impermeant fluorescent dyes smaller than 900 Da, like propidium iodide or YoPro-1. Thus, membrane-impermeable dyes can enter a cell through large channel openings [78, 79]. This feature has been crucial in revealing CSD-induced Panx1 activity in the mouse and rat brain [10, 18]. Because CSD-induced perturbations last approximately 2 min, Panx1 opening is transient as shown by propidium iodide influx to neurons [10]. Nevertheless, this timeframe proves adequate for promptly initiating inflammasome formation (Fig. 1B) and activating caspase-1 in neurons, as detected 5 [10] and 15 [30] minutes after a single CSD induced by pinprick or optogenetically by two independent laboratories. This observation holds true for both male and female mice, which is crucial to emphasize because migraine prevalence is significantly higher in females, and females experience autoimmune and autoinflammatory diseases more frequently [80, 81]. However, most experimental research on migraine has historically focused on male animals, though this trend is changing. Notably, NLRP3 has recently been identified as the NLRP subtype responsible for forming the inflammasome in neurons. Its inhibitor MCC950 effectively suppressed caspase-1 cleavage induced by CSD [30].
Membrane-impermeant fluorescent dyes can also enter through P2x7 receptor (P2rx7) channel pore, which can open in a large conductance state, possibly induced by extracellular ATP reaching high levels during CSD [84]. Notably, the P2x7/Panx1 pore inhibitor A438079 [16] as well as disruption of the interaction of P2x7 receptor with Src family kinases by TAT-P2x7 [85] have been shown to reduce the increase in IL-1β expression after CSD. However, the location of P2rx7 channels initiating the inflammatory transcription, whether on neurons or glial cells, remains currently unknown as astrocytes and microglia also harbor Panx1 and P2rx7 as well as Src-family kinases interacting with them [86]. Although increased IL-1β expression after CSD (correlated with the number of CSDs [16]) has long been recognized [45, 47], this represents a distinct process (transcriptional expression) compared to the cleavage and activation of the constitutively present pro-IL-1β by caspase-1 in neurons following the activation of Panx1 channels and inflammasome formation, as discussed earlier.
Strongly supporting the notion that CSD-induced propidium iodide influx to neurons occurs through large channel opening of the neuronal Panx1, this was prevented not only by the non-selective Panx1 blockers carbenoxolone and probenecid but also the selective inhibitor 10Panx peptide [10]. Additionally, RNAi-mediated suppression of Panx1 expression proved to be a successful strategy in inhibiting this process [10]. In line with the involvement of Panx1 channels, Panx1 mRNA in the cortex was reportedly upregulated following a single CSD [32] as well as after synaptic metabolic stress causing Panx1 activation [87]. Of note, P2x7/Panx1 channels present in glial cells may also facilitate CSD generation and propagation, for instance by releasing K+, as suggested by studies using P2x7/Panx1 channel inhibitors in addition to their role in inflammatory signaling [16].
The exact mechanism of how neuronal Panx1 channels open in a large-conductance state after CSD has not been thoroughly investigated. In addition to factors such as high extracellular K+ and neuronal swelling [10, 87], it has been proposed that intense stimulation of NR2A type NMDA receptor subunits by high extracellular glutamate and strong depolarization during CSD activates Src-family kinases [18]. These kinases, in turn, phosphorylate Y308 near the intracellular C-terminal, thereby promoting the opening of Panx1 channels [18]. Indeed, the TAT-Panx308 peptide, which inhibits Y308 phosphorylation by Src-family kinases, has been shown to prevent CSD-induced HMGB1 release [27]. Similarly, the Src-family kinase inhibitor, PP2, or the NR2A–receptor antagonist, NVP–AAM077, when perfused into cerebral ventricles of rats prior to CSD induction, attenuated CSD-induced Panx1 activation in cortices [18].
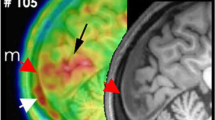
Interestingly, neuronal Panx1 activation as monitored by propidium influx was not limited to the cortex ipsilateral to CSD. It was also observed in the contralateral cortex and subcortical structures such as the dentate gyrus [10, 88] (Fig. 2B). Subsequent validation of this observation included the demonstration of widespread HMGB1 release from neurons and NF-ĸB activation in astrocytes in cortical and subcortical areas of both hemispheres [26]. These effects were less intense in the contralateral hemisphere. Importantly, these experimental observations conform with PET findings that revealed bi-hemispheric cortical as well as subcortical inflammatory tracer uptake in patients suffering from frequent migraine with aura attacks [19, 20] (Fig. 2A). The mechanisms underlying the spread of this phenomenon and its potential association with bilateral headaches following unilateral aura remain unclear. Notably, the significant propidium iodide uptake in dentate gyrus granular neurons, in contrast to neighboring CA sector pyramidal neurons, suggests a propagation via axonal volleys from the entorhinal cortex rather than gray matter or interstitium continuity. These volleys typically fire at the onset of CSD wave before depression of electrical activity [89]. The heightened excitatory firing, akin to observations during epileptiform discharges, has the potential to activate Panx1 channels due to the overactivation of NMDA receptors and rise in extracellular K+ [75, 90]. In support of this notion, when NMDA receptors were inhibited by locally applied MK801 to the cortex contralateral to the site where CSD was generated, HMGB1 release was suppressed in the contralateral (non-CSD) cortex, without any discernible impact on the CSD occurring on the ipsilateral side [26]. Likewise, in familial hemiplegic migraine type 1 mice exhibiting enhanced glutamate release due to a knock-in S218L missense mutation in α1A subunit of presynaptic CaV2 (but not in R192Q knock-in exhibiting less severe phenotype [26]), HMGB1 release in the contralateral cortex was increased [27].
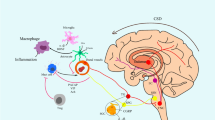
CSD-induced inflammatory activity propagates through the brain, meninges, and skull. A. PET studies utilizing inflammation markers revealed bi-hemispheric cortical as well as subcortical inflammatory tracer uptake in patients suffering from frequent migraine with aura attacks. B. Consistent with clinical observations, Panx1 activity, monitored by propidium iodide (PI) influx to neurons (red fluorescence), was not confined to the cortex (Cx) ipsilateral to CSD but was also evident in the contralateral cortex and subcortical structures such as the dentate gyrus (DG) in the mouse brain. C. Simultaneous tracer uptake ([11C]PBR28) was observed in the affected occipital cortex responsible for generating the aura and the overlying dura, extending to the adjacent bone marrow. These findings suggest that myeloid cells may also contribute to inflammation in addition to the inflammatory mediators released from astrocyte endfeet and dural cells (D), thus underscoring the significance of sustained dural inflammation in migraine headache generation. Lym: lymphocyte, DC: dendritic cell, Mac: macrophage, Mono: monocyte, MC: mast cell. Reproduced from [10, 19, 91] with permission. Illustrations were created using BioRender.com and Servier Medical Art (http://www.servier.com).
While studies involving CSD have been crucial in uncovering and exploring parenchymal inflammatory signaling initiated by the activation of neuronal Panx1 channels, a lingering question remains about whether the same pathway could be triggered by transient neuronal disturbances other than CSD. This potential mechanism could offer insights into migraine without aura arising from brain perturbations, such as sleep deprivation, distinct from migraine without aura caused by factors directly activating meningeal nociceptors. Indeed, experiments creating synaptic stress by inhibiting glycogen use have shown the opening of neuronal Panx1 channels, caspase-1 activation, and the release of HMGB1 in the absence of CSD in mice [87, 92]. This occurrence is attributed to the essential role of glycosyl units derived from glycogen in astrocyte processes, fueling astrocytic uptake of glutamate and K+ during rapidly escalating intense neuronal activity. Migraine triggers, including sleep deprivation or acute psychological stress, induce transcriptional changes in astrocytes [92]. Some of these changes promote glycogen synthesis in astrocyte processes over its utilization, potentially jeopardizing the clearance of glutamate and K+ during high-frequency/prolonged neuronal activity [92]. Consequently, based on experimental evidence, we can hypothesize that migraine triggers hold the potential to activate the parenchymal inflammatory signaling, leading to headaches without the necessity of CSD and, consequently, without the occurrence of aura.
Owing to its upstream role in the initiation of inflammation, Panx1 is being considered as a therapeutic target for treating inflammatory diseases such as rheumatoid arthritis. The aforementioned findings also highlight it as a potential target for prophylaxis of migraine with aura and, perhaps, migraine without aura. In experimental settings, it is possible to inhibit Panx1 or purinergic receptor activity with non-selective pharmacological agents like carbenoxolone, probenecid, mefloquine, flufenamate [93], spironolactone, nitric oxide donors (by S-nitrosylation at Panx1 C346) [79], quinolones and brilliant blue FCF or G to name a few among a growing number of agents [94] (Fig. 1A). Additionally, selective peptides such as 10Panx and specific conventional or mini antibodies can be employed [93]. While carbenoxolone, probenecid, mefloquine, spironolactone, floxacins are clinically registered drugs and brilliant blue G is a commercially used candy additive [79], there are no published reports on their potential effect on migraine at the clinically used doses. Notably, flufenamate was used in the past as a nonsteroidal anti-inflammatory drug for treating menstruation-related migraine [95]. However, the question of whether these agents can achieve effective concentrations in the cortex to inhibit neuronal Panx1 channels after systemic administration of clinically used doses (e.g. carbenoxolone is poorly BBB permeable [96]) and whether any unwanted side effects could overshadow (e.g. spironolactone is 1000-times more potent in blocking mineralocorticoid receptors [97]) their migraine prophylactic action remains unclear (see [93] for review). Just like Panx1 inhibitors, there is growing consideration for inflammasome and caspase-1 inhibitors as potential therapeutic targets for treating inflammatory diseases (reviewed in [98, 99]). This exploration may pave the way for clinical trials involving promising candidates in the treatment of migraines. Of note, the anti-inflammatory agents developed may not only address parenchymal inflammatory signaling but also potentially suppress dural neurogenic inflammation. As a result, these agents could serve a dual purpose by being utilized not only in migraine prophylaxis but also in the treatment of acute migraine attacks. However, it’s important to note that effective doses for the brain and meninges could vary significantly due to factors such as the BBB permeability and the differing abundance of targets to be inhibited. Higher doses could potentially lead to unwanted effects. Additionally, inhibiting widely expressed upstream targets such as inflammasomes carries the risk of undesired immunomodulation, a common concern in drug development, for instance, for rheumatic diseases.
Proinflammatory mediators released from neurons
The CSD-induced formation of the NLRP3 inflammasome complex represents a downstream event triggered by the activation of Panx1 channels in neurons (Fig. 1B). Inflammasome formation serves as a common initial step in various inflammatory conditions, establishing molecular machinery for processing of pro-forms of proinflammatory mediators into their active forms. The assembly of an inflammasome complex involves the clustering of node-like receptors (NLRs) around a hub when detecting pathogen- or cellular damage-associated signals in the cytoplasm. This clustering is completed by the joining of an adapter molecule containing a caspase recruitment domain [100, 101]. Pro-caspase-1 binding to this complex dimerizes and undergoes self-cleavage, releasing active caspase-1. Subsequently, this active enzyme mediates the cleavage of pro-IL-1β and pro-IL-18, generating active IL-1β and IL-18. Besides the formation of the NLRP3 inflammasome complex after CSD and the emergence of the cleaved form of caspase-1 mentioned above, the released active IL-1β from neurons has been identified in CSF [10] and brain rinsing solution [30]. It is worth noting that a technical drawback in studying IL-1β lies in the fact that the available antibodies typically recognize both the pro and cleaved forms of IL-1β. Overcoming this limitation, the detection of secreted active form in CSF provides valuable insights, albeit with technical challenges associated with collecting CSF from small rodents. The release of IL-18 along with IL-1β is likely to occur as generally observed in other cells [102, 103], although its role in the context of CSD has not been explored. In addition to cleavage of existing pro-IL-1β in neurons and release of IL-1β, an increase in IL-1β expression has been detected as early as 10 minutes after a single noninvasive (optogenetically triggered) CSD in the mouse [13] and after potassium chloride or microinjury-induced single CSD in the rat [32]. Multiple CSDs cause a more robust increase in IL-1β transcription, accompanied by the expression of several other pro-inflammatory genes [13, 16]. This transcriptional response was reduced in IL-1 receptor-1 knockout mice, suggesting that it was initiated by IL-1β released from neurons [13]. Supporting a neuronal origin for this inflammatory activity, 10Panx and NLRP3 inhibitor MCC950 ameliorated SD-induced upregulation of IL-1β transcription [30].
Parenchymal IL-1β production could also play a significant role by triggering meningeal nociceptor activation in migraine without aura (i.e. without CSD) [87, 92]. Indeed, migraine without aura attacks are seen in patients with cryopyrin-associated periodic syndromes (CAPS), where IL-1β is overproduced due to mutations in the NLRP3 inflammasome. Further supporting the involvement of parenchymal inflammatory signaling in migraine without aura, elevated levels of IL-1β, prostaglandin E2, tumor necrosis factor-α (TNF-α), IL-6, and nitrite were detected in the internal jugular vein (which primarily drains the brain parenchyma but not the meninges) within the first hour of a migraine without aura attack [61, 104,105,106]. Interestingly, migraine attacks in CAPS patients are suppressed with the IL-1 receptor antagonist anakinra [107,108,109]. Considering the poor BBB penetrance of anakinra, its main site of action could be the dura as IL-1β activates meningeal nociceptors and increases their mechanosensitivity [110, 111]. However, these observations reinforce the idea that agents antagonizing the action of IL-1β could be used in migraine prophylaxis and attack treatment if not limited by potential side effects.
Inflammasome activation is also associated with the translocation of HMGB1 from the nucleus to the cytoplasm [112, 113]. HMGB1, a non-histone protein that binds to DNA, is abundantly expressed in nearly all cells and serves various nuclear functions [114]. However, it transforms into a proinflammatory mediator upon release into the extracellular medium, akin to other alarmin proteins such as IL-33 or S100β [115]. HMGB1 passively leaks from necrotic or damaged cells but it can also be actively transported out of the cell after an inflammatory stimulus such as cell swelling, tissue injury, or infection [116, 117]. In such cases, its three-dimensional structure changes by acetylation, phosphorylation, or methylation of different amino acids [117]. This structural alteration exposes the nuclear export signal necessary for the translocation of HMGB1 from the nucleus to the cytoplasm. HMGB1 can activate various inflammatory pathways including NF-κB in nearby cells expressing receptors for advanced glycation end products (RAGE) and toll-like receptors (TLRs) [117, 118].
Depending on brain region, approximately 40–80% of the neuronal nuclei exhibit loss of HMGB1 immunoreactivity immediately after a single CSD, whereas glial nuclei remain unaffected [10, 26] (Fig. 3A). Optogenetically-induced CSD results in comparable HMGB1 release to pinprick- or Potassium chloride-induced single CSDs, confirming that HMGB1 release is specifically triggered by CSD but not experimental injury [26, 27, 30, 41](Table 1). A recent study demonstrates that, after a single CSD induced optogenetically or by pinprick, HMGB1 is released from neurons within extracellular vesicles (EVs), predominantly having a size compatible with exosomes [41] (Fig. 3B, C). This is in line with the fact that HMGB1 molecule does not have a leader peptide sequence to cross the plasma membrane by conventional protein secretion mechanisms [119, 120]. Interestingly, released exosomes are promptly taken up by astrocyte processes enveloping neuron soma (Fig. 3D), leading to NF-ĸB activation in these cells, which was previously shown to be suppressed by knocking down HMGB1 expression or by inhibiting HMGB1 activity with anti-HMGB1 antibodies or BoxA fragment of HMGB1 applied before CSD [10]. In contrast, microglia do not internalize HMGB1-bearing EVs and exhibit neither NF-ĸB activation nor the conventional inflammatory phenotype even 24 h after CSD [41]. After multiple CSDs, some of the released HMGB1 leaks into CSF, reaching detectable levels with Western blotting [10]. As a result, a slight reduction in HMGB1 levels in cortex extracts can be observed 2–3 h after multiple [12, 30], but not single, CSDs giving the impression that only multiple CSDs could cause HMGB1 release [12]. Consequently, the most reliable parameter to show CSD-induced HMGB1 release appears to be the loss of nuclear HMGB1 immunoreactivity detected by immunohistochemistry [10, 12, 30].
A single CSD triggered by pinprick causes release of HMGB1 from neurons within small EVs, which are subsequently taken up by astrocyte processes. (A) Immunolabeling reveals numerous HMGB1-positive puncta (red, marked by white arrows) in the cytoplasm surrounding the nuclei of cortical neurons, identified by CD171 immunolabeling (green). Insets below delineate the boundaries of neuronal cytoplasm and nucleus, emphasizing the distribution of the puncta. Shedding of HMGB1-labeled puncta from cells, with varying degrees of nuclear HMGB1 immunopositivity loss, is observed as early as 15 min post-CSD. Puncta near the nuclei (white arrows) suggest HMGB1 release within vesicles. Images are maximum projections of confocal z-stacks. Scale bars: 10 μm. (B) Electron microscopic (EM) images of a neuron depict a multivesicular body (light blue) containing several small EVs, one of which carries gold nanoparticles marking HMGB1 1-hour post-CSD. (C) Transmission EM image of EV suspension isolated from mouse brain, predominantly having a size compatible with exosomes. (D) 3D surface reconstruction of a GFP‐positive astrocyte and its process shows that HMGB1-immunopositive puncta (black triangles) are located inside the process. The black rectangle on the left panel indicates the HMGB1‐immunopositive process that is visualized on the right panels from different angles in 3D. P and D denote the proximal and distal ends of the process, respectively. Scale bars: 2 μm. X, Y, and Z axes of the volume are shown for orientation. Reproduced from [41] under Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
Astrocyte and microglia activation and NF-κB pathway
Both astrocytes and microglia express receptors that can respond to HMGB1. However, recent findings reveal that HMGB1 released in EVs selectively initiates an inflammatory signaling in astrocytes without activating the NF-κB system in microglia after a single CSD (Fig. 4). Microglia are known to exhibit an inflammatory phenotype only after multiple CSDs and with a 24-hour delay, dependent on TLR2/4 [52]. The latter may be more relevant to inflammatory activity in patients experiencing frequent migraine with aura attacks, rather than the parenchymal inflammatory signaling mediated by astrocytes after a typical single aura. In addition, the glia limitans formed by astrocyte endfeet, covers the whole cortical surface and perivascular spaces, providing a large surface area for transporting proinflammatory mediators to the pial nociceptors as well as to the CSF. This creates an opportunity for direct access to dural nociceptors in addition to their activation via pial collaterals [4]. Even when microglia are in an active proinflammatory state, their cytokines released into the interstitium seem to reach CSF in perivascular and subarachnoid spaces through a tight extracellular space [121]. Accordingly, astrocytes covering the entire cortical surface and forming an elaborate syncytium among themselves are in a prime position to facilitate communication of inflammatory signaling between the brain parenchyma, pia, and CSF. Conversely, microglia continuously survey the spines and dendrites with their processes, participating in recycling and repairing synapses and, when injured irreparably, in removing them [51, 122,123,124]. In fact, a recent study has demonstrated that neuronal swelling-induced by opening of Panx1 channels leads to ATP release, which attracts microglia processes via P2Y12 receptors exclusively expressed in microglia [125]. These observations raise the possibility that while astrocytes stimulated by HMGB1 activate an inflammatory signaling cascade to excite pial nociceptors, microglia promote repair programs involving the expression of cytoprotective cytokines.
A single CSD or synaptic stress induces the opening of neuronal Panx1 channels, formation of the inflammasome complex, activation of caspase-1, and subsequent release of IL-1β and HMGB1, which induce translocation of NF-ĸB pairs to the nucleus to initiate pro-inflammatory transcription in astrocytes. The pro-inflammatory transcription in astrocytes leads to the induction of enzymes such as COX2 and iNOS or cytokines such as CCL2. The subsequent release of prostaglandins, NO, and cytokines from the astrocyte endfeet along the glia limitans can activate/sensitize the pial nociceptors, thereby contributing to sustaining headache. ATP release from Panx1 channels attracts microglia processes that continuously survey the spines via P2Y12 receptors to repair injured spines. Illustrations were created using Servier Medical Art (http://www.servier.com)
While the inflammatory response is inherently complex and entails multiple pathways, the translocation of NF-κB subunits to the nucleus suggests that transcriptional NF-κB activity in astrocytes likely plays a role for orchestrating this process, from the secretion of pro-inflammatory algesic signals to the CSF, to the anti-inflammatory resolution phase. The latter activity may contribute to the termination of dural neurogenic inflammation and alleviating headache. The NF-κB transcription factor family operates by combining p65, cRel, RelB, p52, and p50 subunits in pairs. Depending on the specific subunit pairs, NF-κB either promotes the expression of pro-inflammatory molecules or anti-inflammatory ones. For instance, while the p65:p50 pair promotes the expression of inflammatory genes such as iNOS, COX2, and TNF-α, the cRel-containing pairs induce the expression of anti-inflammatory/survival genes such as transforming growth factor beta (TGF-β) and Bcl-x [126,127,128]. The relative abundance of transcripts for these pairs determines the overall behavior of the nucleus [129, 130]. Furthermore, NF-κB pairs can influence the transcription of various NF-κB subunits and inhibitory-kappa B (IκB), which plays a role in terminating the transcriptional activity of NF-κB pairs. Our recent studies have revealed that pro-inflammatory NF-κB p65:p50 pairs, as well as anti-inflammatory cRel:p65 pairs are both translocated to astrocyte nuclei shortly after CSD [131]. Interestingly, however, 24 h after CSD, the nuclear p65:p50 pairs disappear while cRel:p65 persist, consistent with a shift from pro-inflammatory to anti-inflammatory transcriptional activity in astrocytes. One of the steps that terminates NF-κB activation is the translocation of IκB to the cell nucleus. Consistent with this, we detected IκB in astrocyte nuclei along with p65 and cRel shortly after CSD. Microglia may also contribute to the resolution of the parenchymal inflammatory signaling by switching to an anti-inflammatory phenotype, however, this remains to be investigated [132].
Clinical outlook and conclusions
Advancements in neuroimaging techniques are promising to be able to directly assess the presence of the mechanisms discussed above in migraine patients. Particularly encouraging is the detection of the meningeal uptake of the inflammatory tracer [11C]PBR28 over the occipital cortex, exhibiting parenchymal uptake in patients suffering from migraine with visual aura [20] (Fig. 2). [11C]PBR28 PET may also provide insight into the relationship between inflammatory signaling and headache in secondary headache disorders such as post-seizure headache. [11C]PBR28 exploits its high affinity against the 18 kDa translocator protein (TSPO) in the outer mitochondrial membrane, an inflammation-specific biomarker in activated glial cells. TSPO-PET imaging is increasingly being utilized for various clinical populations to disclose neuroinflammatory involvement. For example, in patients with chronic neurodegenerative disorders such as amyotrophic lateral sclerosis and Alzheimer’s disease, inflammatory glial activation in the central nervous system (CNS) has been demonstrated starting from the early stages [133, 134]. Despite inconsistent results with [11C]PBR28 [135], another TSPO ligand, [18F]FEPPA, showed a notable increase in glial uptake in patients with depression in relevant regions like the anterior cingulate cortex and hippocampus [136]. These discrepancies underscore the ongoing need for improved PET ligands, as TSPO signals can be confounded by variable binding affinities depending on TSPO gene polymorphisms, issues with TSPO binding specificity, and their in vivo metabolic profiles. Next-generation tracers with enhanced TSPO binding features are in active development [137]. If successful, these improved tracers can play a pivotal role in resolving some of the controversies surrounding the role of meningeal neurogenic inflammation and parenchymal inflammatory signaling in migraine. Moreover, it is worth noting that, inflammatory glial activation in the brain and the spinal cord has also been shown with [11C]PBR28 PET in chronic pain conditions other than migraine, such as chronic low back pain [138, 139] and fibromyalgia [135], suggesting a shared neuroinflammatory element across a heterogeneity of pain-related conditions [140].
In conclusion, neuroinflammatory mechanisms are garnering increasing attention in CNS disorders. The hypothesis of neuroinflammatory signaling following transient perturbations such as CSD or synaptic metabolic stress has received considerable experimental support over the past decade. The demonstration of inflammatory tracer uptake in brain parenchyma as well as the meninges in migraine with aura patients aligns with these experimental findings, reinforcing the notion that inflammatory mechanisms may play a pivotal role in headache generation after brief perturbations and in sustaining the pain. Available evidence suggests that astrocyte endfeet covering the brain surface and perivascular spaces could serve as an extensive interface for transducing parenchymal inflammatory signaling to neurogenic inflammation in the meninges, where pial nociceptors detect the algesic signals and activate the dural nociceptors via collaterals, resulting in release of peptides such as CGRP [6]. These peptides stimulate dural inflammatory cells, inducing secretion of algesic and inflammatory mediators, thereby contributing to sustaining inflammation and nociceptive activity, hence, headache [4, 6, 24]. Supporting the role of dural neurogenic inflammation in headaches, various rodent models have shown that the application of inflammatory substances (e.g., complete Freund’s adjuvant, inflammatory soup) onto the dura causes headache-like behaviors such as peri-orbital allodynia, facial grooming and scratching, along with activation of trigeminal ganglion and nucleus caudalis neurons, as well as trigeminal ganglion satellite cells [34, 35, 141,142,143,144,145]. Additionally, these models exhibit pain-related general behaviors such as freezing and reduced locomotor activity [141]. Of note, the primary factors contributing to female vulnerability for migraine, estrogen and testosterone indeed influence the pain processing networks. Testosterone and estradiol exhibit anti-nociceptive and nociceptive effects, respectively [146,147,148,149]. Interestingly, dural nociceptors in female rodents show heightened sensitization in response to CGRP [150] and, prolactin has been reported to sensitize them for increased CGRP release [151, 152]. While this framework is bolstered by multiple lines of evidence, there remain outstanding questions that require clarification through future research. These include a deeper understanding of the involved molecular pathways and cell types, as well as the mechanisms that render them noxious, as inflammatory reactions in the brain are not always associated with headaches. Central pain-regulating mechanisms, as well as a migraine-specific genetic background, may modulate these mechanisms in inhibitory as well as facilitatory directions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BBB:
-
Blood-brain barrier
- CAPS:
-
Cryopyrin-associated periodic syndromes
- CGRP:
-
Calcitonin gene-related peptide
- COX:
-
Cyclooxygenase
- CSD:
-
Cortical spreading depolarization
- CSF:
-
Cerebrospinal fluid
- HMGB1:
-
High mobility group box protein 1
- IĸB:
-
Inhibitory kappa B
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- MS:
-
Multiple sclerosis
- NF-κB:
-
Nuclear factor-kappa B
- NLR:
-
Nod-like receptor
- NMDA:
-
N-methyl-D-aspartate
- NO:
-
Nitric oxide
- nNOS:
-
Neuronal nitric oxide synthase
- Panx:
-
Pannexin
- PET:
-
Positron emission tomography
- RAGE:
-
Receptor for advanced glycation end products
- TGF-β:
-
Transforming growth factor beta
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TSPO:
-
18 kDa translocator protein
References
Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA (2019) Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 18:795–804
Wiesmann M, Koedel U, Brückmann H, Pfister HW (2002) Experimental bacterial meningitis in rats: demonstration of hydrocephalus and meningeal enhancement by magnetic resonance imaging. Neurol Res 24:307–310
Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, la Marca G, Andrè E, Preti D, Avonto C et al (2012) The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 135:376–390
Carneiro-Nascimento S, Levy D (2022) Cortical spreading depression and meningeal nociception. Neurobiol Pain 11:100091
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017) Pathophysiology of migraine: a disorder of sensory Processing. Physiol Rev 97:553–622
Levy D, Moskowitz MA (2023) Meningeal mechanisms and the migraine connection. Annu Rev Neurosci 46:39–58
Rorabaugh JBJ, Radivojevic A, Dotan O, Ghibellini G, Zeng H, Lu X, Dai F, Angeles T, Szilagyi O, Wang Z, Mallett S, Barash S, Rabinovich-Guilatt L, Goadsby P (2024) Measurement and Modeling of Peripherally Administered Anti-CGRP Monoclonal Antibody in CSF and Brain of Healthy Volunteers. In AAN Annual Meeting
Edvinsson L, Tfelt-Hansen P (2008) The blood-brain barrier in migraine treatment. Cephalalgia 28:1245–1258
Peng KP, May A (2019) Migraine understood as a sensory threshold disease. Pain 160:1494–1501
Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Koçak E, Sen ZD, Dalkara T (2013) Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339:1092–1095
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54:8071–8089
Takizawa T, Shibata M, Kayama Y, Toriumi H, Ebine T, Koh A, Shimizu T, Suzuki N (2016) Temporal profiles of high-mobility group box 1 expression levels after cortical spreading depression in mice. Cephalalgia 36:44–52
Takizawa T, Qin T, Lopes de Morais A, Sugimoto K, Chung JY, Morsett L, Mulder I, Fischer P, Suzuki T, Anzabi M et al (2020) Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex. J Cereb Blood Flow Metab 40:1117–1131
Ghaemi A, Alizadeh L, Babaei S, Jafarian M, Khaleghi Ghadiri M, Meuth SG, Kovac S, Gorji A (2018) Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia 38:626–638
Ghaemi A, Sajadian A, Khodaie B, Lotfinia AA, Lotfinia M, Aghabarari A, Khaleghi Ghadiri M, Meuth S, Gorji A (2016) Immunomodulatory Effect of Toll-Like Receptor-3 ligand poly I:C on cortical spreading depression. Mol Neurobiol 53:143–154
Chen SP, Qin T, Seidel JL, Zheng Y, Eikermann M, Ferrari MD, van den Maagdenberg A, Moskowitz MA, Ayata C, Eikermann-Haerter K (2017) Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 140:1643–1656
Eising E, Shyti R, t Hoen PAC, Vijfhuizen LS, Huisman SMH, Broos LAM, Mahfouz A, Reinders MJT, Ferrari MD, Tolner EA et al (2017) Cortical spreading Depression causes Unique Dysregulation of Inflammatory pathways in a transgenic mouse model of Migraine. Mol Neurobiol 54:2986–2996
Bu F, Nie L, Quinn JP, Wang M (2020) Sarcoma family kinase-dependent Pannexin-1 activation after cortical spreading depression is mediated by NR2A-Containing receptors. Int J Mol Sci 21
Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, Zürcher NR, Akeju O, Bonnier G, Price J, Hooker JM et al (2019) Imaging of neuroinflammation in migraine with aura: a [(11)C]PBR28 PET/MRI study. Neurology 92:e2038–e2050
Hadjikhani N, Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, Zürcher NR, Akeju O, Bonnier G, Price J et al (2020) Extra-axial Inflammatory Signal in Parameninges in Migraine with visual aura. Ann Neurol 87:939–949
Major S, Huo S, Lemale CL, Siebert E, Milakara D, Woitzik J, Gertz K, Dreier JP (2020) Direct electrophysiological evidence that spreading depolarization-induced spreading depression is the pathophysiological correlate of the migraine aura and a review of the spreading depolarization continuum of acute neuronal mass injury. Geroscience 42:57–80
Charles A (2010) Does cortical spreading depression initiate a migraine attack? Maybe not. Headache 50:731–733
Dalkara T, Moskowitz MA (2017) From cortical spreading depression to trigeminovascular activation in migraine. In Neurobiological Basis of Migraine Edited by Dalkara T, Moskowitz MA: Wiley-Blackwell; : 267–284
Erdener ŞE, Kaya Z, Dalkara T (2021) Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine. J Headache Pain 22:138
Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L (2010) Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci 30:14420–14429
Dehghani A, Phisonkunkasem T, Yilmaz Ozcan S, Dalkara T, van den Maagdenberg A, Tolner EA, Karatas H (2021) Widespread brain parenchymal HMGB1 and NF-κB neuroinflammatory responses upon cortical spreading depolarization in familial hemiplegic migraine type 1 mice. Neurobiol Dis 156:105424
Dehghani A, Schenke M, van Heiningen SH, Karatas H, Tolner EA, van den Maagdenberg A (2023) Optogenetic cortical spreading depolarization induces headache-related behaviour and neuroinflammatory responses some prolonged in familial hemiplegic migraine type 1 mice. J Headache Pain 24:96
Takizawa T, Shibata M, Kayama Y, Toriumi H, Ebine T, Koh A, Shimizu T, Suzuki N (2015) Temporal profiles of high-mobility group box 1 expression levels after cortical spreading depression in mice. Cephalalgia 36:44–52
Zhao YF, Tang Y, Illes P (2021) Astrocytic and Oligodendrocytic P2X7 Receptors Determine Neuronal Functions in the CNS. Front Mol Neurosci 14:641570
Chen PY, Yen JC, Liu TT, Chen ST, Wang SJ, Chen SP (2023) Neuronal NLRP3 inflammasome mediates spreading depolarization-evoked trigeminovascular activation. Brain 146:2989–3002
Schain AJ, Melo-Carrillo A, Ashina S, Strassman AM, Burstein R (2020) Celecoxib reduces cortical spreading depression-induced macrophage activation and dilatation of dural but not pial arteries in rodents: implications for mechanism of action in terminating migraine attacks. Pain 161:1019–1026
Volobueva MN, Suleymanova EM, Smirnova MP, Bolshakov AP, Vinogradova LV (2022) A single episode of cortical spreading depolarization increases mRNA levels of Proinflammatory cytokines, calcitonin Gene-related peptide and Pannexin-1 channels in the cerebral cortex. Int J Mol Sci 24
Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8:136–142
Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R (2010) Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 30:8807–8814
Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R (2011) Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69:855–865
Zhao J, Levy D (2015) Modulation of intracranial meningeal nociceptor activity by cortical spreading depression: a reassessment. J Neurophysiol 113:2778–2785
Zhao J, Levy D (2016) Cortical Spreading Depression Promotes Persistent Mechanical Sensitization of Intracranial Meningeal Afferents: Implications for the Intracranial Mechanosensitivity of Migraine. eNeuro 3
Zhao J, Levy D (2018) The CGRP receptor antagonist BIBN4096 inhibits prolonged meningeal afferent activation evoked by brief local K(+) stimulation but not cortical spreading depression-induced afferent sensitization. Pain Rep 3:e632
Schain AJ, Melo-Carrillo A, Borsook D, Grutzendler J, Strassman AM, Burstein R (2018) Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol 83:508–521
Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R (2019) CSD-Induced arterial dilatation and plasma protein extravasation are unaffected by Fremanezumab: implications for CGRP’s role in migraine with aura. J Neurosci 39:6001–6011
Kaya Z, Belder N, Sever-Bahcekapili M, Donmez-Demir B, Erdener ŞE, Bozbeyoglu N, Bagci C, Eren-Kocak E, Yemisci M, Karatas H et al (2023) Vesicular HMGB1 release from neurons stressed with spreading depolarization enables confined inflammatory signaling to astrocytes. J Neuroinflammation 20:295
Caggiano AO, Breder CD, Kraig RP (1996) Long-term elevation of cyclooxygenase-2, but not lipoxygenase, in regions synaptically distant from spreading depression. J Comp Neurol 376:447–462
Miettinen S, Fusco FR, Yrjänheikki J, Keinänen R, Hirvonen T, Roivainen R, Närhi M, Hökfelt T, Koistinaho J (1997) Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci U S A 94:6500–6505
Yrjänheikki J, Koistinaho J, Copin JC, de Crespigny A, Moseley ME, Chan PH (2000) Spreading depression-induced expression of c-fos and cyclooxygenase-2 in transgenic mice that overexpress human copper/zinc-superoxide dismutase. J Neurotrauma 17:713–718
Jander S, Schroeter M, Peters O, Witte OW, Stoll G (2001) Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab 21:218–225
Yokota C, Inoue H, Kuge Y, Abumiya T, Tagaya M, Hasegawa Y, Ejima N, Tamaki N, Minematsu K (2003) Cyclooxygenase-2 expression associated with spreading depression in a primate model. J Cereb Blood Flow Metab 23:395–398
Thompson CS, Hakim AM (2005) Cortical spreading depression modifies components of the inflammatory cascade. Mol Neurobiol 32:51–57
Viggiano E, Ferrara D, Izzo G, Viggiano A, Minucci S, Monda M, De Luca B (2008) Cortical spreading depression induces the expression of iNOS, HIF-1alpha, and LDH-A. Neuroscience 153:182–188
Zhao J, Blaeser AS, Levy D (2021) Astrocytes mediate migraine-related intracranial meningeal mechanical hypersensitivity. Pain 162:2386–2396
Grinberg YY, Milton JG, Kraig RP (2011) Spreading Depression sends Microglia on Lévy flights. PLoS ONE 6:e19294
Shibata M, Suzuki N (2017) Exploring the role of microglia in cortical spreading depression in neurological disease. J Cereb Blood Flow Metabolism 37:1182–1191
Takizawa T, Shibata M, Kayama Y, Shimizu T, Toriumi H, Ebine T, Unekawa M, Koh A, Yoshimura A, Suzuki N (2017) High-mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. J Cereb Blood Flow Metab 37:890–901
Harriott AM, Chung DY, Uner A, Bozdayi RO, Morais A, Takizawa T, Qin T, Ayata C (2021) Optogenetic spreading Depression elicits Trigeminal Pain and anxiety behavior. Ann Neurol 89:99–110
Cui Y, Takashima T, Takashima-Hirano M, Wada Y, Shukuri M, Tamura Y, Doi H, Onoe H, Kataoka Y, Watanabe Y (2009) 11 C-PK11195 PET for the in vivo evaluation of neuroinflammation in the rat brain after cortical spreading depression. J Nucl Med 50:1904–1911
Christensen RH, Gollion C, Amin FM, Moskowitz MA, Hadjikhani N, Ashina M (2022) Imaging the inflammatory phenotype in migraine. J Headache Pain 23:60
Arngrim N, Hougaard A, Ahmadi K, Vestergaard MB, Schytz HW, Amin FM, Larsson HBW, Olesen J, Hoffmann MB, Ashina M (2017) Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Ann Neurol 82:925–939
Strassman AM, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384:560–564
Zhao J, Levy D (2018) Dissociation between CSD-Evoked metabolic perturbations and meningeal afferent activation and sensitization: implications for mechanisms of Migraine Headache Onset. J Neurosci 38:5053–5066
Houben T, Loonen IC, Baca SM, Schenke M, Meijer JH, Ferrari MD, Terwindt GM, Voskuyl RA, Charles A, van den Maagdenberg AM, Tolner EA (2017) Optogenetic induction of cortical spreading depression in anesthetized and freely behaving mice. J Cereb Blood Flow Metab 37:1641–1655
Akcali D, Sayin A, Sara Y, Bolay H (2010) Does single cortical spreading depression elicit pain behaviour in freely moving rats? Cephalalgia. 30:1195–1206
Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P (2006) Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the Internal Jugular blood of Migraine patients without Aura Assessed Ictally. Headache: J Head Face Pain 46:200–207
Sarchielli P, Floridi A, Mancini ML, Rossi C, Coppola F, Baldi A, Pini LA, Calabresi P (2006) NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia 26:1071–1079
Mouse Brain Atlas [mousebrain.org]
Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J et al (2018) Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci 21:1209–1217
Pulous FE, Cruz-Hernández JC, Yang C, Kaya Ζ, Paccalet A, Wojtkiewicz G, Capen D, Brown D, Wu JW, Schloss MJ et al (2022) Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat Neurosci 25:567–576
Kolabas ZI, Kuemmerle LB, Perneczky R, Förstera B, Ulukaya S, Ali M, Kapoor S, Bartos LM, Büttner M, Caliskan OS et al (2023) Distinct molecular profiles of skull bone marrow in health and neurological disorders. Cell 186:3706–3725e3729
Mazzitelli JA, Pulous FE, Smyth LCD, Kaya Z, Rustenhoven J, Moskowitz MA, Kipnis J, Nahrendorf M (2023) Skull bone marrow channels as immune gateways to the central nervous system. Nat Neurosci 26:2052–2062
Deng Z, He Z, Maksaev G, Bitter RM, Rau M, Fitzpatrick JAJ, Yuan P (2020) Cryo-EM structures of the ATP release channel pannexin 1. Nat Struct Mol Biol 27:373–381
Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R (2005) Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci 21:3277–3290
Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R (2007) Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 146:9–16
Thompson RJ, Macvicar BA (2008) Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin) 2:81–86
Sarrouilhe D, Dejean C, Mesnil M (2017) Connexin43- and pannexin-based channels in Neuroinflammation and cerebral neuropathies. Front Mol Neurosci 10:320
Makarenkova HP, Shah SB, Shestopalov VI (2018) The two faces of pannexins: new roles in inflammation and repair. J Inflamm Res 11:273–288
Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284:18143–18151
Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA (2008) Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–1559
Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68
Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G (2014) The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal 7:ra69
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS et al (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Yeung AK, Patil CS, Jackson MF (2020) Pannexin-1 in the CNS: emerging concepts in health and disease. J Neurochem 154:468–485
Lahita RG (2023) Sex and gender influence on immunity and autoimmunity. Front Immunol 14:1142723
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638
Koval M, Cwiek A, Carr T, Good ME, Lohman AW, Isakson BE (2021) Pannexin 1 as a driver of inflammation and ischemia–reperfusion injury. Purinergic Signalling 17:521–531
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687
Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, Ventureyra EC, Thompson CS (2007) Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res 1168:129–138
Nie L, Ma D, Quinn JP, Wang M (2021) Src family kinases activity is required for transmitting purinergic P2X7 receptor signaling in cortical spreading depression and neuroinflammation. J Headache Pain 22:146
Lohman AW, Weilinger NL, Santos SM, Bialecki J, Werner AC, Anderson CL, Thompson RJ (2019) Regulation of pannexin channels in the central nervous system by Src family kinases. Neurosci Lett 695:65–70
Kilic K, Karatas H, Dönmez-Demir B, Eren-Kocak E, Gursoy-Ozdemir Y, Can A, Petit JM, Magistretti PJ, Dalkara T (2018) Inadequate brain glycogen or sleep increases spreading depression susceptibility. Ann Neurol 83:61–73
Dalkara T, Karatas H, Sen ZD, Gursoy-Ozdemir Y (2009) Cortical spreading depression transiently increases plasmalemma permeability not only in cortical but also in subcortical and brain stem neurons. In SfN Meeting Neuroscience 2009, vol. 339.8/N10
Herreras O, Largo C, Ibarz JM, Somjen GG (1994) Martín Del Río R: role of neuronal synchronizing mechanisms in the propagation of spreading depression in the in vivo hippocampus. J Neurosci 14:7087–7098
Rasmussen R, O’Donnell J, Ding F, Nedergaard M (2020) Interstitial ions: a key regulator of state-dependent neural activity? Prog Neurobiol 193:101802
Kisler K, Zlokovic BV (2022) How the brain regulates its own immune system. Nat Neurosci 25:532–534
Petit JM, Eren-Koçak E, Karatas H, Magistretti P, Dalkara T (2021) Brain glycogen metabolism: a possible link between sleep disturbances, headache and depression. Sleep Med Rev 59:101449
Rusiecka OM, Tournier M, Molica F, Kwak BR (2022) Pannexin1 channels-a potential therapeutic target in inflammation. Front Cell Dev Biol 10:1020826
Navis KE, Fan CY, Trang T, Thompson RJ, Derksen DJ (2020) Pannexin 1 channels as a therapeutic target: structure, inhibition, and Outlook. ACS Chem Neurosci 11:2163–2172
Vardi Y, Rabey IM, Streifler M, Schwartz A, Lindner HR, Zor U (1976) Migraine attacks. Alleviation by an inhibitor of prostaglandin synthesis and action. Neurology 26:447–450
Leshchenko Y, Likhodii S, Yue W, Burnham WM, Perez Velazquez JL (2006) Carbenoxolone does not cross the blood brain barrier: an HPLC study. BMC Neurosci 7:3
Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E et al (2018) Pannexin 1 channels as an unexpected New Target of the anti-hypertensive drug spironolactone. Circ Res 122:606–615
Coll RC, Schroder K, Pelegrín P (2022) NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 43:653–668
Schwaid AG, Spencer KB (2021) Strategies for targeting the NLRP3 inflammasome in the clinical and preclinical space. J Med Chem 64:101–122
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022
Caseley EA, Poulter JA, Rodrigues F, Caseley EA, Poulter JA, McDermott MF (2020) Immunome Project Consortium for Autoinflammatory D: Inflammasome inhibition under physiological and pharmacologicalconditions. Genes Immun 21:211–223
Chan AH, Schroder K (2020) Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217
Tapia VS, Daniels MJD, Palazón-Riquelme P, Dewhurst M, Luheshi NM, Rivers-Auty J, Green J, Redondo-Castro E, Kaldis P, Lopez-Castejon G, Brough D (2019) The three cytokines IL-1β, IL-18, and IL-1α share related but distinct secretory routes. J Biol Chem 294:8325–8335
Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V (2000) Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20:907–918
Sarchielli P, Alberti A, Vaianella L, Pierguidi L, Floridi A, Mazzotta G, Floridi A, Gallai V (2004) Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache 44:961–968
Musubire AK, Cheema S, Ray JC, Hutton EJ, Matharu M (2023) Cytokines in primary headache disorders: a systematic review and meta-analysis. J Headache Pain 24:36
Kitley JL, Lachmann HJ, Pinto A, Ginsberg L (2010) Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology 74:1267–1270
Miyamae T (2012) Cryopyrin-associated periodic syndromes: diagnosis and management. Paediatr Drugs 14:109–117
Keddie S, Parker T, Lachmann HJ, Ginsberg L (2018) Cryopyrin-Associated Periodic Fever Syndrome and the nervous system. Curr Treat Options Neurol 20:43
Sjöström EO, Culot M, Leickt L, Åstrand M, Nordling E, Gosselet F, Kaiser C (2021) Transport study of interleukin-1 inhibitors using a human in vitro model of the blood-brain barrier. Brain Behav Immun Health 16:100307
Zhang X, Burstein R, Levy D (2012) Local action of the proinflammatory cytokines IL-1β and IL-6 on intracranial meningeal nociceptors. Cephalalgia 32:66–72
Vande Walle L, Kanneganti TD, Lamkanfi M (2011) HMGB1 release by inflammasomes. Virulence 2:162–165
Lu B, Wang H, Andersson U, Tracey KJ (2013) Regulation of HMGB1 release by inflammasomes. Protein Cell 4:163–167
Stros M (2010) HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta 1799:101–113
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Andersson U, Tracey KJ (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29:139–162
Bertheloot D, Latz E (2017) HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 14:43–64
Paudel YN, Angelopoulou E, Piperi C, Balasubramaniam VRMT, Othman I, Shaikh MF (2019) Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: updates on receptor signalling. Eur J Pharmacol 858:172487
Kwak MS, Kim HS, Lee B, Kim YH, Son M, Shin JS (2020) Immunological significance of HMGB1 post-translational modification and Redox Biology. Front Immunol 11:1189
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135:387–407
Eikermann-Haerter K, Arbel-Ornath M, Yalcin N, Yu ES, Kuchibhotla KV, Yuzawa I, Hudry E, Willard CR, Climov M, Keles F et al (2015) Abnormal synaptic ca(2+) homeostasis and morphology in cortical neurons of familial hemiplegic migraine type 1 mutant mice. Ann Neurol 78:193–210
Gehrmann J, Mies G, Bonnekoh P, Banati R, Iijima T, Kreutzberg GW, Hossmann KA (1993) Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathol 3:11–17
Eyo UB, Wu LJ (2019) Microglia: lifelong patrolling immune cells of the brain. Prog Neurobiol 179:101614
Weilinger NL, Yang K, Choi HB, Groten CJ, Wendt S, Murugan M, Wicki-Stordeur LE, Bernier LP, Velayudhan PS, Zheng J et al (2023) Pannexin-1 opening in neuronal edema causes cell death but also leads to protection via increased microglia contacts. Cell Rep 42:113128
Bunting K, Rao S, Hardy K, Woltring D, Denyer GS, Wang J, Gerondakis S, Shannon MF (2007) Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J Immunol 178:7097–7109
Gilmore TD, Gerondakis S (2011) The c-Rel transcription factor in Development and Disease. Genes Cancer 2:695–711
NF-kB Target Genes https://www.bu.edu/nf-kb/gene-resources/target-genes/
Shih R-H, Wang C-Y, Yang C-M (2015) NF-kappaB Signaling pathways in neurological inflammation: a Mini Review. Front Mol Neurosci 8
Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP (2020) NF-κB-Mediated neuroinflammation in Parkinson’s Disease and potential therapeutic effect of polyphenols. Neurotox Res 37:491–507
Kaya Z (2019) Resolution of Inflammation Triggered by Cortical Spreading Depression and Its Contribution to Migraine Headache Pathophysiology. PhD Thesis. Hacettepe University, Graduate School of Health Sciences, Basic Neurological Sciences Program
Walker DG, Lue LF (2015) Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther 7:56
Alshikho MJ, Zürcher NR, Loggia ML, Cernasov P, Reynolds B, Pijanowski O, Chonde DB, Izquierdo Garcia D, Mainero C, Catana C et al (2018) Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Ann Neurol 83:1186–1197
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW et al (2013) In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136:2228–2238
Dahoun T, Calcia MA, Veronese M, Bloomfield P, Reis Marques T, Turkheimer F, Howes OD (2019) The association of psychosocial risk factors for mental health with a brain marker altered by inflammation: a translocator protein (TSPO) PET imaging study. Brain Behav Immun 80:742–750
Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, Meyer JH (2018) Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 5:339–347
Adhikari A, Zhang MR, Tiwari AK (2022) Acetamidobenzoxazolone scaffold as a promising translocator protein (18 kDa, TSPO) marker for neuroinflammation imaging: Advancement in last decennial period. Drug Dev Res 83:1519–1533
Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR et al (2015) Evidence for brain glial activation in chronic pain patients. Brain 138:604–615
Torrado-Carvajal A, Toschi N, Albrecht DS, Chang K, Akeju O, Kim M, Edwards RR, Zhang Y, Hooker JM, Duggento A et al (2021) Thalamic neuroinflammation as a reproducible and discriminating signature for chronic low back pain. Pain 162:1241–1249
Alshelh Z, Brusaferri L, Saha A, Morrissey E, Knight P, Kim M, Zhang Y, Hooker JM, Albrecht D, Torrado-Carvajal A et al (2022) Neuroimmune signatures in chronic low back pain subtypes. Brain 145:1098–1110
Spekker E, Tanaka M, Szabó Á, Vécsei L (2021) Neurogenic inflammation: the participant in Migraine and recent advancements in Translational Research. Biomedicines 10
Burgos-Vega CC, Quigley LD, Trevisan Dos Santos G, Yan F, Asiedu M, Jacobs B, Motina M, Safdar N, Yousuf H, Avona A et al (2019) Non-invasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia 39:123–134
Reducha PV, Bömers JP, Edvinsson L, Haanes KA (2023) Rodent behavior following a dural inflammation model with anti-CGRP migraine medication treatment. Front Neurol 14:1082176
Lukács M, Haanes KA, Majláth Z, Tajti J, Vécsei L, Warfvinge K, Edvinsson L (2015) Dural administration of inflammatory soup or complete Freund’s adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J Headache Pain 16:564
Oshinsky ML, Gomonchareonsiri S (2007) Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47:1026–1036
Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK (2006) Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 26:5777–5785
Alarcón-Alarcón D, Cabañero D, de Andrés-López J, Nikolaeva-Koleva M, Giorgi S, Fernández-Ballester G, Fernández-Carvajal A, Ferrer-Montiel A (2022) TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat Commun 13:6304
Barcelon E, Chung S, Lee J, Lee SJ (2023) Sexual dimorphism in the mechanism of Pain Central Sensitization. Cells 12
Craft RM (2007) Modulation of pain by estrogens. Pain 132(Suppl 1):S3–s12
Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G (2019) Dural Calcitonin Gene-related peptide produces female-specific responses in Rodent Migraine models. J Neurosci 39:4323–4331
Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, Mecklenburg J, Goffin V, Wajahat N, Price TJ, Akopian AN, Dussor G (2021) Meningeal CGRP-Prolactin Interaction evokes female-specific migraine behavior. Ann Neurol 89:1129–1144
Mason BN, Kallianpur R, Price TJ, Akopian AN, Dussor GO (2022) Prolactin signaling modulates stress-induced behavioral responses in a preclinical mouse model of migraine. Headache 62:11–25
Acknowledgements
Not applicable.
Funding
Turgay Dalkara’s research is funded by the Turkish Academy of Sciences. Some of our studies cited here was funded by the Scientific and Technological Research Council of Turkey to TD (TÜBİTAK, 118S435). Şefik Evren Erdener is supported by Turkish Academy of Sciences Outstanding Young Scientist Award Program (TÜBA-GEBİP).
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting the manuscript. Z.K. prepared Fig. 4. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dalkara, T., Kaya, Z. & Erdener, Ş.E. Unraveling the interplay of neuroinflammatory signaling between parenchymal and meningeal cells in migraine headache. J Headache Pain 25, 124 (2024). https://doi.org/10.1186/s10194-024-01827-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-024-01827-x