Abstract
Background
Neurovascular contact (NVC) is the main cause of primary trigeminal neuralgia (PTN); however, cases of PTN without NVC are still observed. In this study, the Meckel cave (MC) morphology in PTN were analyzed by radiomics and compared to healthy controls (HCs) to explore the cause of PTN.
Methods
We studied the 3.0T MRI data of 115 patients with PTN and 46 HCs. Bilateral MC was modeled using the 3D Slicer software, and the morphological characteristics of MC were analyzed using the radiomics method.
Results
The right side incidence rate in the PTN group was higher than the left side incidence. By analyzing the flatness feature of MC, we observed that the affected side of the PTN was lower than that of the unaffected side, the right MC of the PTN and HC was lower than that of the left MC, the MC of the affected side of the left and right PTN without bilateral NVC was lower than that of the unaffected side.
Conclusions
By providing a method to analyze the morphology of the MC, we found that there is an asymmetry in the morphology of bilateral MC in the PTN and HC groups. It can be inferred that the flatness of the MC may be a cause of PTN.
Similar content being viewed by others
Key points
Flatness of the Meckel cave may be a cause of primary trigeminal neuralgia.
There is asymmetry in the morphology of bilateral Meckel cave of primary trigeminal neuralgia and healthy controls.
The shape features of the Meckel cave can be accurately quantified and analyzed by radiomics method.
Introduction
Trigeminal neuralgia (TN) is a chronic neuropathic pain disorder [1] that can lead to poor quality of life and even suicide [2]. TN can be classified as primary TN (PTN), which is either classical or idiopathic depending on the degree of neurovascular contact (NVC), or as secondary TN, which is caused by pathology rather than NVC [3]. Asymptomatic NVC in patients with PTN [4] and high prevalence of NVC in individuals without TN [5] suggest that NVC is not the only pathogeny of PTN, which is worthy of further study.
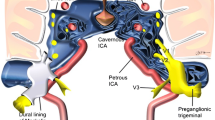
Anatomically, the intracranial trigeminal nerve is divided into the root entry zone (REZ) and the Meckel cave (MC) segments after emerging from the brain stem. Many studies [1,2,3] have proven that morphological changes (atrophy or displacement) with NVC of the trigeminal nerve in the REZ are related to symptomatic PTN. However, no significant correlation has been observed between PTN and NVC without morphological changes. Some unknown etiological factors are likely to play an important role [6]. The MC, an important structure after the REZ of the trigeminal nerve, is hence worth studying.
Previous studies on the trigeminal nerve morphology of PTN have focused on the REZ and lacked emphasis on the MC. The MC is an important part of the trigeminal nerve, which is composed of numerous small fibers and the trigeminal ganglion consisting of a small amount of solid tissue [7]. Some cases studies [8, 9] have reported that ipsilateral hypoplastic MC, which may lead to morphological atrophy, as well as crowded MC, may produce symptoms consistent with TN. Percutaneous balloon compression (PBC) can effectively relieve the pain symptoms of patients with PTN by changing the shape of the MC, which expands after balloon dilation; moreover, the balloon shape is a parameter that has a very strong impact on outcome [10, 11]. Therefore, it can be inferred that the morphological characteristics of the MCs may be related to PTN.
Here, we hypothesized that MCs with narrow morphological characteristics are related to PTN. Magnetic resonance imaging (MRI) provides the best way to evaluate the morphological characteristics of PTN [6], and radiomics can automatically quantify the phenotypic characteristics of medical imaging [12]. The aim of the present study was to collect 3.0T MRI data of patients with PTN and healthy controls (HCs) to accurately model the bilateral MC with the 3D Slicer software and then analyze the MC morphology using radiomics to explore the cause of PTN.
Materials
Data source
The study was approved by the Medical Ethics Committee of the Guangdong Second Provincial General Hospital in Guangzhou, China. Written informed consent was obtained from each subject. From January 2018 to January 2020, 115 PTN patients (mean age: 62.89 ± 10.43 years, 70 female) were recruited from the Department of Neurosurgery of the Second Provincial General Hospital of Guangdong. The PTN diagnosis was based on the Third edition of International Classification of Headache Disorders [13]. Exclusion criteria were secondary TN, other primary headache disorders (e.g., migraine or tension-type headache) and past surgical treatment of PTN (such as microvascular decompression, PBC, and radiofrequency). We also recruited 46 healthy volunteers as HC (mean age: 49.28 ± 10.96 years, 31 female). Table 1 lists the demographics and patient characteristics.
Magnetic resonance imaging
MRI examinations were performed by a Philips Achieva 3.0T dual-source MRI scanner (Philips Healthcare, Amsterdam, The Netherlands). Balanced turbo field echo (BTFE) and enhanced T1 high-resolution isotropic volume excitation (ETHRIVE) sequences were obtained using a fast field echo (FFE) pulse sequence with repetition time (TR) = 25ms, echo time (TE) = 4.1ms, flip angle (FA) = 30°, acquisition matrix = 256 × 256, and slice thickness = 1.0mm. NVC was defined on the analysis of imaging with no perceptible CSF signal intervening between the vascular structure and the cisternal segment of the trigeminal nerve.
Image processing
The dcm2niigui (http://people.cas.sc.edu/rorden/mricron/stats.html) was used to convert the original raw digital imaging and communications (DICOM) in medicine data of head MRA ETHRIVE and BTFE sequences into nifti (Neuroimaging Informatics Technology Initiative) format. Images were registered and fused using Co-register (Estimate & Re-slice) of the SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and opened by the 3D Slicer software (v4.11, https://www.slicer.org/) [14].
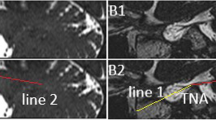
The segment-editor function of the 3D Slicer software was used to model and correct the bilateral MC in BTFE sequence through axial, coronal, and sagittal planes, and then registered ETHRIVE sequence was opened to overlap the BTFE sequence. At last, we observed the MC wall with ETHRIVE sequence, determined the edge of the MC from the axial, coronal, and sagittal planes, and recalibrated the edge of the MC again. (Fig. 1) All MRI studies and the manual plotting process were reviewed and analyzed by an experienced neuroradiologist and a neurosurgeon, who were blinded to the diagnosis and the laterality of the headache. When discrepancies existed, the two assessors reached a consensus for statistical analysis.
Morphological features acquisition
Through the radiomics (revision:3b2c531) plug-in of the 3D Slicer software, we analyzed bilateral 322 MCs and got 13 morphological features of shape [15] including:
1. Voxel volume (using the following formula:
where the volume of the MC Vvoxel is multiplying the number of voxels in the region of interest (ROI) by the volume of a single voxel Vk.),
2. Surface area (using the following formula:
where aibi and aici are edges of the ith triangle in the mesh, formed by vertices ai, bi and ci. To calculate the surface area, first the surface area Ai of each triangle in the mesh is calculated (1). The total surface area is then obtained by taking the sum of all calculated subareas (2).),
-
1.
3. Maximum 3D diameter (is defined as the largest pairwise Euclidean distance between ROI surface mesh vertices. Also known as Feret diameter.),
-
2.
4. Maximum 2D diameter slice (is defined as the largest pairwise Euclidean distance between ROI surface mesh vertices in the row-column (generally the axial) plane.),
-
3.
5. Maximum 2D diameter column (is defined as the largest pairwise Euclidean distance between ROI surface mesh vertices in the row-slice (usually the coronal) plane.),
-
4.
6. Maximum 2D diameter row (is defined as the largest pairwise Euclidean distance between ROI surface mesh vertices in the column-slice (usually the sagittal) plane.),
-
5.
7. Minor axis length (using the following formula:
least axis=\(4\sqrt{{{\uplambda }}_{least}}\)
This feature yield the smallest axis length of the MC-enclosing ellipsoid and is calculated using the largest principal component λleast. In case of a 2D segmentation, this value will be 0.),
8. Major axis length (using the following formula:
major axis=\(4\sqrt{{\uplambda}\text{major}}\)
This feature yield the largest axis length of the ROI-enclosing ellipsoid and is calculated using the largest principal component λmajor.),
9. Least axis length (using the following formula:
least axis=\(4\sqrt{{{\uplambda }}_{least}}\)
This feature yield the smallest axis length of the ROI-enclosing ellipsoid and is calculated using the largest principal component λleast. In case of a 2D segmentation, this value will be 0.),
10. Flatness (using the following formula:
Flatness=\(\sqrt{\frac{{{\uplambda }}_{least}}{{{\uplambda }}_{major}}}\)
Flatness shows the relationship between the largest and smallest principal components in the MC shape. Here, λmajor and λleast are the lengths of the largest and smallest principal component axes. The values range between 1 (non-flat, sphere-like) and 0 (a flat object, or single-slice segmentation).
The model of the MC was independently performed by two neurosurgeons who were proficient in using the 3D Slicer software, and the morphological data were averaged.
Statistical analysis
Chi-test were performed to assess differences in gender; Independent sample T test were performed to assess differences in age; Binomial test was used to test the left and right sides of PTN; Linear regression analysis were used to assess differences in morphological features of the MC (voxel volume, surface area, maximum 3D diameter, maximum 2D diameter slice, maximum 2D diameter column, maximum 2D diameter row, minor axis length, major axis length, least axis length, flatness); Paired sample T test were used to assess differences in morphological features of the bilateral MC of two groups respectively; and Kendall correlation analysis were used to assess correlation in the morphological features of the MC in PTN and HC groups using IBM SPSS Version 22.0 (IBM Corp., Armonk, New York, USA). P < 0.05 was considered statistically significant.
Results
Differences of demographic characteristics and morphological features of the MC between PTN and HC groups
The demographic characteristics of PTN and HC groups and the 10 morphological features of 322 MCs were analyzed. The right side incidence rate in the PTN group was higher than the left side incidence (66.96 % vs. 33.04 %, P < 0.01) (Table 1).
The voxel volume of the MC ranged 94.23 ~ 1589.90mm3, the surface area ranged 131.16 ~ 958.39mm2 (Fig. 2), the maximum 3D diameter ranged 8.42 ~ 19.70mm, the maximum 2D diameter slice ranged 7.28 ~ 16.88mm, the maximum 2D diameter column ranged 7.37 ~ 19.10mm, the maximum 2D diameter row ranged 8.03-19.64mm, the minor axis length ranged 6.50 ~ 12.46mm, the major axis length ranged 7.32 ~ 18.46mm, the least axis length ranged 2.84 ~ 5.98mm, there was no significant difference between PTN and HC groups (Table 2).
Morphology of MC with different morphological characteristics. (A) The red MC’s voxel volume is 94mm3, the yellow is 347mm3, the green is 601mm3, the light blue is 904mm3, the navy blue is 1136mm3 and the purple is 1589mm3.(B) The red MC’s surface area is 131mm2, the yellow is 335mm2, the green is 482mm2, the light blue is 569mm2, the navy blue is 784mm2 and the purple is 853mm2. (C) The red MC’s flatness is 0.25, the yellow is 0.32, the green is 0.37, the light blue is 0.42, the navy blue is 0.47 and the purple is 0.54.(D) The flatness of MC was compared between different groups.**P < 0.01; *P < 0.05
By analyzing the flatness feature of the MC, we found that the flatness of MC ranged from 0.25 ~ 0.54, the affected side of the PTN was lower than that of the unaffected side (P < 0.05), the right MC of HCs and patients with PTN was lower than left MC (P < 0.01). (Fig. 2)(Table 2).
Differences in morphological features of bilateral MC in PTN and HC groups
By analysing the morphological features of MC in the HC, we found that in some features, the left side and right side were significantly different. These differences included the maximum 3D diameter (P < 0.05), maximum 2D diameter column (P < 0.05), least axis length (P < 0.05), and flatness (P < 0.01) (Table 2).
As for the morphological characteristics of MC in PTN, we also found that there were significant differences between the left side and the right side in some features. These differences included the surface area (P < 0.01), maximum 3D diameter (P < 0.01), maximum 2D diameter slice (P < 0.01), maximum 2D diameter column (P < 0.01), maximum 2D diameter row (P < 0.05), minor axis length (P < 0.01), major axis length (P < 0.01), least axis length (P < 0.05), and flatness (P < 0.01) (Table 2).
Correlation analysis of MC Flatness features in PTN and HC groups
The flatness of HC was positively correlated with the least axis length (r = 0.325, P < 0.01) and negatively correlated with the major axis length (r=-0.263, P < 0.01), maximum 3D diameter (r = -0.217, P < 0.01), maximum 2D diameter slice (r = -0.164, P < 0.05), maximum 2D diameter row (r = -0.169, P < 0.05). The most significant correlation was least axis length, followed by major axis length.
The flatness of PTN was positively correlated with the least axis length (r = 0.351, P < 0.01), major axis length (r =-0.238, P < 0.01), and negatively correlated with the maximum 3D diameter (r = -0.210, P < 0.01), maximum 2D diameter slice (r = -0.152, P < 0.01), maximum 2D diameter column (r = -0.109, P < 0.05) and maximum 2D diameter row (r = -0.147, P < 0.05). The most significant correlation was least axis length, followed by major axis length (Fig. 3).
Scatter plot of correlation coefficient of flatness. (A) Scatter plots of the flatness positively correlated with the least axis length in the HC group (r = 0.325, P < 0.01). (B) Scatter plots of the flatness negatively correlated with the major axis length in the HC group (r = -0.263, P < 0.01). (C) Scatter plots of the flatness positively correlated with the least axis length in the TN group (r = 0.351, P < 0.01). (D) Scatter plots of the flatness negatively correlated with the major axis length in the TN group (r = -0.238, P< 0.01).
Differences in morphological features of the MC in PTN group without bilateral NVC
Table 3 summarizes the NVC of PTN group. There were 9 patients with left PTN and 9 patients with right PTN without bilateral NVC. The flatness of MC of the left PTN on the affected side was lower than that of the unaffected side (P < 0.051), the flatness of MC of right PTN on the affected side was lower than that of the unaffected side (P < 0.05). (Table 4)(Fig. 4) Other morphological features were no significant differences.
Comparative analysis of Flatness with bilateral MCs under different NVC conditions. (A) No NVC on both sides. The MC of the affected side of the left PTN without bilateral NVC was lower than that of the unaffected side (0.39 ± 0.04 vs 0.42 ± 0.04, P < 0.051), the MC of the affected side of the right PTN without bilateral NVC was lower than that of the unaffected side (0.35 ± 0.03 vs 0.39 ± 0.04, P < 0.05). (B) NVC on both sides. There was no significant difference between groups. (C) NVC on affected side and no NVC on unaffected side. There was no significant difference between groups. (D) NVC on unaffected side and no NVC on affected side. There was no significant difference between groups.*P < 0.051; ** P < 0.05; ns:P > 0.05; NVC: neurovascular contact;L: Left; R: Right; AS: Affected side; US: Unaffected side.
Discussion
The MC is an important anatomical part of the intracranial trigeminal nerve after the REZ. The MC is the dural space formed by the trigeminal nerve root, which is enclosed by the dural membrane of the posterior fossa in the middle fossa and crosses the rocky ridge; moreover, it contains the trigeminal ganglion and the trigeminal cistern [16]. Morphological changes in the MC are very important in the diagnosis [17,18,19,20,21,22] and treatment [23, 24] of intracranial diseases, such as radiofrequency thermocoagulation and PBC of PTN [25,26,27,28,29]. Ipsilateral hypoplastic MC and crowded MC have been suggested to produce symptoms consistent with TN [8, 9] At present, there is little research on the morphology of MC in patients with PTN and healthy people, and therefore a lack of statistical data comparing the MC morphology between these two groups.
In this study, we introduced the method of MC morphological features extraction based on radiomics. In the identification of MC anatomy by MRI, most studies [8, 17,18,19] have only used one of the T1 or T2 sequences. T1 enhanced sequence is helpful to determine the wall of the MC [30], but lacks the sensitivity of cerebrospinal fluid and nerves in the MC. T2 sequence can distinguish the MC content sensitively but cannot display the wall of the MC clear enough. We used the fusion of ETHRIVE and BTFE sequences of 3.0T MRI to model the MC, which can clearly show not only the structure in the MC but also the MC wall.
Radiomics is an automatic algorithm to quantify the phenotypic characteristics of medical imaging, which can be used after installing PyRadiomics plug-in through the 3D Slicer software [12] and is widely used in medical science research [31,32,33,34]. In this study, we obtained morphological features of the MC by radiomics, which can be divided into two categories. One is the feature that describes the size of the MC, including voxel volume, surface area, maximum 3D diameter, maximum 2D diameter slice, maximum 2D diameter column, maximum 2D diameter row, minor axis length, major axis length, and least axis length. The other feature describes the shape features of the MC, including sphericity and flatness.
The morphological results (voxel volume and surface area) of the MC in this study were significantly higher than those of an autopsy [35], which is due to the loss of MC cerebrospinal fluid in the cadaver head as well as the fixation of the corpse.
The precise acquisition of MC morphological data through the radiomics method is beneficial for the preparation of PBC for PTN. PBC requires the puncture of the MC and filling up of the balloon to compress the semilunar ganglion nerve [36]. The accurate evaluation of the MC volume before PBC can not only provide a reference for the balloon filling volume but also prepare appropriate balloon volume for some special MC volumes. (The maximum volume of MC in this study was 1589.90 mm3.)
The shape analysis of the MC is difficult to quantify and is generally described as an ellipse or open-ended three-fingered glove [37]. By contrast, the flatness was obtained in this study using radiomics and was accurately quantified and analyzed.
In the HC group, the maximum 3D diameter and maximum 2D diameter column of the right MC were greater than those of the left MC (P < 0.05), whereas the least axis length and flatness were less than those of the left MC (P < 0.05). In the PTN group, the surface area, maximum 3D diameter, maximum 2D diameter slice, maximum 2D diameter column, maximum 2D diameter row, minor axis length, and major axis length of the right MC were greater than those of the left MC (P < 0.05), while the least axis length and flatness were less than those of the left MC (P < 0.05), suggesting that the shape of the bilateral MC was asymmetric. The MC on the right side was flatter and larger than that on the left side, especially in the PTN group. To our knowledge, the bilateral asymmetry of the MC has been rarely described in the literature among healthy people and patients with PTN. There is an asymmetry between the two hemispheres of the human brain, including the cortical projection area associated with the trigeminal nerve, such as the posterior central gyrus, cingulate gyrus, and paracentral gyrus [38,39,40]. Whether the asymmetry of the MC shape is related to the volume or thickness of the cerebral cortex deserves further study.
The complicated pathogenesis of PTN has always been a focus and is unclear, to a large extent, in medical knowledge. Although the most common pathogenic mechanism is NVC in the REZ [41], asymptomatic vessel-nerve contact is present in 92 % of patients, and there is no NVC in 3.1–17 % of PTN patients [4]. Therefore, this theory does not apply to all the cases [42]. A study [43] reported 6 cases having whiplash-associated disorder with specific symptoms of trigeminal neuralgia-like pain, and some cases had hyperesthesia. Therefore, it may be classified as painful post-traumatic trigeminal neuropathy rather than PTN based on the third edition of the International Classification of Headache Disorders [13].
By analyzing the flatness features of the MC, we found that the affected side of the PTN was lower than that of the unaffected side (P < 0.05), and that the affected MC side of the left PTN without bilateral NVC was lower than that of the unaffected side (P < 0.051). Moreover, the affected MC side of the right PTN without bilateral NVC was lower than that of the unaffected side (P < 0.05). These results suggest that flatness was still associated with PTN even after NVC exclusion. Further analysis found that the right MC of HCs and patients with PTN was lower than that of the left MC (P < 0.01) and that the prevalence of PTN on the right side was higher than that on the left side (P < 0.01), which was consistent with the literature [2, 44]. Our current study suggests that this finding is more than a mere chance association, and that the flatness of the MC may be associated with PTN, which is likely to explain the high prevalence of the right PTN.
Based on the results of this study, we found that the right MC is larger and flatter than the left MC, which may lead to increased density or compression of the trigeminal nerve at the right MC. This compression of the trigeminal nerve can lead to PTN. Ipsilateral MC dysplasia may produce symptoms consistent with TN [8] and crowded MC may also lead to TN [9] Therefore, we believe that the flatness of the MC may be a cause of PTN.
The limitation of this study is that the P-value of the MC flatness results on the affected MC side of the left PTN without bilateral NVC was 0.0509. Despite being very close, it did not reach the statistical level of significance (P < 0.05), which was related to the insufficient sample size of this study. In addition, the mean ages of the PTN and HC groups had a significant difference, which was related to the age of onset of TN and the small number of individuals in the HC group. Further research will expand the sample size of the HC group and select the corresponding ages for comparison. We also only analyzed the morphological characteristics of the MC without further analysis of the trigeminal nerve in the MC. The next step is to increase the number of samples and analyze the trigeminal nerve components in the MC to explore the etiology of PTN.
Conclusions
By providing a method to analyze the morphology of the MC, we found that there is an asymmetry in the morphology of bilateral MC in the PTN and HC groups. It can be inferred that the flatness of the MC may be a cause of PTN.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- HC:
-
healthy controls
- MRI:
-
magnetic resonance imaging
- MC:
-
Meckel cave
- NVC:
-
neurovascular contact
- PBC:
-
percutaneous balloon compression
- PTN:
-
primary trigeminal neuralgia
- REZ:
-
root entry zone
- TN:
-
trigeminal neuralgia
- BTFE:
-
balanced turbo field echo
- ETHRIVE:
-
enhanced T1 high-resolution isotropic volume excitation
- ROI:
-
Region of interest
References
Bendtsen L, Zakrzewska JM, Heinskou TB et al (2020) Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 19(9):784–796
Cruccu G, Di Stefano G, Truini A (2020) Trigeminal Neuralgia. N Engl J Med 383(8):754–762
EAN guideline on trigeminal neuralgia. European Journal of Neurology. 2019
Peker S, Dinçer A, Necmettin Pamir M (2009) Vascular compression of the trigeminal nerve is a frequent finding in asymptomatic individuals: 3-T M R imaging of 200 trigeminal nerves using 3D CISS sequences. Acta Neurochir (Wien) 151(9):1081–1088
Selçuk P, Özlem K, İbrahim Ü, Necmettin PM (2006) Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2:354–9
Bendtsen L, Zakrzewska JM, Abbott J (2019) al. e. European Academy of Neurology guideline on trigeminal neuralgia. European journal of neurology 26:831–849
Rubinstein D, Stears R, Stears J (1994) Trigeminal nerve and ganglion in the Meckel cave: appearance at CT and MR imaging. Radiology 193(1):155–159
Cleary DR, Handwerker J, Ansari H, Ben-Haim S (2019) Three Cases of Trigeminal Neuralgia with Radiographic Absence of Meckel’s Cave. Stereotact Funct Neurosurg 97(4):249–254
Panda A, Lehman V, Garza I, Diehn F. Trigeminal Neuralgia Due to Meckel′s Cave Crowding in the Setting of Possible Idiopathic Intracranial Hypertension without Papilledema. Annals of Indian Academy of Neurology. 2020
Konstantinos K, Gastón S, Göran L, Jaleh W, Bengt L (2010) Factors that influence outcome of percutaneous balloon compression in the treatment of trigeminal neuralgia. Neurosurgery. 4:925–34
Scranton RA, Shah K, Cohen-Gadol AA (2019) Alternative customized instrumentation and technique for percutaneous balloon compression rhizotomy for trigeminal neuralgia. J Neurosurg 132(6):1–4
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 77(21):e104–e107
Headache Classification Committee of the International Headache Society (IHS) The International Class ification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211
Lin J, Zhang Y, Peng R et al (2019) Preoperative imaging and microscopic navigation during surgery can avoid unnecessarily opening the mastoid air cells through craniotomy using the retrosigmoid approach. World Neurosurgery 121:e15–e21
Zwanenburg A, Leger S, Vallières M, Lck S. Image biomarker standardisation initiative. arXiv eprints. 2016:arXIv:1612.07003
Rigante L, Herlan S, Tatagiba MS, Stanojevic M, Hirt B, Ebner FH (2016) Petrosectomy and Topographical Anatomy in Traditional Kawase and Posterior Intradural Petrous Apicect omy (PIPA) Approach: An Anatomical Study. World Neurosurg 86:93–102
Wright J, Wycoco V (2017) Asymmetric Meckel cave enlargement: a potential marker of PHACES syndrome. American Journal of Neuroradiology 38(6):1223–1227
Kamali A, Sullivan K, Rahmani F et al (2020) Indentation and Transverse Diameter of the Meckel Cave: Imaging Markers to Diagnose Idiopathic Intracranial Hypertension. American Journal of Neuroradiology 41(8):1487–1494
Aaron GP, Illing E, Lambertsen Z et al (2017) Enlargement of Meckel’s cave in patients with spontaneous cerebrospinal fluid leaks. Int Forum Allergy Rhinol 7(4):421–424
Moisak GI, Matsko DE, Chernov SV et al (2018) What Tactics Should a Surgeon Choose to Treat a Black Extracerebral Tumor? A Case Report of Psammomatous Melanotic Schwannoma of Meckel’s Cave and Literature Review. World neurosurgery 116:337–342
Taghipour M, Saffarrian A, Ghaffarpasand F, Azarpira N (2017) Dumbbell-Shape Hydatid Cyst of Meckel Cave Extending to Cerebellopontine Angle and Middle Fossa; Surgical Technique and Outcome of Rare Case. World neurosurgery 104:1049. e7-1049. e10
Strub GM, Moore JE, Huang AT, Stevenson AW, Reiter ER (2013) Chronic facial pain and Meckel cave masses as the initial presentation of neurosarcoidosis: a case report. ENT: Ear Nose Throat Journal 92(12):558–565
Truong HQ, Sun X, Celtikci E et al (2018) Endoscopic anterior transmaxillary “transalisphenoid” approach to Meckel’s cave and the middle crania l fossa: an anatomical study and clinical application. J Neurosurg 130(1):227–237
Hughes JD, Kapurch J, Van Gompel JJ et al (2018) Diagnosis and Outcome of Biopsies of Indeterminate Lesions of the Cavernous Sinus and Meckel’s Cave: A Retrospective Case Series in 85 Patients. Neurosurgery 83(3):529–539
Bond JD, Xu Z, Zhang H, Zhang M (2021) Meckel’s Cave and Somatotopy of the Trigeminal Ganglion. World Neurosurg 148:178–187
Linjingzhi Z (2017) Penruoyu, et al. Double targets percutaneous radiofrequency thermocoagulation in treatment of primary trigeminal neuralgia. Chinese Journal of Neuromedicine 5(16):508–512
Linjingzhi W (2014) Luoweishi, et al. Cardiovascular responses during stereotactic assisted percutaneous radiofrequency thermocoagulation therapy for primary trigeminal neuralgia. Chinese Journal of Neurosurgical Disease Research 13(1):67–71
Linjingzhi Z, Huangkaimin, Zhangyang Z (2015) Clinical research of eight areas of trigeminal nerves with electrical slimalation. Chinese Journal of Neuromedicine 14(012):1271–1274
Lin J, Zhang y, Lu J et al (2020) Near field potential of trigeminal nerve entering pontine area. Guangdong Medical Journal 14(41):1432–1436
Tang Z, Feng X, Qian W, Song J (2011) Evaluation of magnetic resonance imaging criteria for Meckel’s cave lesion: logistic regression analy sis and correlation with surgical findings. Clin Imaging 35(5):329–335
Bailly C, Bodet-Milin C, Bourgeois M et al. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers (Basel). 2019;11(9)
Huang YQ, Liang CH, He L et al (2016) Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastas is in Colorectal Cancer. J Clin Oncol 34(18):2157–2164
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762
Sun R, Limkin EJ, Vakalopoulou M et al (2018) A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 19(9):1180–1191
Sabanc PA, Batay F, Civelek E et al (2011) Meckel’s cave. World Neurosurg 76(3–4):335 –335 41;
Sterman-Neto H, Fukuda CY, Duarte KP et al (2021) Balloon compression vs radiofrequency for primary trigeminal neuralgia: a randomized, controlled tria l. Pain 162(3):919–929
Malhotra A, Tu L, Kalra VB et al (2018) Neuroimaging of Meckel’s cave in normal and disease conditions. Insights Imaging 9(4):499–510
Liang X, Zhao C, Jin X et al (2021) Sex-related human brain asymmetry in hemispheric functional gradients. NeuroImage 229:117761
Shuwei L, Yunxia L, Yuchun T (2010) The construction, asymmetry and genetic correlation of 4D digital brain atlas. JOURNAL OF SHANDONG UNIVERSITY(HEALTH SCIENCES) 51(1):33–41
Guadalupe T, Kong X-Z, Akkermans SE, Fisher SE, Francks C (2021) Relations between hemispheric asymmetries of grey matter and auditory processing of spoken syllables in 281 healthy adults. Brain Struc Funct. 4:1–12
Love S, Coakham HB (2001) Trigeminal neuralgia: pathology and pathogenesis. Brain 124(Pt 12):2347–2360
Revuelta-Guti¨¦rrez R, L¨®pez-Gonz¨¢lez MA, Soto-Hern¨¢ndez JL (2006) Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surg Neurol 66(1):32–36
Samim F, Epstein JB (2019) Orofacial Neuralgia Following Whiplash-Associated Trauma: Case Reports and Literature Review. SN Comprehensive Clinical Medicine 1(4):627–632
Giorgio L, Khadija R, Emer O et al (2020) Trigeminal neurovascular contact in SUNCT and SUNA: a cross-sectional magnetic resonance study. Brain 143(12):3619–3628
Acknowledgements
None.
Funding
Yiquan Ke received funding from the Natural Science Foundation of China (No. 81772651).
Jinzhi Lin received funding from the Medical Scientific Research Foundation of Guangdong Province (No. A2019399).
Author information
Authors and Affiliations
Contributions
Jinzhi Lin: data collection, data analysis and interpretation, writing and revising the manuscript. Yong Zhang, Wuming Li, Jianhao Yan: data collection, writing and revising the manuscript. Yiquan Ke: drafting of the study, writing and revising the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of the Guangdong Second Provincial General Hospital in Guangzhou, China. Written informed consent was obtained from each subject.
Consent for publication
The scientific guarantor of this publication is Yiquan Ke.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, J., Zhang, Y., Li, W. et al. Flatness of the Meckel cave may cause primary trigeminal neuralgia: a radiomics-based study. J Headache Pain 22, 104 (2021). https://doi.org/10.1186/s10194-021-01317-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-021-01317-4