Abstract
Among the ecological roles that sponges play in marine ecosystems, one of the highlights is their ability to host a wide diversity and abundance of epibenthic organisms. However, of the different marine environments, this role has been less investigated in seagrass-dwelling sponges. In this study, the main objective was to determine whether the structure of the associated faunal assemblages in two common sympatric species of seagrass-dwelling sponges (Amorphinopsis atlantica and Haliclona implexiformis) vary depending on the volume and morphology of the host sponge as well as the environment to which both sponges are exposed. Even though the collection sites had the same habitat type (seagrass meadows composed by Thalassia testudinum and Halodule wrightii) and depth, there were substantial differences in faunal composition (ANOSIM test, R = 0.86) between both sponge species. The value of the data on species richness, diversity, and abundance of associated organisms was significantly higher in the individuals of A. atlantica than in those of H. implexiformis. These differences in the community structure of associated fauna could be influenced by the differential growth form of the hosts (e.g. growth form and oscula diameter) as well as their distinct environmental preferences (sites with different degrees of exposure to wind-generated waves and levels of human disturbance). This study contributes to the knowledge on the functional role that sponges play in seagrass meadows, one of the world’s most endangered ecosystems. Furthermore, it underlines the importance of examining both, the sponge morphology and the local environmental conditions, to explain spatial variations in the macrofaunal assemblages associated with sponges.
Similar content being viewed by others
Background
Sponges are considered the oldest living animal phylum [1] and are well known to play important ecological functions in marine benthic ecosystems [2]. One of these, is its capability to establish interspecific relationships with a wide diversity of organisms (including species of flora and fauna and micro- and macro-organisms) [3]. Given its complex three-dimensional morphology, presence of internal channels and oscular openings that interconnect with the external environment, sponges can host a great diversity and abundance of epi and endobionts [4,5,6,7,8,9], trait for which they have been regarded as “living hotels” [4] and one of the richest marine benthic biotopes [7].
Furthermore, it has also been determined that composition of sponge-associated fauna can vary among sponge species or, even, between individuals of the same species. This variability may depend on many factors, including the sponge size [4, 10], morphology and anatomy [6,7,8], geographic location [5, 9], depth [11], seasonality [12], and the habitat where they live [13, 14]. Regarding the latter, recent research has found that the composition and abundance of sponge-associated fauna can vary (even on a small spatial scale) between individuals of the same sponge species (e.g. in Halichondria melanadocia) depending on the habitat [13]. This inter-habitat variability has been attributed to different factors such as the substrate type and orientation and the different degree of exposure to factors such as light, sedimentation, predation and the surrounding fauna, as well as to the different morphology that the sponge develop in each habitat [14]. However, despite the efforts made in this field, there are relatively few studies addressing the environmental condition effects on the structure of the sponge-associated faunal assemblages.
In the Mexican coasts of the Gulf of Mexico (Tropical Western Atlantic) there are extensive seagrass meadows [15], in which the biodiversity of associated invertebrates has still been poorly investigated [16, 17]. Particularly, within the estuarine system of Laguna de Terminos, located west of the Yucatan peninsula, there are shallow seagrass meadows (Thalassia testudinum, Syringodium filiforme and Halodule wrigtii) whose permanence has been threatened by both, natural (e.g. tropical storms and coastal erosion) and human-induced stress (e.g. oil industry spills, urban development and overfishing) [18,19,20]. Associated to these habitats, the sponge species Haliclona (Reniera) implexiformis (Hechtel 1965) (Haplosclerida: Chalinidae) and Amorphinopsis atlantica Carvalho, Hajdu, Mothes & van Soest, 2004 (Halichondrida: Halichondriidae) are commonly found living in coexistence. However, according to preliminary observations they seem to have distinct environmental preferences. Whereas A. atlantica is more frequent in areas relatively exposed to wind-generated waves and closer to the urban area (with a high degree of human disturbance), H. implexiformis is more frequent in more protected areas and further away from the urban zone. Therefore, since these species appear to have different morphological characteristics and environmental preferences, it is expected to find differences in their associated faunal assemblages.
Therefore, the aims of this study were (i) to determine and compare the species richness, species diversity and abundance of macrofauna (including epi- and endo-bionts) associated with these sponge species in a tropical estuarine system of the southern Gulf of Mexico and (ii) to determine whether these ecological parameters vary as a function of the sponge size. Furthermore, given that these sponges seem to show different environmental preferences in the study area, the possible influence of environmental conditions on the structure of the associated faunal assemblages was discussed.
Materials and methods
Study area and specimen’s collection
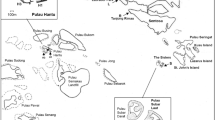
This study was conducted in the estuarine system of the Laguna de Terminos Flora and Fauna Protection Area, Campeche, Mexico (at the southern Gulf of Mexico) (Fig. 1). Within this system, two collection sites were chosen where the sponges A. atlantica and H. implexiformis are commonly found (Fig. 2). These sites correspond to shallow (0.5–1.0 m depth) seagrass meadows (composed by a mix of Thalassia testudinum Banks ex König, 1805 and Halodule wrightii Ascherson, 1868) located in the inner part of Isla del Carmen. The collection site for A. atlantica (18°38’24.07"N, 91°47’49.86"W) was relatively close (at about 100 m of distance) to the urban area of Ciudad del Carmen, and given its location on the coastline, this site was regarded as the exposed site (ES). The collection site for H. implexiformis (18°44’31.62"N, 91°32’13.66"W) was located 27 km from the urban area and due it was located in an area relatively less exposed to waves it was named as protected site (PS).
A total of 10 specimens of each sponge species were collected by snorkeling in April 2019. Before collection, sponges were covered with a plastic bag to prevent escape of the mobile fauna. In the laboratory, the osculum diameter (mm) was measured (10 oscula per individual of each sponge species) with a ruler and the volume (mL) of each sponge was measured by the volumetric displacement method [21]. The individuals were dissected into small pieces under a stereomicroscope (Stemi 305, Carl Zeiss Microscopy GmbH) to extract all the associated macroinvertebrates that were present. In the same way, the seawater in the bags was filtered in a sieve (mesh size of 0.5 mm) to recover any macroinvertebrates that might have detached from the sponge during transport [9]. The organisms were fixed with 4 % formaldehyde 24 h before being transferred to 70% alcohol for preservation. The macroinvertebrates were identified to the lowest taxonomic level possible using specialized faunal guides for the region [22,23,24,25,26,27,28]. Then, individuals of each species were counted, and the abundance was expressed as the total number of individuals per sponge, and the density as the average (± SD) number of individuals per liter of sponge tissue. The species richness was expressed as the total number of species (or taxa) per sponge individual [14].
Environmental parameters
In order to characterize the environmental conditions at the two collection sites, temperature (ºC), salinity, dissolved oxygen (mg/L), total chlorophyll concentration (µg/L), total suspended solids in the water column (mg/L), transparency (m) and sedimentation rate (g of dry weight/m2/day) were measured.
Temperature, salinity, dissolved oxygen, and total chlorophyll concentration were directly measured with a YSI-model eXO2 (Multiparameter Sonde). Total suspended solids (TSS) was determined by filtering (using 1.5 µm pore opening fiberglass filters) of a known volume of water and drying at 60 °C in a stove for 72 h (see detailed method in [29]). Transparency of the water column was measured using a Secchi disc. Sedimentation rate was measured at each site with a trap system consisting of four sets of plastic bottles (height 23 cm, internal diameter 2.2 cm, and ratio height/diameter 10.4/1), whose opening was vertically positioned at 30 cm from the bottom. Sediment traps remained for 30 days before being taken to the laboratory. The trapped material was repeatedly rinsed with distilled water to remove salts and was dried at 70 °C for 48 h before being weighed (dry weight, g) (see the detailed method in [30]).
Water motion was estimated using the plaster dissolution method, which measures loss in weight of plaster spheres during a determined period (see details in [31]). At each site, four plaster spheres 5 cm in diameter were placed at about 20 cm above the bottom over 20 days [32]. It has been suggested that loss of mass of spheres is independent of flow direction and speed fluctuations [33] but is linearly related with flow speed [34,35,36] and water temperature. The water motion was expressed as the average (± SE) dissolution rate (%d− 1) at each site.
Data analyses
The normality and homoscedasticity of the data (abundance, density and species richness of associated fauna and environmental parameters) were examined using the Shapiro-Wilk and Levene’s tests, respectively. To compare the sponge volume, oscula diameter, abundance, density, and species richness of associated fauna between both sponge species the Mann–Whitney U test was applied. To determine whether data of abundance and species richness of associated macroinvertebrates vary as a function of the sponge volume (for each sponge species), Spearman rank correlations were performed. These analyses were performed using Statistica 6.0 software. The Shannon–Wiener diversity index (H’) was calculated to determine the specific diversity in each of the individuals of A. atlantica and H. implexiformis that were collected. This index is expressed as a positive number, and in most natural ecosystems it varies between 0.5 and 5.0, although its normal value is between 2 and 3. Values below 2 are considered low in diversity and above 3 are high in species diversity. Taxonomic homogenization was analyzed by partitioning β-diversity into spatial turnover of species composition and nestedness. Total beta diversity, turnover and nestedness were calculated from the abundance data with the beta.pair function using the Sorensen index [37]. The function beta.pair computes 3 distance matrices accounting for the (i) turnover (replacement), (ii) nestedness-resultant component, and (iii) total dissimilarity (i.e. the sum of both components). The spatial turnover was measured as Simpson pair-wise dissimilarity, while the nestedness-resultant dissimilarity, was measured as the nestedness-fraction of Sorensen pair-wise dissimilarity. This method estimates the total dissimilarity as Sorensen pair-wise dissimilarity (a monotonic transformation of beta diversity). These calculations were performed in R 4-0.3 [38].
A non-metric multi-dimensional scaling (nMDS) analysis, coupled with an analysis of similarities (ANOSIM) test, was conducted to examine differences in the sponge-associated macrofaunal assemblages between both sponge hosts. For these analyses, a Bray-Curtis similarity matrix was created based on the abundance data (number of individuals per sponge) of species/taxa recorded [39]. These multivariate analyses were made using the Primer 6.0 program (Plymouth Marine Laboratory, UK).
Results
Environmental parameters
The data of the environmental parameters measured in both sites are shown in the Table 1. The following parameters were significantly higher in ES: sedimentation rate (Mann-Whitney U test, p < 0.05), water motion (Mann–Whitney U test, p < 0.05) and SST (Student t-test, p < 0.01). Whereas in PS: the DO and water transparency (Student t-test, p < 0.01, in both cases). Chlorophylls did not vary significantly between sites (Mann-Whitney U test, p > 0.05) as did temperature and salinity.
Volume and associated fauna in the two sponge hosts
The average osculum diameter in A. atlantica (3 ± 0.8 mm) was significantly lower (Mann–Whitney U test, p < 0.01) than in H. implexiformis (6 ± 1.5 mm). The average volume of A. atlantica individuals (344 ± 101 mL) was significantly higher (Mann–Whitney U test, p < 0.05) than that of H. implexiformis (128.5 ± 62.1 mL). In A. atlantica, a total of 1,410 macrofaunal organisms (equivalent to 914 individuals/L of sponge) were found, which were divided into 55 different taxa (Fig. 3). In this sponge, arthropods were the most abundant group (amphipods of family Gammaridae = 32.33 % and tanaidaceans of family Leptocheliidae = 15.46 %) followed by polychaetes (family Terebellidae = 5.60%) and echinoderms (represented by the species Ophiactis savignyi) (5.46%) (Fig. 4). Regarding H. implexiformis, a total of 202 macrofaunal organisms (or 441.70 individuals/L of sponge) were found, which were divided into 25 different taxa (Fig. 3). Polychaetes of family Syllidae were the most abundant (40.59 %) in this sponge species, followed by arthropods with family Gammaridae (19.31 %) and echinoderms with the species O. savignyi (9.90%). The complete list of species/taxa groups found in each sponge is shown in Table 2.
The abundance and species richness of associated fauna were significantly higher in A. atlantica than in H. implexiformis (Mann–Whitney U test, p < 0.01, in both cases). However, when we compared the density of associated organisms (sponge ind/L) as a standard measure, independent on the sponge size, no significant differences were found (Mann-Whitney U test, p > 0.05) between both sponge species. This result indicates that, regardless of the sponge size, the concentration of the associated fauna (epi- and endo-bionts) was similar between the examined individuals of both sponge species.
Diversity and similarity between the sponge‐associated faunal assemblages
In the individuals of A. atlantica, the Shannon-Wiener diversity index (H’) ranged from 1.59 to 2.80 and was on average (± SD) (2.28 ± 0.36) higher than that of H. implexiformis (0.76–1.65, average = 1.32 ± 0.27). Regarding the overall beta diversity (βSOR) of associated macrofaunal assemblages (including that of both sponge species) it was of 0.88. This overall diversity was better explained by spatial species turnover (βSIM = 0.77) than by changes in richness related to nestedness (βSNE = 0.11).
According to the nMDS analysis, the twenty sponge samples examined were separated in two main groups (2D Stress = 0.13, Similarity = 20%), which were significantly different (ANOSIM, Global R = 0.862, p < 0.05) (Fig. 5). In group A were all the samples of A. atlantica and in group B all those of H. implexiformis.
Spearman rank order correlations
In both sponge species, there was no significant correlation between the volume and the density of associated fauna (A. atlantica, ρ = –0.88, p > 0.05, n = 10; H. implexiformis, ρ = –0.69, p > 0.05, n = 10). Only in H. implexiformis, the volume of individuals correlated positively (ρ = 0.76, p < 0.01, n = 10) with the Shannon-Wiener diversity index and the abundance with the species richness (ρ = 0.81, p < 0.01, n = 10).
Discussion
The abundance and species richness reported here in A. atlantica and H. implexiformis were within the range of that found in other sponge species. For example, in seven sponge species from the Aegean Sea (such as Geodia cydonium, Agelas oroides, Petrosia ficiformis, Spongia officinalis, Ircinia fascculata, I. muscarum and Verongia aerophoba), the abundance of associated macrofauna ranged from 312 to 3838 individuals/sponge and the species richness from 61 to 151 species/sponge [6]. Also, in the sponge Halichondria (Halichondria) melanadocia (from the southern the Gulf of Mexico) a total of 3272 individuals of macroinvertebrates were found in 66 sponge individuals, belonging to 40 taxonomic groups [14]. The Shannon diversity index (H’) values recorded here in both sponge species were also within the range of that reported in other sponge species (e.g. Mycale microsigmatosa = 3.0, [9]; Ircinia strobilina = 4.5, I. felix = 3.5 and Aplysina fistularis = 0.3, [40]; Aplysina archeri = 1.14 and A. lacunosa = 1.47, [41].
On the other hand, although there were 15 shared species between both sponge species (from a total of 65 species), multivariate analyzes showed that these associated faunal assemblages were significantly different. In addition, the beta diversity analysis revealed that the differences recorded in the species richness between both host sponges species were a reflection of spatial turnover, i.e., the associated assemblage of a sponge species was not a subset of the other sponge species [42].
It is well known that morphological characteristics of sponges may partly explain the inter and intra-species variations in the structure of the associated assemblages [6, 13, 43]. In this regard, despite the morphological differences that exist between the examined individuals of H. implexiformis (massive-branched form with several tube-like oscular projections) and A. atlantica (massive-encrusting form with a wide basal area and small oscula), a complexity index or surface area was not determined to relate it with the differences found in the sponge-associated faunal assemblages [6]. Among the morphological characteristics of sponges, the osculum size has also been regarded as good predictor of abundance and richness of associated organisms, i.e., the larger oscula can provide them with greater shelter and protection from predation [3, 11, 44]. The size of these openings can also define the size of the endobionts that enter the sponge, and consequently, influence in the structure of the sponge-associated assemblages [45]. In this case, although A. atlantica had smaller oscula than H. implexiformis, it had the greatest species richness and abundance. This exception to the rule has also been reported previously in other sponge species [46], and in this study the oscula size can help explain the size of the dominant associated group. That is, the dominant group in H. implexiformis were the polychaetes, whose size range (0.1–2.3 mm diameter) was relatively larger than the dominant organisms found in A. atlantica (amphipods, 1–5 mm diameter). In this study we did not measure the sponge canal volume and diameter, two factors known to influence macrofaunal abundance and species diversity in sponges [47].
Moreover, the relationship between the sponge volume and the abundance of its associated fauna has been positive for many sponge species, and can be explained by the fact that that larger sponges usually have more space for associated organisms than smaller ones [41, 48]. However, in this study the volume of both sponge species was not correlated with the density of their associated fauna. This lack of correlation is consistent with that found in other species such as Verongia aerophoba from the North Aegean Sea [49]. Only for H. implexiformis, the volume of individuals correlated positively with the Shannon-Wiener diversity index. The positive relationship between the sponge volume and the diversity index (H’) of associated organisms has also been documented in other sponge species (e.g. Sarcotragas muscarum Schmidt, 1864 and its associated-polychaete assemblages) [50] and could be related with the Theory of Island Biogeography [51]. In H. implexiformis, the positive relationship recorded between abundance and species richness of the associated fauna may be indicative of a high degree of equality of the abundance distribution of species associated with this sponge species.
In addition to the influence of host morphology, this study also provides evidence about the possible environmental influence on the differences found in the structure of the associated faunal assemblage between both sponge species. For example, the different degree of exposure to wind-generated waves that the sites had could also have played an important role in the differences found in the composition, richness, and abundance of sponge-associated fauna. In this regard, a previous study conducted in the same study area revealed significant variations in the abundance, richness, and composition of the seagrass-dwelling macroinvertebrates between sites with different exposure degree to wind-generated waves [17]. Despite being the same habitat and depth in both sites, the collection site of A. atlantica was the most exposed and physical parameters such as hydrodynamism, sedimentation rate and TSS were significantly higher than in the H. implexiformis collection site. Here, it is also important to mention that most of the A. atlantica individuals were found half-buried, which may be product of the greater sedimentation regime registered in that site. This closeness of A. atlantica to the sea-floor could provide a greater possibility for inhabitants of this microhabitat (soft sediments) to find and to live inside the sponge, as has been suggested in other sponge species (e.g. Mycale [Zygomycale] parishii [Bowerbank, 1875]) [44].
It is also important to mention that part of the fauna associated with these sponges (20 % of the recorded here in A. atlantica and 16 % of that recorded in H. implexiformis) had already been previously reported in the surrounding seagrasses of the study area [17]. Therefore, the differences in the associated fauna with these two sponge species could also be due, in part, to the spatial variability of epibenthic assemblages of the surrounding habitat [17].
Another difference between the sites, in addition to the degree of exposure to waves, was the relative proximity to the urban area. The A. atlantica collection site is an area with relatively high levels of human disturbance, as it is often affected by garbage accumulation, untreated urban wastewater discharges, as well as by wastewater from the adjacent local slaughterhouse (E. Ávila pers. com.). Although little is known about the effect of environmental pollution on the fauna associated with sponges [9], the possibility that inter-site differences in environmental quality may have influenced the variability recorded in the assemblages associated with these two species of sponges is not ruled out. In a study conducted on the sponge Mycale microsigmatosa Arndt, 1927 (from Rio de Janeiro State, SE Brazil), where collection sites with different environmental quality were included (one of them under strong influence of polluted waters by domestic wastes, oil, and heavy metals) and no differences were found in the community structure of their associated fauna [9]. Therefore, to verify this effect more studies are necessary where other environmental parameters such as chlorophyll concentration, biochemical oxygen demand and organic matter content on the water column and sediments.
Finally, due to individuals of both sponge species differ in morphology and that they were collected from different sites, there is potential interference between these factors to explain the variability found in the assemblages of associated fauna, i.e., the effect of site and the species morphology. As mentioned before, several studies have supported both factors as responsible for the variability of the associated biota [5, 7, 14, 46]. However, in this case we consider that the effect of the site may be more important, since according to previous studies, the organisms that live in association with a particular sponge species come mainly from the surrounding benthic assemblage [13, 14, 46]. The fact that both sponge species from this study shared a considerable proportion of associated macrofauna with the surrounding habitat support the importance of site and the local environmental conditions.
Conclusions
In this study the ecological role of two common seagrass-dwelling sponge species (Amorphinopsis atlantica and Haliclona implexiformis) as hosts for other marine organisms was examined in a tropical coastal lagoon of the southern Gulf of Mexico. In both sponge species the data of abundance, species richness and diversity of associated macroinvertebrates were within the range reported for other sponge species from other regions of the world. It was also found that these assemblage descriptors of the associated fauna varied between both species of sponges, being significantly higher in A. atlantica, a species that inhabits in a relatively more exposed site (to wind-generated winds and levels of human disturbance). However, due to the fact that each of the sponges inhabit a different environment and that they show morphological differences (e.g. growth form and oscula diameter) it was impossible to imply causality or even a correlation between the parameters. Differences in their assemblage structure should be addressed through a reciprocal transplant experiment, which will allow us to examine the changes in species composition due to an induced change in environmental conditions. The data generated in this study may also serve as a baseline for future studies focused on the conservation and ecological restoration of habitats of seagrass meadows in the study area.
Availability of data and materials
The data sets used in this study are available from the corresponding author on reasonable request.
References
Antcliffe JB, Callow RH, Brasier MD. Giving the early fossil record of sponges a squeeze. Biol Rev. 2014;89(4):972–1004.
Bell JJ. The functional roles of marine sponges. Estuar Coast Shelf Sci. 2008;79(3):341–53.
Wulff JL. Ecological interactions of marine sponges. Can J Zool. 2006;84(2):146–66.
Pearse AS. Inhabitants of certain sponges at Dry Tortugas. Publ Carnegie Inst Wash. 1932;435:123–4.
Pearse AS. Notes on the inhabitants of certain sponges at Bimini. Ecology. 1950;31(1):149.
Koukouras A, Voultsiadou-Koukoura E, Chintiroglou H, Dounos C. Benthic bionomy of the north Aegean Sea. III A comparison of the macrobenthic animal assemblages associated with seven sponge species. Cah Biol Mar. 1985;26:301–19.
Klitgaard AB. The fauna associated with outer shelf and upper slope sponges (Porifera, Demospongiae) at the Faroe Islands, northeastern Atlantic. Sarsia. 1995;80(1):1–22.
Neves G, Omena E. Influence of sponge morphology on the composition of the polychaete associated fauna from Rocas Atoll, northeast Brazil. Coral Reefs. 2003;22(2):123–9.
Ribeiro SM, Muricy G, Omena EP. Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar Coast Shelf Sci. 2003;57(5):951–9.
Frith DW. Animals associated with sponges at North Hayling, Hampshire. Zool J Linn Soc. 1976;58(4):353–62.
Peattie ME, Hoare R. The sublittoral ecology of the Menai Strait: II. The sponge Halichondria panicea (Pallas) and its associated fauna. Estuar Coast Shelf Sci. 1981;13(6):621–35.
Cuartas EI, Excoffon AC. La fauna acompañante de Hymeniacidon sanguinea (Grant, 1827) (Porifera: Demospongiae). Neotropica. 1993;39(101–102):3–10.
Ávila E, Ortega-Bastida AL. Influence of habitat and host morphology on macrofaunal assemblages associated with the sponge Halichondria melanadocia in an estuarine system of the southern Gulf of Mexico. Mar Ecol. 2015;36(4):1345–53.
Ávila E, Briceño-Vera AE. A Reciprocal inter-habitat transplant reveals changes in the assemblage structure of macroinvertebrates associated with the sponge Halichondria melanadocia. Estar Coasts. 2018;41(5):1397–409.
Espinoza-Avalos J. Distribution of seagrasses in the Yucatan Peninsula, Mexico. Bull Mar Sci. 1996;59(2):449–54.
Ávila E, Ávila-García AK, Cruz-Barraza JA. Temporal and small-scale spatial variations in abundance and biomass of seagrass-dwelling sponges in a tropical estuarine system. Mar Ecol. 2015;36(3):623–36.
Ávila E, Yáñez B, Vazquez-Maldonado LE. Influence of habitat structure and environmental regime on spatial distribution patterns of macroinvertebrate assemblages associated with seagrass beds in a southern Gulf of Mexico coastal lagoon. Mar Biol Res. 2015;11(7):755–64.
Noreña-Barroso E, Gold-Bouchot G, Sericano JL. Polynuclear aromatic hydrocarbons in American oysters Crassostrea virginica from the Terminos Lagoon, Campeche, Mexico. Mar Pollut Bull. 1999;38(8):637–45.
Carvalho FP, Villeneuve JP, Cattini C, Rendón J, de Oliveira JM. Ecological risk assessment of PCBs and other organic contaminant residues in Laguna de Terminos. Mexico Ecotoxicol. 2009;18(4):403–16.
Abascal-Monroy IM, Zetina-Rejón MJ, Escobar-Toledo F, López-Ibarra GA, Sosa-López A, Tripp-Valdez A. Functional and structural food web comparison of Términos Lagoon, Mexico in three periods (1980, 1998, and 2011). Estuar Coasts. 2016;39(4):1282–93.
Rützler K. Sponges in coral reefs. In: Stoddart DR, Johannes RE, editors Coral reefs: research methods: monographs on oceanographic methodology, 5 UNESCO. 1978.
Breedy O, Murillo M. Isópodos (Crustacea: Peracarida) de un arrecife del Caribe de Costa Rica. Rev Biol Trop. 1995;219–229.
Ortiz M, Jimeno A. Guía ilustrada para la identificación de las familias y los géneros de los Anfípodos del suborden Gammaridea de la Península Ibérica. Graellsia. 2001;57(2):3–93.
García-Cubas A, Reguero M. Catalogo ilustrado de moluscos del Golfo de Mexico y Mar Caribe. UNAM; 2007.
de León-González JA, Bastida-Zavala JR, Carrera-Parra LF, García-Garza ME, Peña-Rivera A, Salazar-Vallejo SI, et al. Poliquetos (Annelida: Polychaeta) de México y América Tropical. Univ Autón Nuevo León Monterrey México. 2009.
Laguarda-Figueras A, Hernández-Herrejón LA, Solís-Marín FA, Durán-González A. Ofiuroideos del Caribe mexicano y Golfo de México. ICMyL-UNAM Conabio México DF. 2009.
Mazé RA. Orden Amphipoda. Revista IDE@-SEA. 2015;(82):1–10. http://sea-entomologia.org/IDE@/revista_82.pdf. Accessed 28 Oct 2020.
Vilas-Fernández C. Órdenes Mysida y Lophogastrida. Revista IDE@-SEA. 2015;(81):1–10. http://sea-entomologia.org/IDE@/revista_81.pdf. Accessed 28 Oct 2020.
McAllister CD, Parsons TR, Strickland JDH. Primary productivity and fertility at station “P” in the north-east Pacific Ocean. ICES J Mar Sci. 1960;25(3):240–59.
Carballo JL, Ávila E, Enríquez S, Camacho L. Phenotypic plasticity in a mutualistic association between the sponge Haliclona caerulea and the calcareous macroalga Jania adherens induced by transplanting experiments. I: morphological responses of the sponge. Mar Biol. 2006;148(3):467–78.
Gambi MC, Buia MC, Casola E, Scardi M. Estimates of water movement in Posidonia oceanica beds: a first approach. In: International workshop on Posidonia beds. GIS Posidonie Paris; 1989. p. 101–112.
Naranjo SA, Carballo JL, Garcia-Gomez JC. Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain). Possible marine bioindicators? Mar Ecol Prog Ser. 1996;144:119–31.
Denny MW. Biology and the mechanics of the wave-swept environment. Princeton: Princeton University Press; 1988.
Muus BJ. A field method for measuring “exposure” by means of plaster balls: a preliminary account. Sarsia. 1968;34(1):61–8.
Komatsu T, Kawai H. Measurements of time-averaged intensity of water motion with plaster balls. J Oceanogr. 1992;48(4):353–65.
Maldonado M, Young CM. Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Mar Ecol Prog Ser. 1996;138:169–80.
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F, Logez M. betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.5.2. 2020. https://CRAN.R-project.org/package=betapart.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/.
Clarke KR, Warwick RM. Change in marine communities: an approach to statistical analysis and interpretation. 2nd Edition, PRIMER-E, Ltd., Plymouth Marine Laboratory, Plymouth. 2001.
Carrera-Parra LF, Vargas-Hernández JM. Comunidad críptica de esponjas del arrecife de Isla de Enmedio, Veracruz, México. Rev Biol Trop. 1996;311 – 21.
Villamizar E, Laughlin RA. Fauna associated with the sponges Aplysina archeri and Aplysina lacunosa in a coral reef of the Archipielago de Los Roques, National Park, Venezuela. En: Fossil and recent sponges. Springer; 1991. p. 522–542.
Baselga A. Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr. 2010;19:134–43.
Abdo DA. Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Mar Biol. 2007;152(4):845–54.
Wendt PH, Van Dolah RF, O’Rourke CB. A comparative study of the invertebrate macrofauna associated with seven sponge and coral species collected from the South Atlantic Bight. J Elisha Mitchell Sci Soc. 1985;101:187–203.
Beepat SS, Appadoo C, Marie DEP, Paula JPM, Çinar ME, Sivakumar K. Macrofauna associated with the sponge Neopetrosia exigua (Kirkpatrick, 1900) from Mauritius. West Indian Ocean J Mar Sci. 2014;13(2):133–42.
Koukouras A, Russo A, Voultsiadou-Koukoura E, Arvanitidis C, Stefanidou D. Macrofauna associated with sponge species of different morphology. Mar Ecol. 1996;17(4):569–82.
Koukouras A, Russo A, Voultsiadou-Koukoura E, Dounas C, Chintiroglou C. Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. Int Rev Gesamten Hydrobiol Hydrogr. 1992;77(4):609–19.
Duarte L, Nalesso R. The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuar Coast Shelf Sci. 1996;42(2):139–51.
Voultsiadou-Koukoura HE, Koukouras A, Eleftheriou A. Macrofauna associated with the sponge Verongia aerophoba in the North Aegean Sea. Estuar Coast Shelf Sci. 1987;24(2):265–78.
Cinar ME, Ergen Z. Polychaetes associated with the sponge Sarcotragus muscarum Schmidt, 1864 from the Turkish Aegean coast. Ophelia. 1998;48(3):167–83.
MacArthur RH, Wilson EO. Island biogeography. Princeton; 1967.
Acknowledgements
We thank Hernán Alvarez-Guillén, Andres Reda-Deara and Alejandro Gómez-Ponce for their assistance with field samplings. Antony E. Briceño-Vera thanks to Consejo Nacional de Ciencia y Tecnología (CONACYT) by its student grant. Dr. Julio Cesar Canales-Delgadillo for their help with beta diversity analysis.
Funding
This study was funded by the Instituto de Ciencias del Mar y Limnología (internal project no. 618), with complementary funding from the project PAPIIT-IN203419, UNAM.
Author information
Authors and Affiliations
Contributions
AEBV participated in the field work, in the sample and data processing and in the writing of the manuscript. EA design the work, drafted and substantively revised the manuscript. MARS participated in the writing of the manuscript, data analysis and design of figures and tables. ARM participated in the sample processing, analysis and interpretation of environmental data. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Briceño-Vera, A.E., Ávila, E., Rodríguez-Santiago, M.A. et al. Macrofaunal assemblages associated with two common seagrass‐dwelling demosponges (Amorphinopsis atlantica and Haliclona implexiformis) in a tropical estuarine system of the southern Gulf of Mexico. Helgol Mar Res 75, 1 (2021). https://doi.org/10.1186/s10152-021-00546-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10152-021-00546-z