Abstract
Biomass-based adhesives, which are environmentally friendly and sustainable materials enabling low formaldehyde wood composites, have garnered interest. Therefore, palm kernel shells (PKS), available as industrial agricultural residue and rich in lignin, are mixed in form of fine particles with glycerol and citric acid, and tested as a candidate for binder in plywood production. The study focused on examining the effects of two factors: the quantity of adhesive used and the pressing temperature. Glycerol and citric acid are low-cost non-toxic chemicals that activate the functional groups and induce changes in the PKS component during hot pressing. Consequently, the mixtures with PKS as fine particles could cross-link with rubberwood veneer, forming a plywood panel with shear strength and bending strength that meet the requirements outlined in ISO 12466-2: part 2, and in Thai industrial standard (TIS 178-2549) for indoor use. The properties of plywood were primarily influenced by the pressing temperature rather than by the quantity of adhesive. Specifically, the temperatures 180 °C and 200 °C enhanced the extent to which the molten binder penetrated the rubberwood surface, consequently improving the mechanical properties and water resistance of the bonding.
Similar content being viewed by others
Introduction
Plywood, a type of wood-based composite panels, has consistently been embraced by consumers as an excellent material for building construction, as well as for furniture or packaging. The growth potential in plywood manufacturing as well as for other wood composites is dependent on the stringent limits to environmental impacts. Specifically, concerns arise regarding formaldehyde emissions from the binders used. Hence, various bonding techniques and formaldehyde-free adhesives have been under investigation [1].
The cross-linking to form a panel of wood veneer layers, or of other shapes such as particles and fibers, primarily occurs through the combined effects of heat, moisture, and compression. This technique is referred to as self-bonding or binderless manufacturing [2,3,4]. All chemical components in wood or other lignocellulosic raw materials, mainly lignin, hemicellulose, and cellulose, along with various extractive components, influence the bonding strength of the resulting binderless board product. Additionally, the network of lignin and furan products, formed through the conversion of sugar monomers from carbohydrate polymers, enhances the bonding strength [5, 6]. Building upon these findings, wood and other lignocellulosic materials in the form of fine particles have also been utilized as natural adhesives in the production of veneer laminate boards. Kenaf (Hibiscus cannabinus L.) core powder, with a grain size of 10 μm, that was directly applied on the veneer surface before assembly and the hot compression process, was demonstrated in fabricating three-ply sugi (Cryptomeria japonica D. Don) plywood when subjected to hot pressing at 5 MPa pressure and 200 °C for a duration of 20 to 30 min [7]. On the other hand, the central portion of the oil palm trunk, rich in carbohydrate polymers such as starch and holocellulose, provides an opportunity for bonding with rubberwood veneer when hot pressed at 180 °C to 200 °C for a short 5 min pressing time. The bond strengths in both dry and wet conditions were improved when blended with citric acid [8]. Besides the use of fine particles, also chemicals extracted from lignocellulose materials, such as lignin and tannin, are still developed for plywood manufacturing [9]. Yang et al. [9] found that milled wood lignin (MWL) isolated from eucalyptus wood particles exhibits an excellent bonding performance when used to bond plywood from poplar veneers. Hot pressing at 190 °C and 1.5 MPa for 8 min gave a good bond strength surpassing the standard requirement of 0.7 MPa. Although isolated lignin holds potential for use as adhesive in plywood production, the extraction incurs prohibitive costs. Therefore, the utilization of lignocellulose materials in the form of fine particles as wood adhesives is comparatively attractive. This approach aligns with the desired adhesive properties: non-toxicity, affordability, a simple manufacturing process, a long shelf life, and ease of use. Importantly, the raw material should be continuously available on a commercial scale. In Southeast Asia, countries such as Indonesia, Malaysia, and Thailand have easy access to palm oil industrial residues, including empty fruit bunches (EFB), mesocarp fibers, and palm kernel shells (PKS). These biomass residues are primarily used as fuel in energy plants. Considering their chemical compositions, PKS stands out as an excellent source of lignin, containing around 45.5% lignin, 17.5–24.1% hemicellulose, and 24.6–29.9% cellulose [10, 11]. Unfortunately, the direct utilization of fine particles of lignocellulose as an adhesive results in a poor water resistance, along with the lignin exhibiting a lower reactivity than carbohydrate polymers such as hemicellulose and cellulose. Therefore, its bonding ability is improved with other chemicals [7, 12].
A previous study has reported the use of citric acid and glycerol with active functional groups as such modifiers. Citric acid possesses three carbonyl groups, while glycerol has three hydroxyl groups. Interestingly, both citric acid and glycerol are non-toxic and affordable chemicals; they are currently considered as renewable raw materials for the synthesis of formaldehyde-free wood adhesives [13, 14]. Citric acid in pure form, or in combination with glycerol and other chemicals such as glucose or tannin, has given good bonding results in plywood production [15, 16]. At elevated temperatures surpassing the melting point of citric acid, esterification occurs between the carboxyl groups in the citric acid and hydroxy groups in the glycerol as well as in wood polymers, encompassing both lignin and carbohydrates. In the case of lignin, cross-linking presents an opportunity for esterification to take place at the γ position in the β-O-4 and β-5 linkages, as well as at the hydroxy group in its aromatic structure. Carbohydrate polymers, including cellulose, hemicellulose, and starch, undergo reactions particularly yielding furan products that further cross-link with citric acid to form a bond network [17,18,19]. Azerêdo et al. [13] reported that an adhesive synthesized from glycerol and citric acid in the presence of tin (II) 2-ethylhexanoate as the catalyst, was preheated at 120 °C for 10 min before application at 150 g/m2. The pair of veneers with the adhesive was then compressed at 120 °C and 2 MPa for 20 h. This gave a shear strength of 2.3 MPa for the bond between two plies of eucalyptus species (Eucalyptus sp.). In addition to their use as wood adhesives, citric acid and glycerol also excel in functioning as plasticizers of lignin and other composite polymers.
Therefore, the goal of this study was investigating the ability of a fine-particle binder mixture of palm kernel shell, glycerol, and citric acid to bond plywood. The study examined experimentally the impacts of adhesive quantity (g/m2) and pressing temperature, while maintaining a fixed pressure of 5 MPa and a pressing time of 10 min, on bonding performance and other properties of the resulting plywood.
Materials and methods
Materials
PKS residues from palm oil extraction were sourced from industries in Surat Thani province, located in southern peninsular Thailand. The PKS raw material underwent purification to remove contaminants such as tiny fibers and sand. Afterward, it was cleaned and dried at the low 50 °C temperature for 24 h. The dried PKS was then ground into fine particles using an electric grain mill (Rukthai locally made machine, Thailand). The particles that passed through a 0.10 mm sieve were used in this study. Glycerol and citric acid, both of analytical grade, were used without further purification. The conditioned rubberwood veneers, prepared using the rotary-cut method, had dimensions of 350 × 350 mm and an average thickness of 1.5 ± 0.2 mm. The average moisture content was approximately 11% for plywood production.
Preparation of PKS powder adhesive
To prepare a bio-based wood adhesive with fine solid particulates, the process began by dissolving in water 15% of citric acid based on the oven-dry weight of PKS. The amount of distilled water was determined to achieve the target moisture content of 30% in the blend with PKS. Once the citric acid was completely dissolved, glycerol was added in a 1:3 (w/w) ratio to PKS with continuous stirring until a clear solution formed. The acidic solution of glycerol was sprayed onto the pulverized PKS, and then the blend was kept in a covered beaker at room temperature for 1 h before use as a binder.
The thermal behavior was determined through thermogravimetric (TG) analysis and differential scanning calorimetry (DSC). In both samples, consisting of the PKS raw material and its mixture with glycerol and citric acid, approximately 5 g underwent decomposition characterization by TG analysis conducted with a TGA-Q500 apparatus. The samples were heated under identical conditions in an air atmosphere, from 50 to 600 °C at a heating rate of 10 °C/min. Furthermore, the two samples were analyzed using DSC analysis (Mettler-Toledo, Switzerland) by heating from 50 to 300 °C. The temperature was increased at a rate of 10 °C/min under nitrogen flushing with a flow rate of 20 mL/min.
Plywood preparation
To assess the performance of PKS mixed with glycerol and citric acid in plywood fabrication, the effects of the adhesive quantity per bond area (120, 160, and 200 g/m2) and pressing temperature (160, 180, and 200 °C) on plywood properties were studied. Different amounts of the blended PKS powder were applied to the surface of conditioned rubberwood veneer by a shaking stainless screen. The three layers of veneers, assembled with blended PKS powder, were compressed using a laboratory hot press to create the plywood board under a fixed maximum pressure of 5 MPa for 10 min at various pressing temperatures. Each treatment was done with five replications. All finished plywood boards were kept at room temperature for a few days before cutting of test specimens.
Bonding performance analysis
The specimen size and testing method for measuring the tensile shear strength, which was used to determine the bondability of PKS mixed with glycerol and citric acid, were adapted from ISO 12466-1: part 1 and ISO 12466-2: part 2 [20, 21]. The tensile shear strength of each plywood board was evaluated under both dry and wet conditions using a universal testing machine (10ST, Tinius Olsen, UK) equipped with a 5kN load cell at a constant crosshead speed of 3 mm/min. In the dry condition, the conditioned specimens were tested directly for tensile strength. In the wet condition, the specimens were soaked in cold water at 20 ± 3 °C for 24 h before undergoing testing. The tensile shear strength for both conditions was calculated as the maximum load (N) divided by the shear support area (mm2). The percentage of wood failure was visually evaluated in accordance to ISO 12466-1: plywood bonding quality part 1 [20]. Both testing conditions were applied to five samples taken from each plywood board.
The characterizations of bondline area in rubberwood plywood was assessed by stereo microscope (ZEISS Stemi 508). The changes in functional groups of PKS mixed with glycerol and citric acid were investigated before and after curing under the hot pressing at 200 °C for 10 min by Fourier transform infrared spectroscopy (FT-IR). The cured binder, obtained from the bond area between the laminates of the plywood specimen after testing the shear tensile strength in dry and wet conditions, was redried at a low temperature of 50 °C until it reached a constant weight before analyzing the functional groups. The same procedure was applied to the original PKS raw material and to a mixture of PKS, glycerol, and citric acid. Then, all the redried specimens were ground into fine particles and combined with potassium bromide (KBr) in a ratio of 1:35 before being subjected to a Vector 70 FT-IR spectroscope (Bruker, Germany). Scanning was performed in the range from 4000 to 400 cm⁻1 with 32 scans per spectrum.
Testing other mechanical and physical properties
Each plywood board was cut into a suitable specimen size for physical and mechanical property testing, and the specimens were kept in a conditioning chamber with controlled 65% relative humidity and 20 °C temperature, until they reached a constant weight. The physical properties determined were density, water absorption (WA) and thickness swelling (TS), which were evaluated using the same specimen size with 50 mm (width) × 50 mm (length) × thickness of board. The weight and dimensions of each specimen were measured before and after immersing in cold water at 21 ± 3 °C for 2 and 24 h. The density testing was performed with two specimens per board, while water absorption and thickness swelling testing were conducted with three specimens per board.
The specimens in parallel to the grain of the veneer’s surface layer, had dimensions of 50 mm (width) × 200 mm (length) and the board thickness was used for determining the modulus of rupture (MOR) and modulus of elasticity (MOE) in a three-point bending test. Specimens were fixed on the support span of the Universal testing machine before applying a load with a fixed 10 mm/min head speed until the specimen failed. Each plywood board was tested with two specimens.
Formaldehyde emissions of plywood
The rubberwood veneer was of ordinary industrial type, and these plywood panels were bonded with a mixture of PKS, glycerol, and citric acid. This bonding occurred at various pressing temperatures and a maximum pressure of 5 MPa for 10 min, with the adhesive amount fixed at 200 g/m2. These specimens were used for analyzing formaldehyde emissions according to the perforator method [22].
Results and discussion
The thermal properties of PSK fine particles mixed with glycerol and citric acid
The thermal degradation of the green adhesive prepared from PKS mixed with glycerol in a 3:1 (w/w) ratio, along with 15% citric acid based on the amount of PKS, was compared to the PKS original material for responses to thermogravimetric analysis (TGA) and differential thermogravimetry (DTG), as presented in Fig. 1. Clearly the glycerol and citric acid activated the degradation of PKS, as the TGA curve showed larger weight loss than for pure original PKS. The weight loss of PKS was rapid in the temperature range from 254 to 414 °C, exhibiting a high degradation rate with two peaks on the DTG curve at 278 °C and 363 °C. The high peak at 278 °C is mainly associated with thermal decomposition of hemicellulose and amorphous cellulose, while the peak at 363 °C mainly corresponds to lignin [23]. Huang et al. [24] found that the isolated lignin of PKS had a high decomposition rate at 280 °C and 373 °C. In the case of PKS mixed with glycerol and citric acid, the TGA curve continually decreased from around 122 °C to 417 °C, indicating a high decomposition rate across three temperature ranges on the DTG curve. The first stage, occurring from 122 to 221 °C with the highest peak at 173 °C, was related to the degradation of citric acid [25]. The second temperature range, from 221 to 337 °C, corresponded to the thermoset polymer products formed through the esterification of citric acid and glycerol [19]. Glycerol decomposes at around 290 °C [26]. The temperature response can also be complicated by the degradation of PKS components, and the last stage occurs from 337 to 417 °C with a peak at 317 °C.
Figure 2 presents the DSC analysis of PKS blended with glycerol and citric acid compared to the original PKS powder. Both samples had an endothermic peak at similar ranges of temperature. However, the PKS blended with glycerol and citric acid had the highest peak at around 92 °C, and this was shifted to 87 °C in the case of the original PKS powder. This was due to the citric acid that normally shows melting at 153–155 °C, and retarded the melting point of PKS [27]. The tiny broad peak around 223 °C is related to decomposition of citric acid and esterified product of glycerol and citric acid [19]. Furthermore, the activation energy for melting was reduced from 383 J/g to 176 J/g when glycerol and citric acid were added. This was due to the plasticizing function of glycerol and citric acid [28].
The bonding characterization of plywood
The fine particles of PKS, when mixed with glycerol and citric acid, are a candidate for bonding rubberwood veneer to fabricate plywood, and the performance was assessed through tensile shear strength testing, as depicted in Fig. 3. The results indicate that, in the case of dry condition testing, the bonding performance of PKS mixed with glycerol and citric acid was significantly influenced by both the quantity of adhesive used and the pressing temperature (p < 0.05). It was observed that increasing both parameters improved the dry tensile shear strength of plywood. However, only the pressing temperature significantly affected the increase in wet tensile shear strength. Applying temperatures of 180 °C and 200 °C enhanced the water resistance of the bondline, thereby increasing the wet tensile shear strength of plywood. Unfortunately, a pressing temperature of 160 °C resulted in relatively lower average shear strength in dry condition testing, and all the plywood samples experienced delamination during a 24-h water soaking test. This is likely a result of inadequate heat and pressing duration, which may not have been sufficient to initiate and complete the esterification process between citric acid and the hydroxy groups found in glycerol and/or the wood polymer. It is worth noting that the esterification of citric acid with other compounds typically occurs at its melting point, which is around 155 °C [19]. This fact also provides an explanation for why applying pressing temperatures of 180 °C and 200 °C enhanced the shear strength in wet condition testing of plywood. The percentage of wood failure, related to the tensile shear strength, was approximately 40% when using pressing temperatures of 180 °C and 200 °C for all binder quantities studied in case of dry condition testing. The wet condition testing gave wood failure in the range from 20 to 30% accordance to ISO 12466-1: plywood bonding quality part 1. The highest dry tensile shear strength was 1.13 MPa achieved with binder quantity of 200 g/m2 and a pressing temperature of 200 °C. However, under these conditions, the tensile shear strength decreased by 52% after soaking in water for 24 h, resulting in a wet tensile shear strength of 0.53 MPa.
Typically, the bond strength is affected by the degree of adhesive penetration in the interphase region. Figure 4 presents photographs of the bond area within the veneer layer of the plywood structure, after hot pressing in various conditions that were studied. The results clearly demonstrate that when pressing at 160 °C was employed, the adhesive penetration was shallower compared to those at 180 °C and 200 °C. Penetration is characterized by a distinct dark-brown color observed through visual inspection. The observed effect is likely a result of the high heat which promoted the melting of components in PSK mixed with glycerol and citric acid, allowing for a deeper thorough penetration into the rubberwood veneer. Furthermore, the PKS components in presence of glycerol and citric acid might be liquefied to small substages that further move between particles and rubberwood surfaces. In thermal-acidic conditions, lignin and hemicellulose are easily converted to liquor products, and the rate of this conversion reaction is accelerated by elevating the temperature [29]. Hence, it is conceivable that the chemical components of rubberwood were also degraded, particularly in the interphase region. All these factors collectively contributed to the improvement in bond strength.
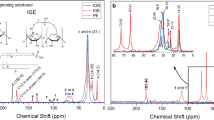
Figure 5a presents the FT-IR spectra of original PKS raw material, the binder mixture of PKS, glycerol and citric acid, including the cured binder taken from the dry and wet condition of the shear tensile strength testing specimens. Upon examination of the PKS mixed with glycerol and citric acid for its spectral patterns, the addition of glycerol and citric acid to the PKS promoted some functional groups, especially the hydroxyl groups (–OH) and the carboxylic groups (–COOH). These two functional groups exhibit a great ability to bond with wood polymers. The O–H stretching exhibited a broader spectrum around 3401 cm−1 in the FT-IR analysis of PKS mixed with glycerol and citric acid, which is slightly shifted from the PKS original material that exhibited a peak around 3399 cm−1. Meanwhile, the C=O stretching and C–O stretching in carboxylic groups (–COOH) are represented by distinct peaks around 1736 cm−1 and 1239 cm−1, respectively. The peak at 1736 cm−1 overlaps with the C=O stretching found in the structure of hemicellulose and lignin [30]. Interestingly, the strong peaks corresponding to the O–H stretching and the C=O stretching were reduced in both cases of the cured binder. This observation corroborates the polycondensation of PKS components with glycerol and/or citric acid, as well as with rubberwood polymers. Citric acid contains three carboxylic groups esterified with the hydroxy groups of glycerol and/or wood polymers to form the ester product, showing the carbonyl ester overlap with the C=O stretching of PKS component, as well as the C=O stretching in the citric acid structure [31]. In addition, the peak around 1635 cm⁻1 that corresponded to the citric acid structure was clearly reduced after the binder was cured. The FT-IR spectrum of glycerol does not show a peak around 1736 cm⁻1, but it reveals the peak of O–H stretching around 3322 cm⁻1 [32]. According to a previous study, using citric acid and glycerol as a binder in particleboard production forms ester linkages, shown around 1731 cm⁻1, improving the water resistance of the board [31]. Ando and Umemura [18] found that the carbonyl peak of esterification between citric acid and wood polymer was around 1740 cm⁻1. Hence, the ester bond was possibly shifted to 1736 cm⁻1 in this study. This is supported by the FT-IR spectrum around 1736 cm−1 of the cured binder from wet condition testing that had stronger peaks than in the spectrum of PKS original material. Normally, the ester linkages formed by citric acid can resist water [31].
From the data above and the review of prior literature, it is apparent that the bonding reaction between the binder components and the rubberwood veneer was complicated. Figure 5b indicates the potential bonding reaction of plywood. It was possibly supported by hydrogen bonding between hydroxy groups present in both PKS and rubberwood [3]. Ester linkages formed as the three carboxylic groups of citric acid reacted with hydroxy groups in glycerol and with the polymer structures of PKS and rubberwood [13, 15, 18]. Additionally, the acidic solution of glycerol and citric acid that penetrated into PKS and rubberwood likely activated liquefaction reactions, converting components into smaller molecular weight compounds such as sugar monomers, which further transformed into furan products such as furfural or 5-HMF during hot compression. Furan products could also cross-link with free functional groups of citric acid, and with components of PKS and rubberwood. Furthermore, glycerol could undergo etherification with lignin and other degraded carbohydrate polymers [18, 19, 29, 33]. Ether bonding (C–O–C stretching) was observed from a peak in the range from 1070 to 1250 [34], which likely overlapped with the spectra of PKS.
Other mechanical and physical properties of plywood
Static 3-point bending strength was employed to evaluate the MOR and MOE of plywood bonded under various conditions with a mixture of fine particles of PKS, glycerol, and citric acid. The results are presented in Fig. 6. The pressing temperature significantly (p < 0.05) affected the MOR and MOE of plywood, while the adhesive quantity did not show significant effects on these properties. Plywood showed an increased MOR with higher pressing temperatures. The highest MOR was achieved when the pressing temperature was set at 200 °C. Visually it was observed that the failures of plywood during bending were often due to cracking at the bonded interface. This indicates that the bending strength of plywood is supported by bonding strength. Furthermore, the densification and chemical changes of the rubberwood veneer's surface layer resulting from hot pressing can also enhance the mechanical properties of plywood [35, 36].
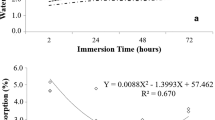
Table 1 summarizes the physical properties of plywood bonded with PKS mixed with glycerol and citric acid. The results reveal that the factors pressing temperature and adhesive quantity did not significantly affect the density, TS, or WA of plywood. The veneer layers of all plywood samples hot pressed at 160 °C separated (delaminated) when immersed in water. This means that the cross-linking was not sufficiently strong to resist the ingress of water molecules, particularly as hydrogen bonds might be formed by the self-bonding of PKS and/or rubberwood, and the binder was not completely cured. Furthermore, using higher pressing temperatures in the range from 180 to 200 °C not only facilitated the esterification of citric acid and glycerol but also promoted the degradation of wood polymers, especially hemicellulose. Hemicellulose typically possesses a high water-absorption capacity [37]. Consequently, employing high pressing temperatures might cause the degradation of hemicellulose and thus enhance the water resistance of plywood. The average moisture content of plywood was significantly influenced by pressing temperature (p < 0.05), with a slight reduction observed in the case of a 200 °C pressing temperature. The densification of rubberwood veneer during hot compression under a maximum pressure of 5 MPa resulted in high-density plywood compared to the density of rubberwood veneer (0.619 ± 0.016 g/cm3). The compression ratio was approximately 17% based on the uncured board. Pressing temperatures in the range from 180 to 200 °C could maintain the form of plywood, resulting in TS ranging from 4.91 to 6.02% and WA ranging from 23.81 to 30.77% after soaking in water for 2 h. Unfortunately, both values increased when soaking for 24 h, but they were lower than in a previous report [15].
Formaldehyde emissions from plywood
Originally, wood and other lignocellulose materials contain small amounts of formaldehyde, with the concentration depending on the wood species and the heat treatment done [38]. The rubberwood veneer raw material showed a formaldehyde emission of 0.71 mg/100 g, which is close to the formaldehyde concentration in mature wood of Scots pine (Pinus sylvestris L.) [39]. Formaldehyde can be generated from extractives, lignin, and carbohydrate polymers in wood during hot pressing [40]. Therefore, plywood bonded with 200 g/m2 of PKS mixed with glycerol and citric acid revealed formaldehyde emissions of 0.81 mg/100 g and 1.05 mg/100 g for pressing temperatures of 180 °C and 200 °C, respectively. However, those formaldehyde emissions from plywood products were compliant with the standard requirement for wood composite products (≦ 8 mg/100 g).
Conclusion
The thermal degradation of PKS fine particles was accelerated by the addition of glycerol and citric acid. According to DSC analysis, the natural binder composed of a mixture of PKS, glycerol, and citric acid required a comparatively low activation energy to soften under heat. However, the esterification reaction of citric acid or glycerol cross-linked the fine particles of PKS and rubberwood, and this required elevated pressing temperatures in the range from 180 to 200 °C for the creation of plywood with excellent tensile shear strength, strong mechanical properties, and water resistance of bondline. Based on the parameters of this study, the plywood bonded with a mixture of fine particles of PKS, glycerol, and citric acid has the potential to be classified as indoor grade according to both the ISO 12466-2: Part 2 and the TIS 178-2549 standard. It is recommended to use adhesive quantities of 160 and 200 g/m2 with a pressing temperature of 180 °C, and 120 to 200 g/m2 with a pressing temperature of 200 °C, to bond the rubberwood veneer.
Availability of data and materials
Not applicable.
Abbreviations
- PKS:
-
Palm kernel shell
- MWL:
-
Milled wood lignin
- EFB:
-
Empty fruit bunches
- TGA:
-
Thermogravimetric analysis
- DTG:
-
Differential thermogravimetry
- DSC:
-
Differential scanning calorimetry
- FT-IR:
-
Fourier transform infrared spectroscopy
- 5-HMF:
-
5-Hydroxymethylfurfural
- MOR:
-
Modulus of rupture
- MOE:
-
Modulus of elasticity
- WA:
-
Water absorption
- TS:
-
Thickness swelling
- TIS:
-
Thai industrial standard
References
Gonçalves D, Bordado JM, Marques AC, Galhano dos Santos R (2021) Non-formaldehyde, bio-based adhesives for use in wood-based panel manufacturing industry-a review. Polymers 13(23):4086. https://doi.org/10.3390/polym13234086
Cristescu C, Sandberg D, Ekevad M, Karlsson O (2015) Influence of pressing parameters on mechanical and physical properties of self-bonded laminated beech boards. Wood Mater Sci Eng 10(2):205–214. https://doi.org/10.1080/17480272.2014.999703
Duan X, Piao X, Xie M, Cao Y, Yan Y, Wang Z, Jin C (2021) Environmentally friendly wood-fibre-based moulded products with improved hydrophobicity and dimensional stability. Coll Surf A Physicochem Eng Asp 627:126998. https://doi.org/10.1016/j.colsurfa.2021.126998
Saari N, Lamaming J, Hashim R, Sulaiman O, Sato M, Arai T, Kosugi A, Nadhari WNAW (2020) Optimization of binderless compressed veneer panel manufacturing process from oil palm trunk using response surface methodology. J Clean Prod 265:121757. https://doi.org/10.1016/j.jclepro.2020.121757
Chen M, Zheng S, Wu J, Xu J (2023) Study on preparation of high-performance binderless board from Broussonetia papyrifera. J Wood Sci 69:17. https://doi.org/10.1186/s10086-023-02092-3
Kurokochi Y, Hasegawa W, Sato M (2019) The effects of wetting and scratching pretreatment of veneers on shear strength of binderless plywood made from sugi (Japanese cedar, Cryptomeria japonica). J Wood Sci 65:15. https://doi.org/10.1186/s10086-019-1795-3
Ando M, Sato M (2009) Manufacture of plywood bonded with kenaf core powder. J Wood Sci 55:283–288. https://doi.org/10.1007/s10086-009-1022-8
Choowang R, Luengchavanon M (2021) Oil palm trunk powder blended with citric acid directly used as wood adhesive by one step liquefaction reaction. Ind Crops Prod 170:113809. https://doi.org/10.1016/j.indcrop.2021.113809
Yang G, Gong Z, Luo X, Chen L, Shual L (2023) Bonding wood with uncondensed lignins as adhesives. Nature 621:511–515. https://doi.org/10.1038/s41586-023-06507-5
Chan YH, Quitain AT, Yusup S, Uemura Y, Sasaki M, Kida T (2018) Optimization of hydrothermal liquefaction of palm kernel shell and consideration of supercritical carbon dioxide mediation effect. J Supercrit Fluids 133:640–646. https://doi.org/10.1016/j.supflu.2017.06.007
Rashid T, Gnanasundaram N, Appusamy A, Kait CF, Thanabalan M (2018) Enhanced lignin extraction from different species of oil palm biomass: kinetics and optimization of extraction conditions. Ind Crops Prod 116:122–136. https://doi.org/10.1016/j.indcrop.2018.02.056
Chen X, Xi X, Pizzi A, Fredon E, Du G, Gerardin C, Amirou S (2020) Oxidized demethylated lignin as a bio-based adhesive for wood bonding. J Adhes 97(9):873–890. https://doi.org/10.1080/00218464.2019.1710830
Azerêdo MS, Nunes MABS, Figueiredo LRF, Oliveira JE, Tonoli GD, de Barros S, Medeiros ES (2022) Environmentally friendly adhesives derived from glycerol-based polymers. J Adhes Sci Technol 36(1):98–108. https://doi.org/10.1080/01694243.2021.1915619
Yang M, Rosentrater KA (2021) Cradle-to-gate life cycle assessment of structural bio-adhesives derived from glycerol. Int J Life Cycle Assess 26:799–806. https://doi.org/10.1007/s11367-020-01733-9
Sutiawan J, Hermawan D, Massijaya MY, Kusumah SS, Lubis MAR, Marlina R, Purnomo D, Sulastiningsih IM (2022) Influence of different hot-pressing conditions on the performance of eco-friendly jabon plywood bonded with citric acid adhesive. Mater Sci Eng 6:400–409. https://doi.org/10.1080/17480272.2021.1884898
Li C, Lei H, Wu Z, Xi X, Du G, Pizzi A (2022) Fully biobased adhesive from glucose and citric acid for plywood with high performance. ACS Appl Mater Interfaces 14(20):23859–23867. https://doi.org/10.1021/acsami.2c02859
Ando D, Umemura K (2021) Bond structures between wood components and citric acid in wood-based molding. Polymers 13(1):58. https://doi.org/10.3390/polym13010058
Ando D, Umemura K (2021) Chemical structures of adhesive and interphase parts in sucrose/citric acid type adhesive wood-based molding derived from Japanese cedar (Cryptomeria japonica). Polymers 13(23):4224. https://doi.org/10.3390/polym13234224
Zhao Z, Sakai S, Wu D, Chen Z, Zhu N, Huang C, Sun S, Zhang M, Umemura K, Yong Q (2019) Further exploration of sucrose citric acid adhesive: investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers 11(12):1996. https://doi.org/10.3390/polym11121996
ISO 12466-1 (2007) Plywood-bonding quality-Part 1: test methods. International Organization for Standardization, Geneva
ISO 12466-2 (2007) Plywood-bonding quality-Part 2: requirements. International Organization for Standardization, Geneva
ISO/DIS 12460-5 (2013) Wood-based panels—determination of formaldehyde release. Geneva
Kawamoto H (2017) Lignin pyrolysis reactions. J Wood Sci 63:117–132. https://doi.org/10.1007/s10086-016-1606-z
Huang Y, Liu H, Yuan H, Zhan H, Zhuang X, Yuan S, Yin X, Wu C (2018) Relevance between chemical structure and pyrolysis behavior of palm kernel shell lignin. Sci Total Environ 633:785–795. https://doi.org/10.1016/j.scitotenv.2018.03.238
Segovia F, Blanchet P, Auclair N, Essoua Essoua GG (2020) Thermo-mechanical properties of a wood fiber insulation board using a bio-based adhesive as a binder. Buildings 10(9):152. https://doi.org/10.3390/buildings10090152
Ítavo LCV, Ítavo CCBF, Petit HV, Dias AM, da dos Santos MC, de Souza ADV, Goularte SR, Leal ES, de Mello JAT, Niwa MVG, de Moraes GJ (2017) Kinetics of thermal decomposition processes and kinetics of degradation in rumen liquor of glycerin derived from biodiesel production. Ind Crops Prod 104:1–6. https://doi.org/10.1016/j.indcrop.2017.04.016
Romeo I, Olivito F, Tursi A, Algieri V, Beneduci A, Chidichimo G, Maiuolo L, Sicilia E, De Nino A (2020) Totally green cellulose conversion into bio-oil and cellulose citrate using molten citric acid in an open system: synthesis, characterization and computational investigation of reaction mechanisms. RSC Adv 10(57):34738–34751. https://doi.org/10.1039/D0RA06542K
Kudahettige-Nilsson RL, Ullsten H, Henriksson G (2018) Plastic composites made from glycerol, citric acid, and forest components. BioResources 13(3):6600–6612. https://doi.org/10.15376/biores.13.3.6600-6612
Jiang X, Li P, Ding Z, Li H, Bing H, Zhang L (2022) Liquefied wheat straw as phenols for bio-based phenolic resins: reaction parameters optimization and chemical routes. Ind Crops Prod 187:15489. https://doi.org/10.1016/j.indcrop.2022.115489
Ikubanni PP, Oki M, Adeleke AA, Adediran AA, Adesina OS (2020) Influence of temperature on the chemical compositions and microstructural changes of ash formed from palm kernel shell. Results Eng 8:100173. https://doi.org/10.1016/j.rineng.2020.100173
Nitu IP, Rahman S, Islam MN, Ashaduzzaman Md, Shams MdI (2022) Preparation and properties of jute stick particleboard using citric acid-glycerol mixture as a natural binder. J Wood Sci 68(1):30. https://doi.org/10.1186/s10086-022-02039-0
Choowang R, Rodpan W, Raknarong J, Noopun N (2023) Glycerol-based liquefied palm kernel shell product and its blend citric acid as bio-based wood adhesive. BioResources 18(4):7877–7888. https://doi.org/10.15376/biores.18.4.7877-7888
Hassanpour M, Abbasabadi M, Gebbie L, Te’o VSJ, O’Hara Ian M, Zhang Z (2020) Acid-catalyzed glycerol pretreatment of sugarcane bagasse: understanding the properties of lignin and its effects on enzymatic hydrolysis. ACS Sustain Chem Eng 8(28):10380–10388. https://doi.org/10.1021/acssuschemeng.0c0183232
Muller LC, Marx S, Vosloo HCM, Chiyanzu I (2019) Functionalising lignin in crude glycerol to prepare polyols and polyurethane. Polym Renew Resour 10(1–3):3–18. https://doi.org/10.1177/20412479198308
Bekhta B, Salca EA, Lunguleasa A (2020) Some properties of plywood panels manufactured from combinations of thermally densified and non-densified veneers of different thicknesses in one structure. J Build Eng. https://doi.org/10.1016/j.jobe.2019.101116
Ferreira BS, Arroyo FN, Kondo MY, Santos HFd, Barreto RL, Dias AMPG, Lahr FAR, Christoforo AL, Campos CId (2022) Physical and mechanical properties of plywood produced with thermally treated Pinus taeda veneers. Forests 13(9):1398. https://doi.org/10.3390/f13091398
Chen C, Tu D, Zhao X, Zhou Q, Cherdchim B, Hu C (2020) Influence of cooling rate on the physical properties, chemical composition, and mechanical properties of heat-treated rubberwood. Holzforschung 74(11):1033–1042. https://doi.org/10.1515/hf-2019-0232
Li N, HuaY WJ, Li J, Wang W (2023) The effect of heat treatment and acetylation on formaldehyde emission in cellulose: a molecular dynamics simulation study. Forests 14:839. https://doi.org/10.3390/f14040839
Weigl M, Wimmer R, Sykacek E, Steinwender M (2009) Wood-borne formaldehyde varying with species, wood grade, and cambial age. Forest Prod J 59(1/2):88–92
Peng WX, Yue X, Chen H, Ma NL, Quan Z, Yu Q, Wei Z, Guan R, Lam SS, Rinklebe J, Zhang D, Zhang B, Bolan N, Kirkham MB, Sonne C (2022) A review of plants formaldehyde metabolism: Implications for hazardous emissions and phytoremediation. J Hazard Mater 436:129304. https://doi.org/10.1016/j.jhazmat.2022.129304
Acknowledgements
The authors would like to acknowledge Associate Professor Seppo Karrila for proofreading our manuscript.
Funding
This research was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Ref. No SIT6601345S).
Author information
Authors and Affiliations
Contributions
Rattana Choowang designed the study concept, acquired data, performed data analysis and interpretation, and drafted the manuscript. Montri Luengchavanon assisted with data acquisition, analysis, and manuscript drafting. Jiraporn Raknarong prepared the raw materials and conducted plywood property testing in certain sections.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
The authors agree to publish the manuscript in the Journal of Wood Science.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Choowang, R., Luengchavanon, M. & Raknarong, J. Employing a mixture of fine-particle PKS, glycerol, and citric acid as an eco-friendly binder for plywood production from rubberwood (Hevea brasiliensis) veneer. J Wood Sci 70, 31 (2024). https://doi.org/10.1186/s10086-024-02145-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-024-02145-1