Abstract
The application of citric acid and glycerol as natural binder was investigated for the manufacturing of jute stick particleboard in this study. The effects of citric acid content (0–30 wt%), citric acid and glycerol mixture (ratio of CA–G), and pressing temperatures on the properties of jute stick particleboard were investigated. Citric acid-bonded jute stick particleboard had good mechanical properties and dimensional stability when citric acid concentration was 20 wt% at pressing temperature of 200 °C. By addition of glycerol concentration (40/60), the properties were further increased. The modulus of rupture (MOR) and thickness swelling (TS) values of CA–G (40/60) bonded jute stick particleboard were 19.67 N/mm2 and 9%, respectively, which satisfy the minimum requirement for type-18 of particleboard JIS A 5908 (2003). FTIR analysis confirmed the formation of ester linkage by polymerization reaction between carboxyl groups and alcohol groups. Citric acid and glycerol polymer reacted with jute stick particles and produced cross-linked networks with enhanced properties, hence improved the adhesiveness during particleboard production. It could be concluded that citric acid and glycerol mixture can be a potential natural binder for the production of jute stick particleboard.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Wood particles and formaldehyde resin as an adhesive are mainly used in particleboard (PB) production. The increasing demand of PB causes forest depletion and formaldehyde emission in the environment over the world [1]. With regard to these environmental concerns, use of agricultural wastes like rice straw [2], bagasse [3], almond shells [4], palm trunks [5], coconut husk [6] as a substitute of wood raw materials has been researched widely in the PB industry. These are lignocellulosic materials that have generated adhesive constituents within the raw materials itself for PB production [7].
Jute grows abundantly in Bangladesh; jute sticks are discarded almost as a waste or used as a burning fuel in rural areas. Jute stick is a lignocellulosic material which contains cellulose, hemicellulose and lignin. Particleboard using jute stick without any synthetic adhesives was successfully developed in our previous study [8]. Due to chemical changes of jute stick during hot pressing, especially pentosans content of raw material decreased with the increased pressing temperatures accelerating the self-bonding of the jute stick binderless particleboard (JBPB). The mechanical properties satisfied the international standard, whereas the dimensional stability did not satisfy the standard. The surface lamination with baggage pith under controlled temperature could be a good way for improving the dimensional stability of JBPB. However, controlling the thickness of laminated board is quite hard and needs much attention.
In recent years, many studies dealing with replacing formaldehyde adhesives with natural adhesives such as citric acid [9]; maleic acid and glycerin [10]; and glycerol [11] are seen as enhancement of the PB properties. Citric acid (CA) is a natural organic polycarboxylic acid [12]. It has three carboxyl groups which have the potential to create intermolecular di-ester linkage during reaction between carboxyl groups and hydroxyl groups of the polysaccharide [13]. During esterification, two adjacent carboxylic acid groups dehydrate to form an intermediary, which is a five-membered cyclic anhydride and react with hydroxyl groups [14]. Thus CA acts as a cross-linking agent to improve the properties of wood-based composites [15]. Umemura et al. [16] found firstly that the application of CA as the main bonding agent for the fabrication of bark molding with Acacia mangium bark powder. Using 20 wt% of CA, the molding products exhibited good physical and mechanical properties. Widyorini et al. [17] fabricated PB from bamboo bonded with CA at adhesive content of 0, 15, and 30 wt%. The authors confirmed that the addition of CA significantly improved the physical and mechanical property.
Previous studies mentioned that 20–30 wt% CA concentration is recommended for achieving required properties of wood-based composite products [18, 19], whereas 8–15 wt% urea formaldehyde (UF) resin is usually used for PB manufacturing [20]. Huge amount of CA sometimes makes the material brittle and decreased the strength of PB. The use of glycerol as a catalyst on CA-bonded board may solve the above-mentioned problems [21, 22]. Glycerol is expected to provide additional hydroxyl groups and reacted with carboxylic acid of CA [23]. CA and glycerol mixture under heat and pressure produces a three-dimensional cross-inked polymer by esterification reaction without any catalyst [24]. Hence, it is expected that the addition of glycerol in CA may improve the properties of the board. Therefore, the objective of the study was to optimize the citric acid concentration, citric acid and glycerol ratio, and pressing temperatures for the manufacturing of jute stick particleboard (JSPB).
Materials and methods
Preparation of materials
Jute sticks (Corchorus capsularis) were collected from the local market of Khulna district, Bangladesh. The jute sticks were air-dried for 3 weeks. The sticks were then cut into small pieces, and the small pieces were converted into smaller particles using laboratory-scale grinder. The particles were screened and those that remained between sizes of 0.5–2 mm (passed through 10 to 35 meshes) were used as a raw material. The particles were dried in an oven at 80 ºC for 24 h and the moisture content of particles was less than 4%. The particles were stored in airtight bag until further use.
Preparation of citric acid and glycerol solution
Citric acid (anhydrous) and glycerol (99.5%) purchased and used without further purification. The CA was dissolved in water at a concentration of 60 wt% and the content (wt%) were adjusted to 10%, 20%, and 30% based on oven-dried weight of jute stick particles. CA and glycerol were dissolved in water by constant stirring in a hot water bath at a temperature of 100 ºC for 60 min [25] under a certain ratio. The mixture ratios of CA and glycerol (CA–G) were 100/0, 70/30, 60/40, 50/50, and 40/60 under the concentration of CA adjusted to 60 wt%. After that, the solution was left for at least 3 h for further use. The viscosity and pH of CA–G mixture solution are shown in Table 1.
Manufacturing of jute stick particleboard
Oven-dried jute stick particles were sprayed with different CA content (0–30 wt%) and sprayed particles were then dried in an oven at 80 °C for 24 h. The particles were hand-formed into a mat by using a forming box, followed by hot pressing at a temperature of 180 °C for 8 min. To investigate the effect of pressing temperature, 20 wt% of CA content was used in manufacturing of JSPB followed by hot pressing at a temperature of 180–220 °C for 8 min. As a reference, 15 wt% of UF resin-bonded particleboard was manufactured at a pressing temperature of 180 °C for 8 min. The manufacturing condition for the production of CA-bonded JSPBs is presented in Table 2. Similarly, for manufacturing the CA–G bonded JSPBs, oven-dried jute stick particles were sprayed with different mixing ratios of CA–G with the adhesive content of 20 and 30 wt%. The sprayed particles were then oven-dried at 100 °C for 24 h and hot-pressed at 200 °C for 8 min. The manufacturing condition for the production of CA–G bonded JSPBs is presented in Table 3. The dimensions of JSPBs were 300 × 200 × 6 mm. The target density for all the JSPBs was around 0.9 g/cm3 by controlling the thickness. Three JSPBs were manufactured in each condition. Prior to testing, all of the JSPBs were conditioned for a week at 25 °C and 60% relative humidity (RH).
Evaluation of mechanical properties and dimensional stability of JSPB
The mechanical properties and dimensional stability of the JSPB were evaluated in accordance with the Japanese Industrial Standard for Particleboards [26]. The static bending test was conducted at 300 × 50 × 6 mm specimens from each JSPB using a three-point bending test through a universal testing machine (SHIMADZU AG-50KNXplus, Japan) over an effective span of 150 mm at a loading speed of 10 mm/min. Five 50 × 50 mm test specimens were prepared from each sample of JSPB for internal bonding (IB) tests. Five specimens of the same size from each sample of JSPB were prepared for water absorption (WA) and thickness swelling (TS) tests for 24 h water immersion.
Fourier transform infrared spectroscopy (FTIR)
Jute stick and manufactured CA–G adhesive bonded jute stick particleboard (JSPB) were ground into a fine powder and dried in an oven at 60 °C for 12 h. Infrared spectrum data were obtained through FTIR spectrometer (Spectrum Two PerkinElmer, USA). Data were recorded between wave numbers of 4000 cm−1 and 1000 cm−1 using the universal attenuated total reflectance (UATR) method.
Results and discussion
Effects of citric acid content and pressing temperatures
Figure 1 shows the effects of CA content on the mechanical properties and dimensional stability of JSPB pressed at 180 ºC. The properties increased with the addition of CA content from 0 to 30 wt%. The average modulus of rupture (MOR) and modulus of elasticity (MOE) of 0 wt% CA-bonded JSPB (binderless particleboard) were 12.02 and 3135 N/mm2, respectively. The average MOR and MOE values were 17.01 and 3774.42 N/mm2, respectively, when 30 wt% CA was added. Compared to the case 0 wt% of CA-bonded JSPB, the improvements in MOR and MOE were 41.51% and 20.4%, respectively. Similarly, 30 wt% of CA-bonded JSPB had the highest IB strength value of 0.78 N/mm2 which was two times higher than the value of 0 wt% CA-bonded JSPB. When 10 wt% CA was used, IB value was of 0.32 N/mm2. Higher IB values were recorded as the content of CA increased. High content of CA may increase the bond contract between the particles [27]. Citric acid contains three carboxyl groups and when these carboxyl groups react with higher amount of hydroxyl group containing in jute stick, then the formation of ester linkage occurs which is the key factor behind the bonding mechanism of the CA-bonded JSPB [16, 27]. Higher amount of ester linkages with increasing the CA content resulted in improved adhesiveness of the JSPB [28]. Thus CA acted as cross-linking agent for JSPBs fabrication.
Similar trend was observed for the dimensional stability of the JSPB. The average WA and TS of JSPB with a 0 wt% CA content (binderless particleboard) were 96.18% and 74.29%, respectively. With the addition of 30 wt% of CA content, the WA and TS decreased to 61.19% and 52.64%, respectively. These results confirm the findings of Prasetiyo et al. [29] that the dimensional stability of CA-bonded corn stalk PB improved with the increase of CA content. Huaxu et al. [30] found that better dimensional stability of rubber wood PB bonded with 20 wt% of CA. The high percentage of WA and TS for 0 wt% of CA-bonded JSPB was due to the highly hydrophilic nature of JS particles as it contains an abundance of hydroxyl groups [8, 29]. When 30 wt% of CA added, amount of particles in the boards decreased as the density of the board was fixed. Thus reaction force decreased due to compressibility of the board which led to reduction in the swell or shrink [27]. Pressing temperature is another vital parameter that could affect the properties of JSPB. McSweeny et al. [31] found that that pressing temperature was an important component of the reaction between the carboxyl groups and hydroxyl groups to form ester groups. Umemura et al. [32] confirmed that 20 wt% of CA content with lower pressing temperatures may be suitable for the formation of ester linkage.
Figure 2 shows the effects of pressing temperatures on the properties of JSPB with 20 wt% of CA content. The results showed that the mechanical properties of the CA-bonded JSPB increased when the pressing temperature increased from 180 to 200 °C then decreased at 220 °C. The average MOR and MOE values of JSPB at 200 °C pressing temperature were 20.48 and 4524.35 N/mm2, respectively. Compared to the JSPB at 180 °C, the improvements in MOR and MOE were 30.45% and 40.36%, respectively. The same trend was also observed for the IB strength. For example, IB values increased from 0.39 N/mm2 to 0.87 N/mm2 when pressing temperature increased from 180 to 200 °C, whereas it decreased to 0.31 N/mm2 at 220 °C. The IB value of JSPB at 200 °C was about 2.5 times high than that of JSPB at 180 °C. The results indicated that better adhesiveness of the JSPB had occurred when pressing temperature elevated to 200 °C. Citric acid was decomposed to different unsaturated acids when it was heated at 175 °C [33]. During hot pressing, CA could have easily created linkages with lignocelluloses component to attain good adhesiveness above this temperature [15]. The JSPB manufactured at 180 °C could not be reached an effective temperature among the particles of the JSPB. The esterification reaction would be accelerated at 200 °C and particle-to-particle adhesion improved which resulted in good bonding of JSPBs. However, at 220 °C, the low mechanical properties were found because of the formation of volatile components at high pressing temperature (220 °C) may interfere the adhesiveness of the JSPBs.
Similarly, WA and TS decreased with increasing the pressing temperatures. JSPB at 200 °C had lowest WA and TS of 27.86% and 14.97%, respectively. Cross-linkage between CA and JS particles increased along with increasing the pressing temperature [15]. Another reason might be the higher amount of cellulose and hemicelluloses content in JS particles contributed to the much amount of hydroxyl groups in the esterification reaction [8]. The increased amount of ester linkage weakening the expansion of the particles when JSPB pressed at 200 °C, hence, contributed to the improved dimensional stability of the material [19]. UF resin-bonded board pressed at 180 °C had the higher mechanical properties and dimensional stability than that of CA-bonded JSPB at 180 °C. However, comparing the values with 200 °C, CA-bonded JSPBs exhibited higher mechanical properties and dimensional stability than UF-bonded board pressed at 180 °C (Fig. 2). Umemura et al. [16] found that addition of 20 wt% CA on wood and bark-molding product exhibited good performance and mechanical properties are improved with increasing the temperature [19]. Hence, it can be said that CA would be used as an adhesive for manufacturing of JSPB with an excellent bonding performance. Therefore, the pressing temperature of 200 °C with 20 wt% of CA content was found to be an optimized condition for good adhesiveness of the JSPB fabrication.
Effects of citric acid and glycerol mixture on the properties of jute stick particleboard
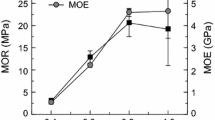
Figure 3 shows the effects of glycerol (G) addition in CA concentration on mechanical properties and dimensional stability of JSPB pressed at 200 °C. The results showed that the properties increased gradually as the glycerol ratio increased from 30 to 60 in a 20 wt% of CA content. The MOR and MOE value of the only CA-bonded JSPB (100/0) was 20.48 and 4524.35 N/mm2, respectively, indicating CA acted as an excellent bonding performance in JSPB production. In case with a ratio CA to glycerol of 40/60 in a 20 wt% of CA content, the MOR and MOE values were 19.67 and 3596.98 N/mm2, respectively. However, further increasing the ratio did not show any additional improvement. The corresponding values for 30 wt% of CA content were 7.24 and 1532.63 N/mm2, respectively, which is lower than that of other JSPBs. The same trend was found for dimensional stability of JSPBs as shown in Fig. 3. The TS of only CA-bonded JSPBs (100/0) was 14.97%. By increasing glycerol ratio 60 in a 20 wt% CA concentration (40/60), the TS considerably reduced to 9.11%. The IB value of the only CA-bonded JSPB (100/0) was 0.87 N/mm2 and with a ratio CA to glycerol of 40/60 in a 20 wt% of adhesive content, the corresponding value was 0.73 N/mm2. In a case of 30 wt% adhesive content with the same CA to glycerol, these values decreased into 0.24 N/mm2. Meanwhile, 20 wt% of adhesive content acted as a potential binding agent compared to 30 wt% of adhesive content in CA–G bonded JSPB fabrication.
CA and glycerol contain 3 carboxyl groups and 3 alcoholic hydroxyl groups which can react and form three-dimensional polymeric structure under heat and pressure without the presence of catalyst or solvent [34]. Glycerol generally consists of two primary and one secondary hydroxyl group which acted as trifunctional monomers [35]. Functional group was increased considerably due to the addition of glycerol in CA concentration. Berube et al. [36] explained that unreacted carboxylic groups from the CA–G polymer are able to bond with free hydroxyl groups. Higher concentration of glycerol have higher amount of carboxylic acid which indicates the higher efficiency of the reactions taking place. Mariano-Torres [37] confirmed that higher glycerol generated a higher efficiency of reaction, and managed to saturate more citric acid protons thus produced higher cross-linking polymer. Yao et al. [38] also reported that glycerol can easily react with citric acid and acted as a cross-linking extender. Thus, CA–G polymer could serve as a potential binding agent for the JSPB fabrication.
By increasing glycerol concentration and decreasing CA concentration in CA–G composition, a higher number of free hydroxyl groups produced in cross-linked polymer networks which are suitable for chemically functionalized in jute stick particles [39]. Tham et al. [40] found that the polymer of CA and glycerol is suitable for cross-linking the alcohols carrying components. A possible cross-link by melt polymerization reaction was proposed which might be triggered by the condensation reaction at the revealed optimal CA–G adhesive content; hence, exhibited satisfactory bonding properties [22, 36]. Therefore, JSPB bonded with 40:60 or 50:50 molar ratio of CA:glycerol which might form a larger cross-linking network than 70:30 ratios which exhibited enhanced mechanical properties of the JSPB. JSPB bonded with CA to glycerol 40/60 showing remarkable improvement in dimensional stability compared to only CA-bonded JSPB and other combinations. The improved dimensional stability of CA–G bonded JSPB (40/60) was due to the reduction of hydroxyl groups as well as formation of higher amount of three-dimensional networking. Segovia et al. [21] reported the same findings from the wood fiber insulation board using a crude glycerol and CA mixture as bio-based adhesive. Better physical and mechanical properties of insulation boards using 1:1 mol/mol ratios of CA–G at 20 wt% of adhesive contents were reported.
Adhesive mixture of 40:60 (CA:G) produced more acidic polymer compared to the other CA–G ratio which promoted the efficiency of reaction in this study. Mariano-Torres et al. [37] confirmed that the potentiality of the reactions sensitive to change the pH. The good wetting of the particles by the CA–G matrix had formed good chemical bond between the particles and matrix, which resulted in better mechanical strength [36, 41]. The sufficiency and effectiveness of the matrix in covering the surfaces of the particles had created the cross-link between the matrix and particles for better mechanical bonding throughout the hydrogen and covalent bonds. The excess adhesive beyond 20 wt% loading had reduced the strength and made the particleboard brittle. The agglomeration of jute particles at higher loadings of matrix had decreased the reinforcement-matrix bond; hence lowering the magnitude of MOR. As a result, it led to poor interfacial bonding between the particles and CA–G matrix; hence decreasing the MOR.
Figure 4 shows the FTIR spectrum of jute stick particles and the manufactured CA–G adhesive bonded JSPB as a function of different ratio of citric acid and glycerol (CA–G). The peaks at 3200–3500 cm−1 attributed to the H-bonded OH- groups stretching due to glycerol and jute stick [25, 42], indicating the presence of free OH groups [36]. The peak intensity was lower in raw jute stick, however it clearly appeared in CA-bonded JSPB. The intensities decreased for the peak from CA-to-glycerol ratio of (40:60) bonded JSPB than that of other ratio JSPB. It indicated that the free OH groups reacted with CA to form hydrogen bond which enhances the dimensional stability of CA–G bonded JSPB [18]. The peaks at around 1030–1035 cm−1 attributed to C–O stretching of the carboxyl group from citric acid which indicated that the carboxyl groups participating in esterification reaction [42]. The peak at around 1709–1738 cm−1 was attributed to C=O stretching derived from the carboxyl group and/or C=O ester group [21, 43]. The spectra from the samples exhibit a strong peak at 1731 cm−1. The peaks derived from CA-to-glycerol ratio of 40/60 and 50/50 bonded JSPB appeared strongly than that of the peak from other ratio JSPB. This indicated that the presence of a higher concentration of ester linkage due to the increased glycerol addition in the CA–G adhesive mixture. The mechanism behind the formation of ester linkage involves a reaction between the functional groups of CA with hydroxyl groups of glycerol and then the functional groups of the carboxylic acid and the hydroxyl groups from the CA–G polymer and/or jute stick. Feng et al. [44] analyzed the cross-linking esterification reaction process of polycarboxylic acid with wood and proved this mechanism by FTIR. Thus the formation of more ester linkage indicated the reduction of hydroxyl groups, reducing the affinity of the CA–G bonded JSPB to shrink or swell.
Conclusion
The aim of the study was to develop particleboard from jute sticks using citric acid and glycerol as a natural binder. The effects of CA concentration, CA and glycerol ratio, and pressing temperatures on mechanical properties and dimensional stability of jute stick particleboard were evaluated. The citric acid-bonded JSPBs possessed better properties with a 20 wt% CA concentration under the pressing condition of 200 ºC for 8 min. The dimensional stability increased considerably as the glycerol ratio increased in 20 wt% of CA concentration under the same pressing condition. By addition of citric acid:glycerol concentration (40/60), the MOR and TS of JSPB were 19.67 MPa and 9%, respectively, which met the minimum requirement for type-18 of particleboard JIS A 5908 (2003). Fourier transform infrared analysis showed that the presence of ester groups which was higher as the glycerol ratio increased from 30 to 60 in a 20 wt% CA concentration. This indicated the formation of strong ester linkage networks through polymerization reaction of carboxyl groups and alcohol groups; hence, improved the properties of JSPBs. Jute stick particleboard bonded with CA and glycerol mixture is completely free from formaldehyde emission and can be used for a variety of applications.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- JSPB:
-
Jute stick particleboard
- JBPB:
-
Jute stick binderless particleboard
- CA:
-
Citric acid
- CA–G:
-
Citric acid and glycerol mixture
- MOR:
-
Modulus of rupture
- MOE:
-
Modulus of elasticity
- IB:
-
Internal bonding
- WA:
-
Water absorption
- TS:
-
Thickness swelling
- FTIR:
-
Fourier transform infrared spectra
References
Salthammer T, Mentese S, Marutzky R (2010) Formaldehyde in the indoor environment. Chem Rev 110(4):2536–2572
Kurokochi Y, Sato M (2015) Properties of binderless board made from rice straw: the morphological effect of particles. Ind Crops Prod 69:55–59
Nonaka S, Umemura K, Kawai S (2013) Characterization of bagasse binderless particleboard manufactured in high-temperature range. J Wood Sci 59(1):50–56
Ferrandez-Villena M, Ferrandez-Garcia CE, Garcia Ortuño T, Ferrandez-Garcia A, Ferrandez-Garcia MT (2019) Study of the utilisation of almond residues for low-cost panels. Agronomy 9(12):811
Lee SH, Ashaari Z, Ang AF, Halip JA, Lum WC, Dahali R, Halis R (2018) Effects of two-step post heat-treatment in palm oil on the properties of oil palm trunk particleboard. Ind Crops Prod 116:249–258
Junior CPA, Coaquira CAC, Mattos ALA, de Souza MDSM, de Andrade Feitosa JP, de Morais JPS, de Freitas RM (2018) Binderless fiberboards made from unripe coconut husks. Waste Biomass Valori 9(11):2245–2254
Zhang D, Zhang A, Xue L (2015) A review of preparation of binderless fiberboards and its self-bonding mechanism. Wood Sci Technol 49(4):661–679
Nitu IP, Islam MN, Ashaduzzaman M, Amin MK, Shams MI (2020) Optimization of processing parameters for the manufacturing of jute stick binderless particleboard. J Wood Sci 66(1):1–9
Feng X, Xiao Z, Sui S, Wang Q, Xie Y (2014) Esterification of wood with citric acid: the catalytic effects of sodium hypophosphite (SHP). Holzforschung 68(4):427–433
Uraki Y, Hashida K, Watanabe N, Sano Y, Sasaya T, Fujimoto H (1994) Novel wood processing by maleic acid-glycerol mixture system: Improvement of water resistance and mechanical property of cellulose by the processing. J Wood Chem Technol 14(3):429–449
Mahmood H, Mehmood S, Shakeel A, Iqbal T, Kazmi MA, Khurram AR, Moniruzzaman M (2021) Glycerol assisted pretreatment of lignocellulose wheat straw materials as a promising approach for fabrication of sustainable fibrous filler for biocomposites. Polymers 13(3):388
Lee SH, Md Tahir P, Lum WC, Tan LP, Bawon P, Park BD, Osman Al Edrus SS, Abdullah UH (2020) A review on citric acid as green modifying agent and binder for wood. Polymers 12(8):1692
Abdillahi H, Chabrat E, Rouilly A, Rigal L (2013) Influence of citric acid on thermoplastic wheat flour/poly (lactic acid) blends. II. Barrier properties and water vapor sorption isotherms. Ind Crops Prod 50:104–111
Hashem M, Sharaf S, Abd El-Hady MM, Hebeish A (2013) Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse-hydrogel-ZnO-nanocomposites. Carbohy Polym 95(1):421–427
Kusumah SS, Umemura K, Guswenrivo I, Yoshimura T, Kanayama K (2017) Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard II: influences of pressing temperature and time on particleboard properties. J Wood Sci 63(2):161–172
Umemura K, Ueda T, Kawai S (2012) Characterization of wood-based molding bonded with citric acid. J Wood Sci 58(1):38–45
Widyorini R, Yudha AP, Adifandi Y, Umemura K, Kawai S (2013) Characteristic of bamboo particleboard bonded with citric acid. Wood Res J 4(1):31–35
Umemura K, Sugihara O, Kawai S (2015) Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: effects of board density and pressing temperature. J Wood Sci 61(1):40–44
Widyorini R, Umemura K, Isnan R, Putra DR, Awaludin A, Prayitno TA (2016) Manufacture and properties of citric acid-bonded particleboard made from bamboo materials. Eur J Wood Wood Prod 74(1):57–65
Stark N, Cai Z (2021) Wood-based composite materials: panel products, glued laminated timber, structural composite lumber, and wood–nonwood composites. In General Technical Report FPL-GTR-282, p 11–1.
Segovia F, Blanchet P, Auclair N, Essoua Essoua GG (2020) Thermo-mechanical properties of a wood fiber insulation board using a bio-based adhesive as a binder. Buildings 10(9):152
L’Hostis C, Thévenon MF, Fredon E, Gérardin P (2018) Improvement of beech wood properties by in situ formation of polyesters of citric and tartaric acid in combination with glycerol. Holzforschung 72(4):291–299
Nilsson RL, Ullsten H, Henriksson G (2018) Plastic composites made from glycerol, citric acid, and forest components. BioResources 13(3):6600–6612
Pramanick D, Ray TT (1988) Synthesis and biodegradation of copolyesters from citric acid and glycerol. Polym Bull 19(4):365–370
Essoua GG, Blanchet P, Landry V, Beauregard R (2016) Pine wood treated with a citric acid and glycerol mixture: biomaterial performance improved by a bio-byproduct. BioResources 11(2):3049–3072
Japanese Standard Association (2003) Japanese Industrial Standard for particle board JIS A 5908. Japanese Standard Association, Tokyo.
Kusumah SS, Umemura K, Yoshioka K, Miyafuji H, Kanayama K (2016) Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: effects of pre-drying treatment and citric acid content on the board properties. Ind Crops Prod 84:34–42
Vukusic SB, Katovic D, Schramm C, Trajkovic J, Sefc B (2006) Polycarboxylic acids as non-formaldehyde anti-swelling agents for wood. Holzforschung 60(4):439–444
Prasetiyo KW, Oktaviani L, Astari L, Syamani FA, Subyakto S, Achmadi SS (2018) Physical-mechanical properties and bonding mechanism of corn stalks particleboard with citric acid adhesive. Journal Ilmu dan Teknologi Kayu Tropis 16(2):131–140
Huaxu Z, Hua LS, Tahir PM, Ashaari Z, Al-Edrus SSO, Ibrahim NA, Abdullah LC, Mohamad SF (2021) Physico-mechanical and biological durability of citric acid-bonded rubberwood particleboard. Polymers 13(1):98
McSweeny JD, Rowell RM, Min SH (2006) Effect of citric acid modification of aspen wood on sorption of copper ion. J Nat Fibers 3(1):43–58
Umemura K, Ueda T, Kawai S (2012) Effects of moulding temperature on the physical properties of wood-based moulding bonded with citric acid. For Prod J 62(1):63–68
Barbooti MM, Al-Sammerrai DA (1986) Thermal decomposition of citric acid. Thermochim Acta 98:119–126
Lichtenthaler FW (2010) Carbohydrates as organic raw materials. Ullmann’s Encycl Ind Chem 05:07
Lang K, Sánchez-Leija RJ, Gross RA, Linhardt RJ (2020) Review on the impact of polyols on the properties of bio-based polyesters. Polymers 12(12):2969
Berube MA, Schorr D, Ball RJ, Landry V, Blanchet P (2018) Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation. J Polym Environ 26(3):970–979
Mariano-Torres JA, López-Marure A, Domiguez-Sánchez MÁ (2015) Synthesis and characterization of polymers based on citric acid and glycerol: its application in non-biodegradable polymers. Dyna 82(190):53–59
Yao W, Wang B, Ye T, Yang Y (2013) Durable press finishing of cotton fabrics with citric acid: enhancement of whiteness and wrinkle recovery by polyol extenders. Ind Eng Chem Res 52(46):16118–16127
Halpern JM, Urbanski R, Weinstock AK, Iwig DF, Mathers RT, Von Recum HA (2014) A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J Biomed Mater Res Part A 102(5):1467–1477
Tham WH, Wahit MU, Kadir MRA, Wong TW, Hassan O (2016) Polyol-based biodegradable polyesters: a short review. Rev Chem Eng 32(2):201–221
Rice RW, D’Onofrio M (1996) Longitudinal gas permeability measurements from eastern white pine, red spruce, and balsam fir. Wood Fiber Sci 28(3):301–308
Fengel D, Ludwig M (1991) Possibilities and limits of the FTIR spectroscopy for the characterization of cellulose. Pt. 1: comparison of various cellulose fibres and bacteria cellulose. Papier 45:45–51
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–27
Fang GZ, Li W, Kang JY (1998) The intermediate of crosslinking reaction between wood and polycarboxylic acid: FTIR Spectra characteristic of cyclic anhydrides reaction intermediate. J Northeast For Univ 26(4):40–43
Acknowledgements
The first author would like to acknowledge the Higher Education Quality Enhancement Project (HEQEP) CP 4035 funded by World Bank and Ministry of Education, the People's Republic of Bangladesh for the fellowship support. The authors are grateful to Professor Dr. Md Nabiul Islam Khan, Forestry and Wood Technology Discipline, Khulna University, Bangladesh for his contribution to graphical presentation.
Funding
Higher Education Quality Enhancement Project (HEQEP) CP 4035 funded by World Bank and Ministry of Education, People's Republic of Bangladesh.
Author information
Authors and Affiliations
Contributions
MIS performed the research plan and IPN collected the data for evaluating the properties of citric acid and glycerol mixture bonded jute stick particleboard. MA and SM performed the literature search. IPN, MIS and MNI performed the data analysis. IPN prepared the first draft of the manuscript. IPN, MIS and MNI were the major contributors for finalizing the manuscript. All authors read and finalized the final manuscript for submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nitu, I.P., Rahman, S., Islam, M.N. et al. Preparation and properties of jute stick particleboard using citric acid–glycerol mixture as a natural binder. J Wood Sci 68, 30 (2022). https://doi.org/10.1186/s10086-022-02039-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-022-02039-0