Abstract
Taiwania (Taiwania cryptomerioides Hayata) has long been regarded as a living fossil from the Tertiary period of Mesozoic Era for its distinguished yellowish-red color with purplish-pink streaks presented in its heartwood. With this elegant appearance that matches the color “red” for good fortune in the Taiwanese culture, Taiwania is supposed to be a popular wood in Taiwan where it is a native species of. Extractives contribute to the properties of wood. It is a fascinating subject to investigate extractives biosynthesis in the process of heartwood formation. Up to date, there is no phytochemistry study of Taiwania sapwood. In this study, three new sesquiterpenoids, Taiwania A (1), Taiwania B (2), and Taiwania C (3), together with 75 known compounds in the Taiwania sapwood. The structures of extractives were determined by analysis of spectroscopic data and comparison with the literatures. This study supported secondary reaction lignans could be found in sapwood that confirmed our previous research on the Taiwania-type of heartwood formation.
Similar content being viewed by others
Introduction
Heartwood is the large part of the wood, and the extractives contained in it are closely related to the properties of the wood, such as strength, durability, color, and odor. The formation mechanism of heartwood has always been of interest and an important research topic for researchers. The heartwood formation could be classified into three types based on distribution patterns of extractives in stem wood of various trees species [1, 2]. Type I heartwood formation, i.e., Robinia-type heartwood formation, where the accumulation of phenolic extractives starts in the transition zone (TZ). In this case, no phenolic precursors were found in the aging sapwood. Type II (Juglans-type) heartwood formation, where the phenolic precursors gradual accumulated centripetally with progressive aging of the sapwood tissues. The extractives that characterize the Type II heartwood were formed in the TZ either by de novo biosynthesis or secondary reactions (oxidation or hydrolysis) of precursor substances. Type III (Taiwania-type) heartwood formation, which most of phenolic compounds are synthesized in sapwood [2].
Taiwania (Taiwania cryptomerioides Hayata) is a native tree species growth in Taiwan. Taiwania also is the highest conifer in East Asia, it can reach 80 m. From 1960s to date, Taiwania is the important plantation species in Taiwan. Due to its excellent durability and processing property, Taiwania is the popular wood material for building and furniture. With regard to phytochemical study of Taiwania, more than 300 compounds, including terpenoids, lignans, isoflavones, and other compounds have been isolated from Taiwania during the past 90 years [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The putative bioactivities compounds of Taiwania, and evaluated the potential usages of the phytochemicals isolated from Taiwania for pharmacological applications were carried on by our research group. We demonstrated that sesquiterpenoids isolated from the heartwood of Taiwania against bacteria, fungi, mite, and termite [24,25,26,27,28]. The diterpenoids also exhibited the antioxidant and anti-inflammatory activities [29, 30]. In the meantime, the lignans of Taiwania presented the potent anti-inflammatory, antiviral, and anticancer activities [26, 31,32,33,34,35,36,37].
The mechanism of heartwood formation in Taiwania is very unique. We found that most of the skeletons of the compounds are already synthesized in the sapwood. Although there have been numerous research reports on the chemical composition of Taiwan cedar, so far, no discussion has been made on its sapwood composition. For understanding the heartwood formation in Taiwania, it is important to clarify the difference of composition between the heartwood and sapwood. This study accurately distinguished sapwood from heartwood in Taiwania, focusing the composition investigation of Taiwania sapwood. Totally, 78 compounds from sapwood of Taiwania, including 3 new skeleton sesquiterpenoids. The results obtained in this study provide a valuable reference for further heartwood formation and metabolites biosynthesis investments.
Materials and methods
General experimental procedures
1H, 13C, and 2D NMR spectra were recorded on a Bruker AVANCE III NMR spectrometer (Bruker, Billerica, Massachusetts, US), acquiring 1H data at 400 MHz and 13C data at 100 MHz, using standard experiments from Bruker pulse programs library. High-resolution mass spectrometry (HR-MS) was determined using an LTQ Orbitrap XL (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). The compositions of the essential oil were analyzed by an ITQ 900 mass spectrometer coupled to a TRACE GC Ultra gas chromatography (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). Methanol (MeOH) extracts were fractionated on silica gel 60 (230–400 mesh ASTM, Merck) and then purified with semi-preparative normal-phase column (luna silica (2), 250 × 10 mm, 5 μm, Phenomenex) on an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, California, U.S.).
Plant materials
A 30-year-old Taiwania used in this study was collected from the Huisun Experimental Forest Station of National Chung-Hsing University in August 2014; and was identified by Prof Yen- Hsueh Tseng, Department of Forestry, National Chung Hsing University. The voucher specimen was deposited in the herbarium of the same university. The sapwood chips (excluding the heartwood and knots) were prepared from a green cut tree and stored in room temperature with avoiding light irradition.
Extraction and isolation
Air-dried sapwood chips (ca. 10 kg) were extracted with MeOH (80 L) for 7 days at ambient temperature three times and concentrated under vacuum to yield the MeOH extract (46 g). The MeOH extract was partitioned between H2O and ethyl acetate (EtOAc) (1:1 for volume) three times to provide EtOAc soluble fraction (18.7 g). The EtOAc soluble fraction was subjected to chromatography using a silica gel (90 g) column eluted with n-hexane–EtOAc which gradient elution by changing 100:0, 95:5, 90:10, 85:15, 80:20, 75:25, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, and 0:100 for 1 L, respectively. The elution was collected by 500 mL to get 1 to 28 fractions. After thin layer chromatography tracing, 1 to 6 fractions were combined to fraction A (1.8 g), 7 to 8 fractions were combined to fraction B (0.5 g), 9 to 11 fractions were combined to fraction C (1.0 g), 12 to 14 fractions were combined to fraction D (0.6 g), 15 to 17 fractions were combined to fraction E (0.7 g), 18 to 20 fractions were combined to fraction F (0.7 g), 21 to 22 fractions were combined to fraction G (0.4 g), 23 to 25 fractions were combined to fraction H (0.6 g), 26 to 27 fractions were combined to fraction I (0.2 g), 28 fraction was fraction J (0.5 g). The fractions were further purified by repeating HPLC using the n-hexane–EtOAc mixture as solvent system through a semi-preparative normal-phase column to give 55 known compounds and 3 new compounds.
Essential oil analysis
The air-dried sapwood chips (350 g) were subjected to hydrodistillation for 8 h using a Clevenger type apparatus. The moisture-free oil which yield 0.01% was obtained by treating with anhydrous Na2SO4. The compositions of the essential oils were analyzed by an ITQ Series GC mass system, equipped with a DB-5MS capillary column (30 m length × 0.25 mm inside diameter × 0.25 μm film thickness, J & W Scientific) and helium as a carrier gas with a flow rate of 1 ml min−1. The injector temperature was 240 ℃ and spilt ratio was 1:200. The oven temperature was start at 40 ℃, and increased by 5 ℃ min−1 to 130 ℃, then rose to 160 ℃ at a rate of 2 ℃ min−1, finally increased to 280 ℃ by 10 ℃ min−1 and held for 10 min. The EI source was 70 eV and 250 ℃. Quantification was obtained from percentage peak areas from the gas chromatogram. A Wiley/NBS Registry of Mass Spectral Data search and authentic reference compounds were used for substance identification. The Kovats retention index (KI), which is a parameter calculated in reference to n-alkanes that converts retention times into system-independent constants, was also confirmed [38]. Chromatography results expressed as area percentages were calculated with a response factor of 1.0.
Results and discussion
Volatile organic compounds analysis of sapwood
Table 1 presents the analysis result of essential composition of Taiwania sapwood. Totally, 25 compounds were identified in sapwood essential oil, including one monoterpenoid, α-terpineol (4); 23 sesquiterpenoids, namely α-copaene (5), α-cedrene (6), β-cedrene (7), β-copaene (8), γ-muurolene (9), α-muurolene (10), γ-cadinene (11), δ-cadinene (12), calamenene (13), α-cadinene (14), α-calacorene (15), elemol (16), globulol (17), cedrol (18), 1,10-di-epi-cubenol (19), epi-cubenol (20), γ-eudesmol (21), δ-cadinol (22), T-muurolol (23), α-eudesmol (24), α-cadinol (25), 8-cedren-13-ol (26), and cadalene (27); and one diterpenoid, ferruginol (28). Among them, α-cadinol (16.74%) was the most abundant compound.
Identification of sapwood non-volatile organic compounds
Three new compounds (1–3) (Fig. 1) and 55 known compounds were identified from sapwood of Taiwania. The known compounds were identified by spectra data and comparing with literature data. The identified known compounds were one fatty acid, i.e., 4,6,6-trimethylheptanoic acid (29) [39]; 6 benzenoids, which were ficusol (30) [40], vanillin (31) [41], trans-p-hydroxycinnamaldehyde (32) [42], 4-(3-hydroxypropyl)-2-methoxyphenol (33) [43], β-hydroxypropiovanillone (34) [44], and 3-methoxy-4-hydroxybenzoic acid (35) [44]; 12 sesquiterpenoids, including (2β,3α)-α-corocalene-2,3-diol (36) [17], epi-cubenol (20) [45], cryptomeridiol (37) [46], cedrol (18) [47], T-cadinol (38) [47], T-muurolol (23) [47], α-cadinol (25) [48], β-eudesmol (39) [49], (4R)-4-hydroxy-1,10-seco-muurol-5-ene-1,10-dione (40) [50], dysodensiol D (41) [51], 1α-hydroxy-4αH-1,2,3,4-tetrahydrocadalen-15-oic acid (42) [52], and 1-hydroxy-1,2,3,4-tetrahydrocadalen-15-oic acid (43) [53]; 6 diterpenoids, i.e., 3β-hydroxysugiol (44) [54], ferruginol (28) [55], hinokiol (45) [56], hinokione (46) [57], sugiol (47) [58], and sandaracopimarinol (48) [59]; 9 steroids, 3-epi-6-deoxocathasterone (49) [60], 7α-hydroxysitosterol (50) [61], 7β-hydroxysitosterol (51) [62], 7-ketositosterol (52) [63], 6β-hydroxystigmast-4-en-3-one (53) [63], ergone (54) [64], stigmast-4-en-3-one (55) [65], stigmastan-3-one (56) [66], and β-sitosterol (57) [67]; 21 lignans and norlignans (Fig. 2), which were (-)-pluviatolide (58) [68], ( +)-pluviatolide (59) [68], ( +)-7-methoxymatairesinol (60) [69], 7′-hydroxymatairesinol (61) [70], arctigenin (62) [71], diphyllin (63) [72], egonol (64) [73], helioxanthin (65) [74], justicidin B (66) [75], (2S,3R)-2-[(1S)-1-hydroxy-1-(3,4-methylenedioxyphen)methyl]-3-(3,4-methylenedioxybenzyl)-4-butanolide (67) [76], lariciresinol (68) [77], matairesinol (69) [78], pinoresinol (70) [79], 9,9′-dihydroxy-3,4-methylenedioxy-3′-methoxy(7-O-4′,8–5′)neolignan (71) [6], 4-[2-(1,3-benzodioxol-5-yl)-7-methoxy-1-benzofuran-5-yl]butanoic acid (72) [80], methyl 2-(1,3-benzodioxol-5-yl)-7-methoxy-1-benzofuran-5-carboxylate (73) [81], salicifoliol (74) [82], savinin (75) [83], Taiwanin C (76) [75], Taiwanin E (77) [84], and hinokinin (78) [85].
It is worthy to note, Taiwanin A was not found in sapwood of Taiwania (Fig. 2). According to the record, Taiwanin A is the unique lignan found in the heartwood of Taiwania; it only has been identified in Taiwania, not in other plants. Kampe and Magel classified the heartwood formation into two types based on distribution patterns of extractives in stem wood of various trees species [1]. (1) Type I (Robinia-type) heartwood formation, where the accumulation of phenolic extractives starts in the transition zoon, while no phenolic precursors were found in the aging sapwood. (2) Type II (Juglans-type) heartwood formation, where the phenolic precursors gradual accumulated centripetally with progressive aging of the sapwood tissues. Our previously study proposed the type III, Taiwania-type of heartwood formation, which was found the secondary reaction for lignans in sapwood [2]. This study further confirms that secondary reaction lignans occurred in sapwood of Taiwania. The biosynthesis of lignans, e.g., matairesinol, hinokinin, savinin, helioxanthin, and Taiwanin E has been synthesized in the sapwood.
New sesquiterpenoids identification
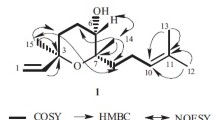
Three new sesquiterpenoids were identified in this study, the structure’s elucidation was reported in the following. Compound 1 was obtained as a colorless oil. The 1H NMR spectrum of 1 (Table 2) displayed resonances for one doublet methyl [δH 1.18 (3H, d, J = 6.0 Hz)], and two oxymethines [δH 3.80 (1H, dd, J = 6.0, 10.3 Hz) and 4.95 (1H, dt, J = 2.2, 10.3 Hz)], an olefinic proton [δH 5.82 (1H, d, J = 6.0 Hz)], an isopropyl group [δH 0.86 (3H, d, J = 6.6 Hz), 0.97 (3H, d, J = 6.6 Hz), and 1.61 (1H, m)]. The 13C NMR and distortionless enhancement by polarization transfer (DEPT) experiments revealed 15 carbon signals, consisting of three methyl, two aliphatic methylene, three aliphatic methine, two oxygenated methine, one olefinic methine, three quaternary olefinic, and one carboxyl carbons. Its high-resolution atmospheric pressure chemical ionization mass spectrometry (HR-APCI-MS) gave a [M + H]+ ion at m/z 267.2663, establishing the molecular formula of 1 as C15H22O4 with five degrees of unsaturation. Ascribing to cnjugated double bond, H-5 exhibited very low field at δH 4.95, and the carbon signals at δC 122.6 (CH), δC 132.8 (C), δC 134.0 (C), δC 157.8 (C), and δC 173.2 (C) indicated the existence of a C = CH, a C = C, and a O = C–OH systems. The remaining two degrees of unsaturation identified 1 as a bicyclic compound. The HMBC (Fig. 3) data showed correlations H-12/C-1, C-11, C-13; H-13/C-1, C-11, C-12, and the COSY (Fig. 4) signals showed coupling between the H-1/H-11; H-11/H-12; H-11/H-13. That confirmed the isopropyl group attached to C-1. From the COSY spectrum showed coupling between the H-1/H2; H-2/H-3; H-5/H-6; H-6/H-7; H-7/H-8; H-7/H-14, and the HMBC signal showed correlations H-5/C-10; H-6/C-14; H-7/C-9; H-8/C-1, C-6, C10; H-14/C-8. Taking the above evidences together, 1 identified as a 5–7 ring compound and pinpointed the location of 4-carboxyl group, OH-5, OH-6, and methyl group (Me)-7. The NOESY (Fig. 5) signals showed correlations of H-5/H-14; H-6/H-14; H-7/H-11 as well as the coupling constant confirmed that H-5, H-6, and Me-7 were β and isopropyl-1 and H-7 were α configuration. Based on these data confirmed the proposed structure of 1 and named Taiwania A, and it is a new skeleton sesquiterpene to the best of our understanding.
Compound 2, a colorless oil, was assigned a molecular formula of C15H22O5 on the basis of HR-APCI-MS and 13C NMR. The 1H NMR and 13C NMR data of 2 (Table 2) were similar to those of 1, indicated that compound 2 was also the same type sesquiterpenoid derivative. Analysis of NMR data revealed that OH-7 of 2 replaced H-7 of 1. This supported by the COSY (Fig. 4) correlations showed H-1/H2; H-2/H-3; H-1/H-11; H-5/H-6; H-11/H-12, H-13, and HMBC (Fig. 3) correlation signals H-8/C-1, C-6, C-10; H-6/C-5, C-7; H-14/C-6, C-7, C-8. The NOESY (Fig. 5) correlations observed H-5/H-14; H-6/H-14. Hence, the structure of 2 is confirmed and named Taiwania B.
The molecular formula of compound 3 was C16H24O6 by electrospray ionization mass spectrometry (ESI-MS) and NMR data, indicated five degree of unsaturation. Sixteen carbon signals were observed in the 13C NMR spectrum of 3 and were assigned by the DEPT experiments displayed four aliphatic methyl, two aliphatic methylene, two aliphatic methine, two oxygenated methine, three oxygenated quaternary, one olefinic methine, one olefinic quaternary, and one carbonyl carbons. The carbon signals at δC 132.8 (CH), δC 134.1 (C), and δC 153.5 (C) indicated the existence of a C=CH and a C=O systems. The carbonyl carbon (C-1′) exhibited very high field at δC 153.5 supported that was carbonate group [–O–C(= O)–O–] [86]. The remaining three degrees of unsaturation identified 3 as a tricyclic compound. Its 1H NMR spectrum of 3 (Table 2) showed the presence of four methyl protons [δH 0.92 (3H, d, J = 6.6 Hz), 0.97 (3H, d, J = 6.6 Hz), 1.27 (3H, s), 1.31 (3H, s)], two oxymethines protons [δH 3.76 (1H, d, J = 9.1 Hz) and 4.66 (1H, d, J = 9.1 Hz)], and an olefinic proton [δH 5.75 (1H, d, J = 1.6 Hz)]. The HMBC (Fig. 3) correlations showed H-12/C-1, C-11, C-13; H-13/C-1, C-11, C-12, and the COSY (Fig. 4) correlations showed H-1/H-11; H-11/H-12 and H-13 confirmed that the isopropyl group attached to C-1. The carbonyl carbon (C-1′) was attached on C-4 and C-5 by the HMBC correlations showed H-5/C-4, C-6, C-2′; H-15/C-3, C-4, C-10. The double bond was assigned to located C-8 and C-9, basing on the HMBC correlation showed H-8/C-1, C-6, C-10. The three hydroxyl groups were located at C-6, C-7, and C-10, respectively, which were assured by the HMBC correlations showed H-6/C-5, C-7, C-14; H-14/C-6, C-7, C-8; H-15/C-3, C-4, C-10. The NOESY (Fig. 5) signals showed the correlations of H-5/H-14, H-15; H-6/H-11, H-12, H-13, H-14, H-15 indicated that isopropyl-1, Me-4, H-5, H-6, and Me-7 were in β orientation. On the basis of these data, compound 3 is assigned the proposed structure and named Taiwania C, and it is as a new natural product.
Conclusion
The biosynthesis and accumulation of the extractives is an important process for the formation of heartwood, and the content and types of the extractives in the heartwood also influence the special properties of wood. Previously, we proposed a new type of Taiwania-type heartwood formation mechanism, i.e., phenolic compounds have completed the secondary reaction in the sapwood, forming a complete structure (Tsao et al. [2]). Although the current research on the phytochemistry of Taiwania is quite complete, there is no research on the sapwood extractives. This study isolated and identified 78 compounds from sapwood of Taiwania, including 1 fatty acid, 6 monoaromatics, 1 monoterpenoid, 34 sesquiterpenoids, 6 diterpenoids, 9 steroids, and 21 lignans and norlignans. Among these, 3 new skeleton sesquiterpenoids, which were Taiwania A, Taiwana B, and Taiwania C were first time identified. During the past decades, a number of studies have reported the metabolites of Taiwania’s wood. However, to our best of knowledge, this is the only study focusing on the elucidation of sapwood compounds. Interestingly, besides 3 new skeleton compounds, all of the 75 known compounds had been reported previously. This study confirmed again that the secondary reaction of lignans occurred in the sapwood of Taiwania. It provided the evidence for type III, Taiwania-type of heartwood formation (Tsao et al. [2]). However, the unique and dominant lignan, Taiwanin A, was not found in the sapwood. The result once again confirmed that all lignans of Taiwania are synthesized in sapwood, except Taiwanin A.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- TZ:
-
Transition zone
- Taiwania:
-
Taiwania cryptomerioides Hayata
- HR-MS:
-
High-resolution mass spectrometry
- MeOH:
-
Methanol
- HPLC:
-
High-performance liquid chromatography
- EtOAc:
-
Ethyl acetate
- KI:
-
The Kovats retention index
- DEPT:
-
Distortionless enhancement by polarization transfer
- HR-APCI-MS:
-
High-resolution atmospheric pressure chemical ionization mass spectrometry
- Me:
-
Methyl group
- ESI-MS:
-
Electrospray ionization mass spectrometry
References
Kampe A, Magel E (2013) New insights into heartwood and heartwood formation. In: Jorg F (ed) Cellular aspects of wood formation. Springer, Heidelberg, pp 71–88
Tsao NW, Sun YH, Chien SC, Chu FH, Chang ST, Kuo YH, Wang SY (2016) Content and distribution of lignans in Taiwania cryptomerioides Hayata. Holzforschung 70:511–518
Lin WH, Fang JM, Cheng YS (1995) Uncommon diterpenes with the skeleton of six-five-six fused-rings from Taiwania cryptomerioides. Phytochemistry 40:871–873
Lin WH, Fang JM, Cheng YS (1996) Diterpenes and related cycloadducts from Taiwania cryptomerioides. Phytochemistry 42:1657–1663
Lin WH, Fang JM, Cheng YS (1998) Diterpenoids and steroids from Taiwania cryptomerioides. Phytochemistry 48:1391–1397
Lin WH, Fang JM, Cheng YS (1999) Lignans from Taiwania cryptomerioides. Phytochemistry 50:653–658
Kuo YH, Chang CI (2000) Podocarpane-type trinorditerpenes from the bark of Taiwania cryptomerioides. J Nat Prod 63:650–652
Kuo YH, Chang CI, Lee CK (2000) Six podocarpane-type trinorditerpenes from the bark of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 48:597–599
Kuo YH, Chien SC (2001) Quinone-type podocarpanes from the bark of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 49:1033–1035
Kuo YH, Chien SC, Huang SL (2002) Four new podocarpane-type trinorditerpenes from the bark of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 50:544–546
Kuo YH, Chyu CF, Lin HC (2003) Cadinane-type sesquiterpenes from the roots of Taiwania cryptomerioides Hayata. Chem Pharm Bull (Tokyo) 51:986–989
Chang CI, Chien SC, Lee SM, Kuo YH (2003) Three novel 5(6–>7) abeoabietane-type diterpenes from the bark of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 51:1420–1422
Chang CI, Tseng MH, Kuo YH (2005) Five new diterpenoids from the bark of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 53:286–289
Chien SC, Chen CC, Chiu HL, Chang CI, Tseng MH, Kuo YH (2008) 18-nor-podocarpanes and podocarpanes from the bark of Taiwania cryptomerioides. Phytochemistry 69:2336–2340
Chien SC, Kuo YH (2004) Two novel 14-Nor-13,14-secopodocarpanes from the bark of Taiwania cryptomeriodes. Helv Chim Acta 87:554–559
Chyu CF, Chiang YM, Lin HC, Kuo YH (2004) Two novel 9,11-seco-11-norabietanes from the roots of Taiwania cryptomerioides. Tetrahedron Lett 45:641–643
Chyu CF, Ke MR, Chang YS, Chien SC, Kuo YH (2007) New cadinane-type sesquiterpenes from the roots of Taiwania cryptomerioides Hayata. Helv Chim Acta 8:1514–1521
Chyu CF, Kuo YH (2007) New lignans from the roots of Taiwania cryptomerioides Hayata. Helv Chim Acta 90:738–747
Chyu CF, Lin HC, Kuo YH (2005) New abietane and seco-abietane diterpenes from the roots of Taiwania cryptomerioides. Chem Pharm Bull (Tokyo) 53:11–14
Xiang Y, Yang SP, Zhan ZJ, Yue JM (2004) Terpenoids and phenols from Taiwania flousiana. Acta Bot Sin 46:1002–1008
Su YC, Ho CL, Wang EIC (2006) Analysis of leaf essential oils from the indigenous five conifers of Taiwan. Flavour Fragr J 21:447–452
Wang SY, Wang YS, Tseng YH, Lin CT, Liu CP (2006) Analysis of fragrance compositions of precious coniferous woods grown in Taiwan. Holzforschung 60:528–532
Majetich G, Shimkus JM (2010) The taiwaniaquinoids: a review. J Nat Prod 73:284–298
Chang ST, Chen PF, Wang SY, Wu HH (2001) Antimite activity of essential oils and their constituents from Taiwania cryptomerioides. J Med Entomol 38:455–457
Chang ST, Cheng SS, Wang SY (2001) Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides). J Chem Ecol 27:717–724
Chang ST, Wang SY, Kuo YH (2003) Resources and bioactive substances from Taiwania (Taiwania cryptomerioides). J Wood Sci 49:1–4
Chang ST, Wang SY, Wu CL, Chen PF, Kuo YH (2000) Comparison of the antifungal activity of cadinane skeletal sesquiterpenoids from Taiwania (Taiwania cryptomerioides Hayata) heartwood. Holzforschung 54:241–245
Ho CL, Yang SS, Chang TM, Su YC (2012) Composition, antioxidant, antimicrobial and anti-wood-decay fungal activities of the twig essential oil of Taiwania cryptomerioides from Taiwan. Nat Prod Commun 7:261–264
Huang GJ, Deng JS, Huang SS, Chang CI, Chang TN, Shie PH, Kuo YH (2011) Anti-inflammatory activities of 6β-acetoxy-7α-hydroxyroyleanone from Taiwania cryptomerioides Hayata ex vivo and in vivo. J Agric Food Chem 59:11211–11218
Wang SY, Wu JH, Shyur LF, KuoYH CST (2002) Antioxidant activity of abietane-type diterpenes from heartwood of Taiwania cryptomerioides Hayata. Holzforschung 56:487–492
Chang ST, Wang DS, Wu CL, Shiah SG, Kuo YH, Chang CJ (2000) Cytotoxicity of extractives from Taiwania cryptomerioides heartwood. Phytochemistry 55:227–232
Cho JY, Park J, Kim PS, Yoo ES, Baik KU, Park MH (2001) Savinin, a lignan from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T cell proliferation. Biol Pharm Bull 24:167–171
Ban HS, Lee S, Kim YP, Yamaki K, Shin KH, Ohuchi K (2002) Inhibition of prostaglandin E2 production by taiwanin C isolated from the root of Acanthopanax chiisanensis and the mechanism of action. Biochem Pharmacol 64:1345–1354
Ho PJ, Chou CK, Kuo YH, Tu LC, Yeh SF (2007) Taiwanin A induced cell cycle arrest and p53-dependent apoptosis in human hepatocellular carcinoma HepG2 cells. Life Sci 80:493–503
Wen CC, Kuo YH, Jan JT, Liang PH, Wang SY, Liu HG, Lee CK, Chang ST, Kuo CJ, Lee SS, Hou CC, Hsiao PW, Chien SC, Shyur LF, Yang NS (2007) Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem 50:4087–4095
Shyur LF, Lee SH, Chang ST, Lo CP, Kuo YH, Wang SY (2010) Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways. Phytomedicine 18:16–24
Wang HC, Tseng YH, Wu HR, Chu FH, Kuo YH, Wang SY (2014) Anti-proliferation effect on human breast cancer cells via inhibition of pRb phosphorylation by taiwanin E isolated from Eleutherococcus trifoliatus. Nat Prod Commun 9:1303–1306
Adams RP (2005) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J Am Soc Mass Spectrom 16:1902–1903
Katritzky AR, Zhang S, Hussein AH, Fang Y, Steel PJ (2001) One-carbon homologation of carboxylic acids via BtCH2TMS: a safe alternative to the Arndt-Eistert reaction. J Org Chem 66:5606–5612
Kuo HT, Peng CF, Huang HY, Lin CH, Chen IS, Tsai IL (2011) Chemical constituents and antitubercular activity of Formosan Pisonia umbellifera. Planta Med 77:736–741
Yang C, Peng W, Yang B, Zhang J, Chen Y (2016) A new sesquiterpenoid from Polyalthia petelotii. Nat Prod Res 30:1565–1570
Konrádová D, Kozubíková H, Doležal K, Pospíšil J (2017) Microwave-assisted synthesis of phenylpropanoids and coumarins: total synthesis of osthol. Eur J Org Chem 2017:5204–5213
Hemelaere R, Carreaux F, Carboni B (2015) A diastereoselective route to trans-2-Aryl-2,3-dihydrobenzofurans through sequential cross-metathesis/isomerization/allylboration reactions: synthesis of bioactive neolignans. Eur J Org Chem 2015:2470–2481
Mo EJ, Ahn JH, Jo YH, Kim SB, Hwang BY, Lee MK (2017) Inositol derivatives and phenolic compounds from the roots of Taraxacum coreanum. Molecules 22:1349
Bohlmann F, Zdero C, Robinson H, King RM (1979) Neue cadinen- und norcadinen-derivate aus Heterotheca grandiflora. Phytochemistry 18:1675–1680
Tebbaa M, Hakmaoui AE, Benharref A, Akssira M (2011) Short and efficient hemisynthesis of α-eudesmol and cryptomeridiol. Tetrahedron Lett 52:3769–3771
Cheng SS, Chung MJ, Lin CY, Wang YN, Chang ST (2012) Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J Agric Food Chem 60:124–128
Lee CK, Chang MH (2000) The chemical constituents from the heartwood of Eucalyptus Citriodora. J Chin Chem Soc 47:555–560
Morita M, Nakanishi H, Morita H, Mihashi S, Itokawa H (1996) Structures and spasmolytic activities of derivatives from sesquiterpenes of Alpinia speciosa and Alpinia japonica. Chem Pharm Bull 44:1603–1606
Kiem PV, Minh CV, Nhiem NX, Cuc NT, Quang NV, Tuan Anh HL, Tai BH, Yen PH, Hoai NT, Ho KY, Kim N, Park S, Kim SH (2014) Muurolane-type sesquiterpenes from marine sponge Dysidea cinerea. Magn Reson Chem 52:51–56
Xie BJ, Yang SP, Yue JM (2008) Terpenoids from Dysoxylum densiflorum. Phytochemistry 69:2993–2997
Delgado G, del Socorro OM, Chávez MI, Ramírez-Apan T, Linares E, Bye R, Espinosa-García FJ (2001) Antiinflammatory constituents from Heterotheca inuloides. J Nat Prod 64:861–864
Egas V, Salazar-Cervantes G, Romero I, Méndez-Cuesta CA, Rodríguez-Chávez JL, Delgado G (2018) Anti-Helicobacter pylori metabolites from Heterotheca inuloides (Mexican arnica). Fitoterapia 127:314–320
Wittayalai S, Sathalalai S, Thorroad S, Worawittayanon P, Ruchirawat S, Thasana N (2012) Lycophlegmariols A-D: cytotoxic serratene triterpenoids from the club moss Lycopodium phlegmaria L. Phytochemistry 76:117–123
Dang J, Wang QL, Tao YD, Mei LJ, Yuan X, Zhao JQ, Shao Y, Zhang L (2017) Terpene from roots of Salvia prattii. Chem Nat Compd 53:781–783
Zhang HF, Zheng XL, Wang L, Xu BY, Yao GD, Guo RX, Shi QW (2014) Abietane, labdane, and Pimarane diterpenoids from the pulp of Torreya nucifera. Chem Nat Compd 50:165–168
Feliciano AS, Medarde M, Lopez JL, Miguel del Corral JM, Puebla P, Barrero AF (1988) Terpenoids from leaves of Juniperus thurifera. Phytochemistry 27:2241–2248
Marcos IS, Beneitez Villamor A, Moro RF, Basabe P, Diez D, Urones JG (2010) Lateral lithiation in terpenes: synthesis of (+)-ferruginol and (+)-sugiol. Tetrahedron 66:7773–7780
Morisawa J, Kim CS, Kashiwagi T, Tebayashi S, Horiike M (2002) Repellents in the Japanese cedar, Cryptomeria japonica, against the pill-bug, Armadillidium vulgare. Biosci Biotechnol Biochem 66:2424–2428
Fujioka S, Noguchi T, Watanabe T, Takatsuto S, Yoshida S (2000) Biosynthesis of brassinosteroids in cultured cells of Catharanthus roseus. Phytochemistry 53:549–553
Shrestha S, Lyu HN, Park JH, Lee DY, Cho JG, Cui EJ, Chung IS, Baek NI (2011) Sterols from the leafy culms of Desmostachya bipinnata. Chem Nat Compd 47:852–853
Gao J, Yue Q, Ji Y, Cheng B, Zhang X (2013) Novel synthesis strategy for the preparation of individual phytosterol oxides. J Agric Food Chem 61:982–988
Wang QY, Cui GX, Wu JC, Chen YG (2015) Steroids from Trigonostemon heterophyllus. Chem Nat Compd 51:1196–1198
Hao JD, Zheng JJ, Chen M, Wang CY (2017) Cytochalasins from the gorgonian-derived fungus Aspergillus sp. XS-2009-0B15. Chem Nat Compd 53:732–735
Gao J, Aisa HA (2017) Terpenoids from Euphorbia soongarica and their multidrug resistance reversal activity. J Nat Prod 80:1767–1775
Luo JR, Ma QY, Zhao YX, Yi TM, Li CS, Zhou J (2009) Palaeophytochemical components from the miocene-fossil wood of Pinus Griffithii. J Chin Chem Soc 56:600–605
Shah H, Khan AA (2017) Phytochemical characterisation of an important medicinal plant, Chenopodium ambrosioides Linn. Nat Prod Res 31:2321–2324
Duan S, Huang S, Gong J, Shen Y, Zeng L, Feng Y, Ren W, Leng Y, Hu Y (2015) Design and synthesis of novel arctigenin analogues for the amelioration of metabolic disorders. ACS Med Chem Lett 6:386–391
Eklund PC, Sundell FJ, Smeds AI, Sjoholm RE (2004) Reactions of the natural lignan hydroxymatairesinol in basic and acidic nucleophilic media: formation and reactivity of a quinone methide intermediate. Org Biomol Chem 2:2229–2235
Moraux T, Dumarçay S, Gérardin P, Gérardin-Charbonnier C (2017) Derivatives of the lignan 7’-hydroxymatairesinol with antioxidant properties and enhanced lipophilicity. J Nat Prod 80:1782–1790
Xu X, Li C, Lei M, Zhu Z, Yan J, Shen X, Hu L (2016) Synthesis and decreasing Aβ content evaluation of arctigenin-4-yl carbamate derivatives. Bioorg Med Chem Lett 26:2988–2991
Ren Y, Lantvit DD, Deng Y, Kanagasabai R, Gallucci JC, Ninh TN, Chai HB, Soejarto DD, Fuchs JR, Yalowich JC, Yu J, Swanson SM, Kinghorn AD (2014) Potent cytotoxic arylnaphthalene lignan lactones from Phyllanthus poilanei. J Nat Prod 77:1494–1504
Reiter C, Capcı Karagöz A, Fröhlich T, Klein V, Zeino M, Viertel K, Held J, Mordmüller B, Emirdağ Öztürk S, Anıl H, Efferth T, Tsogoeva SB (2014) Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells. Eur J Med Chem 75:403–412
Kao TT, Lin CC, Shia KS (2015) The total synthesis of retrojusticidin B, justicidin E, and helioxanthin. J Org Chem 80:6708–6714
Da Silva R, Ruas MM, Donate PM (2007) Complete assignments of 1H and 13C NMR spectral data for arylnaphthalene lignan lactones. Magn Reson Chem 45:902–904
Yamauchi S, Tanakaa T, Kinoshita Y (2001) First highly stereoselective synthesis of (+)-dihydrosesamin, a trisubstituted tetrahydrofuran-type of lignan, by using highly erythro-selective aldol condensation. J Chem Soc Perkin Trans 1 2001:2158–2160
Wang LQ, Zhao YX, Zhou L, Zhou J (2009) Lignans from Gnetum montanum Markgr. f. megalocarpua. Chem Nat Compd 45:424–426
Li HL, Song HC, Zhang Y, Chen YG (2016) Chemical Constituents of the barks of Podocarpus macrophyllus. Chem Nat Compd 52:539–541
Lajter I, Pan SP, Nikles S, Ortmann S, Vasas A, Csupor-Löffler B, Forgó P, Hohmann J, Bauer R (2015) Inhibition of COX-2 and NF-κB1 gene expression, NO production, 5-LOX, and COX-1 and COX-2 enzymes by extracts and constituents of Onopordum acanthium. Planta Med 81:1270–1276
Oztürk SE, Akgül Y, Anil H (2008) Synthesis and antibacterial activity of egonol derivatives. Bioorg Med Chem 16:4431–4437
Rios-Motta J, Avella E (2010) 2-Arylbenzofuran neolignans from the bark of Nectandra purpurascens (Lauraceae). Nat Prod Commun 5:1063–1066
Chang HS, Lee SJ, Yang CW, Chen IS (2010) Cytotoxic sesquiterpenes from Magnolia kachirachirai. Chem Biodivers 7:2737–2747
Da Silva R, Pedersoli S, Lacerda V Jr, Donate PM, de Albuquerque S, Bastos JK, de Matos Araújo AL, Andrade e Silva ML (2005) Complete assignments of 1H and 13C NMR spectral data for benzylidenebenzyl butyrolactone lignans. Magn Reson Chem 43:966–969
Kim T, Jeong KH, Kang KS, Nakata M, Ham J (2017) An Optimized and general synthetic strategy to prepare arylnaphthalene lactone natural products from cyanophthalides. Eur J Org Chem 2017:1704–1712
Xia Y, You J, Zhang YY, Su ZL (2009) Synthesis, anti-virus and anti-tumour activities of dibenzylbutyrolactone lignans and their analogues. J Chem Res 2009:565–569
Laserna V, Fiorani G, Whiteoak CJ, Martin E, Escudero-Adán E, Kleij AW (2014) Carbon dioxide as a protecting group: highly efficient and selective catalytic access to cyclic cis-diol scaffolds. Angew Chem Int Ed 53:10416–10419
Acknowledgements
This study is financially supported by the Ministry of Science and Technology, Taiwan.
Funding
This study is financially supported by the Ministry of Science and Technology, Taiwan (Grant no. 103–2321-B-005–021).
Author information
Authors and Affiliations
Contributions
NWT performed the experiments, analyzed the data. SCC analyzed the data. YHK analyzed the data. SYW designed this study. NWT wrote the manuscript in consultation with SCC and SYW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tsao, NW., Chien, SC., Kuo, YH. et al. Extractives elucidation of Taiwania cryptomerioides sapwood. J Wood Sci 67, 16 (2021). https://doi.org/10.1186/s10086-021-01947-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-021-01947-x