Abstract
Acetylation of softwoods has been largely investigated to increase the dimensional stability and biological resistance of wood. However, the knowledge of this technology has not been applied to tropical hardwood species up to date. The objective of this work was to study the effect of acetylation on nine tropical hardwood species, from forest plantations in Costa Rica, by applying acetic anhydride in three different treatment times (1 h, 2.5 h, 4 h), as well as to evaluate this by Fourier-Transform Infrared Spectroscopy (FTIR). Results showed that weight percentage gain (WPG) of wood varied from 2.2 to 16.8%, with Vochysia ferruginea species showing the highest WPG, and Gmelina arborea and Tectona grandis species exhibiting the lowest WPGs. Tropical woods such as Enterolobium cyclocarpum, Hieronyma alchorneoides and Samanea saman exhibited statistical differences among treatment times, whereas the rest of the species studied showed no significant differences. In general, the most effective acetylation time was 2.5 h for all the species. The ratio of intensity (RI) from the FTIR spectra was greater at the 1732 cm−1, 1372 cm−1 and 1228 cm−1 peaks for all tropical species, associated with lignin. A good correlation between the RI of those peaks and WPG was found; the same was also found between all RIs and each other. Meanwhile, RI associated to the hemicelluloses and lignin (1592 cm−1 and 1034 cm−1 peaks, respectively) showed no correlation with WPG, nor between each other or with the other RIs. Furthermore, it was suggested that RI at 1732 cm−1 (associated to acetyl groups C=O) can be considered as a reliable indicator of the degree of acetylation for tropical hardwood species. Finally, it was observed that tropical hardwoods having more suitable anatomical features, like larger vessel diameter, higher ray width and frequency, and lesser deposits such as gums and tyloses in the vessels, resulted in significantly higher WPGs.
Similar content being viewed by others
Introduction
Wood has been used by the humans for centuries, since it is a natural material, easy-to-work, renewable, widely abundant and sustainable [1, 2]. As a lignocellulosic complex, wood does have a structure of cellular walls made of biopolymers such as cellulose and hemicelluloses as well as phenolic polymers like lignin [1, 3, 4]. Despite its positive characteristics, wood can easily be affected by the increasing presence of moisture in its hierarchical structure, which can lead to its dimensional instability and/or biological deterioration, or degradation by fungi, insects or bacteria [1, 4]. Polymeric constituents of wood, e.g., lignin, cellulose and hemicelluloses, contain a large amount of free hydroxyl groups [1, 2]. Hemicelluloses, accessible or non-crystalline cellulose, and lignin are mainly responsible for moisture uptake [3, 5]. In these polymers, free hydroxyl groups (OH− anions) adsorb and release water depending on the changes in temperature and relative humidity causing cell walls—and all of the structure of wood—to adjust to the presence (or absence) of other OH− anions, such as those of water, thus giving way to changes in dimensional stability of wood, or to potential biological attacks by fungi [1, 3, 6].
The presence and amount of hydroxyl groups, capable of forming hydrogen bonds with water molecules, are crucial for dimensional stability. This takes place in sorption sites (as they are commonly termed) which are mainly present in hemicelluloses, followed by cellulose and lignin [7]. Accessibility of these sorption sites in wood has gained a high interest as methods to improve wood performance by chemical treatments have been implemented [8]. In the case of cellulose, its configuration in microfibril aggregates makes hydroxyl groups on the surface the only possible sorption sites [9], whereas the amount of sorption sites is much greater in hemicelluloses and lignin [7]. During adsorption in these sites, the water molecule with two full-strength covalent bonds can become bound by two relatively strong H-bonds with a pair of nearby –OH groups of the amorphous polysaccharide polymers, in low moisture content. In meanwhile, conjunction in the H-bond network increases with increasing moisture content, gradually allowing the coalescence of water vapour molecules with already absorbed water molecules to form water dimers [8]. Thus, this gain in moisture makes the wood dimensionally unstable.
It is due to the dimensional instability that current research sets the objective of implementing chemical modification to wood for lower water uptake, aiming at achieving higher dimensional stability and increased biological resistance [1, 10,11,12,13,14]. Amongst these chemical modification techniques, a typical one is that of acetic anhydride [5, 15,16,17,18], wherein the OH− anion group in wood polymeric components becomes chemically bound to a residue of the acetate (CH3COO–) of an acetic anhydride molecule [(CH3CO)2O]; this is well known as acetylation of wood [1]. In this process, the OH− anion group is reduced, decreasing hygroscopicity of the wood and thus, increasing its dimensional stability and biological resistance to fungi [4, 19,20,21]. Thybring [19] has clearly stated that decay of acetylated wood cannot progress below the level of 25% moisture content.
As a matter of fact, the composition and distribution of the polymeric constituents in hardwoods differ from those in softwoods, which cause species groups to vary in their sorption sites. Besides these variances, it is true that hardwood species have their own proportion of structural polymeric constituents; therefore, hydroxyl groups are present in different amounts. In hardwood species, hydroxyl groups were reported to be present at percentages of 2.0 to 4.5%, whereas in softwoods, these vary from 0.5 to 1.7% (Rowell, 2016). This difference is attributed to hemicelluloses and lignin compositions: in hardwood species, hemicelluloses contain mostly xylans, whilst hemicelluloses of softwoods contain mostly glucomannans as well as lignin in a lesser amount [10, 22]. Thus, they possess sorption sites at varying proportions [7, 21].
Studies on wood acetylation have indicated that acetylated softwoods achieve a much higher weight gain, as compared with hardwood species [1], despite the fact they are more abundant in hemicelluloses. Nonetheless, hardwoods contain a higher amount of xylans, which do not have a primary hydroxyl group in which to react [23]. Moreover, softwood species contain a higher percentage of lignin, the polymeric component in which the higher percentage of acetylation typically takes place [1, 3].
In addition to the differences in the type and proportion of hemicelluloses, the anatomical structure differs considerably between wood groups: hardwoods are characterised by the presence of conducting elements such as vessels, whereas softwoods are made of tracheids [24]. This distinction causes the flow of liquids to vary largely, between the wood groups [25], thus affecting the acetylation reaction (associated with the liquid flow in wood) as well as other processes performed in the wood cell walls. In fact, most of the research on acetylated wood has been focused on softwoods [4], primarily on pine species [16, 17, 26,27,28].
In spite of these differences, some research work on hardwood species has been carried out. Though, the tropical species remain scarcely known concerning the processes that can be employed to improve their physical and biological properties. One of the few studies is that of Matsunaga et al. [29] on wood species like Paraserianthes falcata, Alstonia macrophylla, Pinus caribaea and Heveabrasiliensis which confirmed that acetylation can increase the dimensional stability of wood, up to 60%. Because of the lack of knowledge on the effects of acetylation on tropical hardwoods, there must be a focusing effort to expand the knowledge of the acetylation on such woods, thus widening their potential applications [30], especially for hardwood species of forest plantations.
In Central America, Costa Rica has been implementing reforestation programs with fast-growing plantations that utilise a variety of tropical hardwood species for lumber production [31]. In these programs, early-age tree harvesting yields juvenile wood [4], which is characterised by dimensional instability. Therefore, acetylation provides a way to improve dimensional stability, increase durability and advance other material properties of tropical hardwood species [4, 5, 11, 20, 32]. Given that plantation species in the tropical regions are valuable, research efforts to upgrade the quality of wood and wood products are very important [33].
However, because of the variations in the anatomical structure of tropical hardwoods, which differ from that of softwoods [34], there is insufficient technical information respecting the potential of tropical species for chemical modification, e.g., acetylation [4, 20, 32, 35]. Hence, the aim of this study was to evaluate the effects of acetylation, using acetic anhydride in liquid phase, on nine tropical hardwood species, commonly cultivated in forest plantations in Costa Rica (Cedrela odorata, Cordia alliodora, Enterolobium cyclocarpum, Gmelina arborea, Hieronima alchornoides, Samanea saman, Tectona grandis, Vochysia ferruginea and Vochysia guatemalensis), and the analysis of key parameters such as solution uptake and weight percentage gain by Fourier-Transform Infrared Spectroscopy (FTIR).
Methodology
Materials
Sapwood, originating from nine Costa Rican fast-growing plantation species, was utilised in the work. It is well established that only sapwood of these tropical hardwood species has a good permeability [25, 36, 37]. Species used included Cedrela odorata, Cordia alliodora, Enterolobium cyclocarpum, Gmelina arborea, Hieronyma alchorneoides, Samanea saman, Tectona grandis, Vochysia ferruginea and Vochysia guatemalensis. The wood materials came from plantations varying from 4- to 8-year-old trees, using three different trees per each species. Once sawn, planks were dried to a moisture content of approx. 12%, and afterwards, 60 samples, measuring 50 mm × 50 mm × 20 mm (radial × tangential × longitudinal), were prepared. The chemical reagents used were acetic anhydride [(CH3CO)2O], at 98% concentration (J.T. Baker, Madrid, Spain), and glacial acetic acid [CH3COOH], at 99% concentration (Químicos Holanda S.A., Costa Rica).
Acetylation process
The acetylation process was carried out in a vacuum-pressure reactor, measuring 10 cm in diameter and 31 cm in length, with a 2.5 l capacity. The process started by introducing 15 samples in the reactor, then applying a vacuum for 15 min at − 70 kPa (gauge mark), after which, the acetic anhydride and glacial acetic acid solution was introduced in a 92:8 volume ratio, respectively. Once inside, the materials were subjected to a 690 kPa pressure for 30 min, then the excess liquid solution was removed, and nitrogen gas was injected in, to serve as the inert medium to control the internal temperature of the wood. For the reaction, temperature was fixed at 120 °C and three different acetylation times were applied for each species, that is, 1 h, 2.5 h and 4 h, labelled as 1 h-acetylation-time, 2.5 h-acetylation-time and 4 h-acetylation-time, respectively. At the end, the treated samples were rinsed, kept in acetone for 1 h, and subsequently left to dry in ambient conditions for 1 week. Following, they were introduced in a conditioning chamber (for 2 weeks), at climatic conditions 20 °C and 65% relative humidity, after which, they were weighed up.
Evaluation of the acetylation process
Per each acetylation time, 15 samples were prepared for each acetylation time, each measuring 50 mm × 50 mm × 20 mm (radial × tangential × longitudinal). Another set of 15 samples was left untreated (controls) to be compared with the acetylated materials. For weight measurement, samples were placed into an oven for 24 h, before and after the acetylation process, to determine the oven-dry weights. The acetylation process was evaluated by determining the uptake of the solution (uptake) (see Eq. 1) and weight percentage gain (WPG), once the process was finished (see Eq. 2), considering the weight of the sample in oven-dry condition as the initial weight.
FTIR
One sample per treatment interval as well as one untreated sample were taken per each species and ground to a size of 420 µm and 250 µm, 40 mesh and 60 mesh, respectively. These samples were oven-dried at 105 °C until reaching a constant weight. This was followed by an FTIR scan using an Nicolet 380 FTIR spectrometer (Thermo Scientific, Mundelein, Illinois, USA) with a single reflecting cell, equipped with a diamond prism. The equipment was configured to perform readings accumulating 32 explorations, with a 1 cm−1 resolution, with background correction before each measurement. The obtained FTIR spectra were processed with Spotlight 1.5.1, HyperView 3.2 and Spectrum 6.2.0 software, developed by Perkin Elmer Inc (Waltham, Massachusetts, USA). Three spectrums were obtained per each acetylation time.
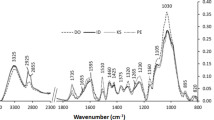
The main vibrations in absorbance (Fig. 1a), where the biggest changes occurred in the wood, were identified (Fig. 1b); present in the peaks at 1034 cm−1 (carbon–oxygen (C–O) stretching), at 1228 cm−1 (carbon–hydrogen (C–H) stretching), at 1372 cm−1 (methyl group (of the acetyl unit) stretching in cellulose and hemicelluloses), at 1592 cm−1 (conjugated carbonyl (C=O) stretching), and at 1732 cm−1 (non-conjugated carbonyl (C=O) stretching). Then, the ratio of the intensity absorbance of those groups at 1034, 1228, 1372, 1592 and 1732 cm−1 was assessed between the control (untreated) samples and the acetylated samples (Eq. 3).
Statistical analysis
First, the data were tested for normality and homogeneity, and strange data or outliers of the variables evaluated were eliminated. Then, a descriptive analysis was carried out: the mean and the standard deviation (SD) were determined for each variable studied. A variance analysis (ANOVA) with a statistical significance level at p < 0.05 was applied to determine the effect of acetylation time (independent variable) on the uptake and WPG (response variables). Tukey’s test was used to determine the statistical significance of the difference between the means of the variables. A second analysis was carried out to establish correlations between the WPG and the IR of the peaks affected by acetylation (1034, 1228, 1372, 1592 and 1732 cm−1). This analysis was performed with the software SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Evaluation of the acetylation process
The uptake of the acetylation solution (uptake) varied from 98.8 to 591.1 l/m3, with V. ferruginea showing the highest values, and G. arborea showing the lowest ones (Table 1). In V. ferruginea, V. guatemalensis, C. alliodora, G. arborea, C. odorata and T. grandis no statistical differences appeared for both parameters (uptake and WPG) amongst the acetylation times (Table 1). No statistically significant differences were observed between the acetylation times for E. cyclocarpum, S. saman and H. alchorneoides (Table 1). In H. alchorneoides, the highest uptake occurred in 1 h-acetylation-time, then in 2.5 h-acetylation-time, whilst the lowest one occurred in 4 h-acetylation-time. The WPG varied from 2.2 to 16.8%; the highest values exhibited by V. ferruginea and the lowest ones shown by G. arborea (Table 1). Wood samples of E. cyclocarpum, H. alchorneoides and S. saman exhibited statistically significant differences amongst the different acetylation times, unlike the other species. Specifically, for E. cyclocarpum and H. alchorneoides woods, the lowest WPGs were observed at the 4 h-acetylation-time (Table 1). Also, S. saman wood showed differences, but its lowest WPG was observed in 1 h-acetylation-time, whereas its highest WPG was observed in 2.5 h-acetylation-time (Table 1). The very low WPG values obtained at the prolonged reaction time (4 h) may be attributed to the thermal degradation of wood polymers.
FTIR analysis
The FTIR spectra (Fig. 1a) showed that in the esterification of the hydroxyl groups by acetic anhydride, the signal intensity of acetyl groups increased at 1732, 1592, 1372, 1228 and 1034 cm−1 (Fig. 1b). The calculation of the ratio of intensity in absorbance (RI) of these signals revealed greater intensity in signals at 1732, 1372 and 1228 cm−1 for all the tropical species studied (Fig. 2). The highest WPGs, after acetylation, occurred in V. ferruginea, V. guatemalensis, E. cyclocarpum, C. alliodora and S. saman woods (Table 1), which showed the highest intensities in the 1732, 1372 and 1228 cm−1 peaks (Fig. 2a–e). On the contrary, species with lower uptakes, for instance, H. alchorneoides, G. arborea, C. odorata and T. grandis species (Table 1) presented the lowest RIs in such signals (Fig. 2f–i). This behaviour was confirmed upon establishing the coefficients of correlation, between the WPG and RI (Table 2), which indicated a positive and significant correlation between the 1732, 1372 and 1228 cm−1 peaks and the WPG values (Fig. 3).
Also, the other RIs that were influenced by acetylation (1592 and 1034 cm−1 peaks) did not present a statistically significant correlation with the WPG of the tropical species tested (Table 2). Markedly, upon establishing correlation between the signal intensities affected by acetylation, the signals with greater RIs (1732, 1372 and 1228 cm−1 peaks) were correlated with each other (Table 2). Signals with the lower RIs (1592 and 1034 cm−1 peaks), on the other hand, did not show correlation with each other, nor did they present any relation with signals of greater RI (Table 2).
For all species, there was an increase in intensity of the bands at the 1 h- and 2.5 h-acetylation-time, whereas at the 4 h-acetylation-time a decrease was noted (Fig. 2a–i). The only exceptions were observed for the wood species S. saman and H. alchorneoides which presented an increase in RI values at the 1 h-acetylation-time, then a noticeable decrease at the 2.5 h-acetylation-time, and finally, a slight increase at the 4 h-acetylation-time (Fig. 2e, f).
Discussion
WPG values of tropical woods, after acetylation, were much lower than those reported for some softwood species, since WPG values around 20% have been reported [1, 5, 16, 27, 38]. This discrepancy may be attributed to the much lower permeability of the tropical species used in this study, since the uptake ranged from 98.8 to 591.1 l/m3 in treated wood (Table 1). Liquid uptake in hardwoods is largely related with the permeability of the wood species [39, 40]. That varies as a function of the anatomical characteristics of each species. Even after wood is formed in the trees, its porosity can be changed by biological deterioration and/or by thermal modification in different mediums.
In hardwood species, liquid flow chiefly occurs along the vessel lumina in longitudinal orientation [40], but this flow can be interrupted by the presence of tyloses and gums into the vessels [40]. Vessels connect longitudinal and radial parenchyma across wall pits, so liquids can then flow through the radial lumina [40]. This flow is favoured when the rays are composed of over 3 series in width, and of more abundant parenchyma [40]. Variations in the anatomy of the tropical species, tested in this work (Table 3), were presented and discussed by Moya et al. [25]. These authors mentioned that the species E. cyclocarpum, H. alchorneoides, S. saman, V. ferruginea and V. guatemalensis have anatomical features of larger dimensions, that is, vessels’ diameter (over 120 µm), rays’ width (from 2 to 10 series of cells or over 252 µm) and rays’ frequency (over 5 rays per tangential mm), as well as some types of parenchyma (Table 3) [25]. Hence, such anatomical features favour the flow of acetic anhydride and acetic acid solutions (Table 3). This is in accordance with the results of the present work, in which, the above-mentioned wood species, indeed, exhibited higher uptake and WPG values (Table 1). Quite the opposite, wood species like C. odorata, G. arborea and T. grandis resulted in greatly lower uptake and WPG (Table 1). This is because, as postulated in here, their anatomical features are very unfavourable to liquid flow (e.g., smaller and less-frequent rays, plenty of gums and tyloses in the vessels, fewer vessel-associated axial parenchyma) (Table 3) [25].
WPGs obtained for the tropical species tested are due to the fact that these species present dissimilar degrees of permeability in their anatomical structures, which in turn influences the acetylation of functional groups. According to Rowell [2], when WPG is close to 4%, due to wood permeability, there is more bonded acetyl in the S2 layer than in the middle lamella; at a WPG of about 10%, acetyl is equally distributed throughout the S2 layer and middle lamella; and with WPG at 20%, there is a slightly higher concentration of acetyl in the middle lamella than in the rest of the cell wall. Then, acetylation of the acetyl groups is expected to be as follows: higher for V. guatemalensis and V. ferruginea, in all the wood structure, with special emphasis in the middle lamella; uniform for E. cyclocarpum, C. alliodora and S. saman in all the wood structure; and lower for H. alchornoides, G. arborea, C. odorata and T. grandis, since acetylation occurs mainly on the S2 layer of the cellular wall, probably over acetyl groups from lignin, which presents the highest degree of acetylation [41].
In most species, reaction time did not have a significant effect on the uptake, except for E. cyclocarpum, S. saman and H. alchorneoides, for which the 4 h-acetylation-time treatment yielded a significantly lower uptake (Table 1). This large decrease may be attributed to the completion of the acetylation reaction well before the time limit. After some time, the reaction slows and levels off, indicating that the reaction is ‘complete’ [41]. Then, once acetylation is fulfilled, degradation of the acetic anhydride may have been taken place, since the temperature used for the process (120 °C) was close to boiling temperature of this compound (139 °C). This suggests that prolonged reaction time was not suitable for species like E. cyclocarpum, S. saman and H. alchornoides, as the wood and the compound itself appeared to be affected in a negative way.
The main changes undergone in acetylated wood can be observed in the peaks at 1228, 1372 and 1732 cm−1 in all species (Fig. 2), which are in agreement with other studies. In fact, according to Stefke et al. [42] and Schwanninger et al. [43], these bands are assigned to valence vibrations of acetyl C=O groups (1745 cm−1), aliphatic C–H deformation vibrations in CH3 (1374 cm−1), the asymmetric C–O stretching vibration from the ester group grafted (C–O–C=O at 1240 cm−1) and C–C plus C–O stretching plus aromatic =C–H in plane deformation vibrations (1265 cm−1 from lignin, which is overlapped by the latter, with an increasing number of acetyl groups) as well as the C–O valence vibration (1034 cm−1) from cellulose, hemicelluloses and lignin. Nonetheless, the other two signals (1034 and 1592 cm−1) are important in those species with a higher uptake value, specifically in V. ferruginea and V. guatemalensis (Fig. 2a, b). This result evidences that acetylation is scarce in the methyl group (of the acetyl unit) stretch (1372 cm−1) and the conjugated carbonyl (C=O) stretch (1592 cm−1) in cellulose and hemicelluloses; in fact, it showed no relation with WPG (Table 2). According to Rowell [11], acetylation occurs mostly in lignin, by 82%, and to a much lesser extent in hemicelluloses and cellulose; therefore, a low signal is to be expected in 1372 and 1592 cm−1 intensities. This outcome is in agreement with the very low acetylation of the acetyl groups occurring in the hemicelluloses and cellulose of the tropical woods tested in this work.
Regarding the main signals in acetylation intervals, the maximum acetylation signal occurs within 1 h for S. samanand H. alchorneoides (Fig. 2c, f), whereas for most species the most efficient acetylation time seemed to be within 2.5 h, as from then on, a decline in the signal takes place, probably due to a thermal degradation of wood upon continued exposure to temperature [41].
Moreover, the RI of peaks, associated to acetylation of lignin (1228, 1372 and 1732 cm−1), was positivity related to WPG (Fig. 3). As for WPG variation (Table 1), and considering all signals are associated to lignin, it is likely that acetyl groups are the same in all species [44], indicating that acetylation of the tropical wood species is almost exclusively associated with the permeability of each species. The variation present in the anatomical structure of the samples is associated to permeability of the hardwood species [39, 40]. Consequently, conductive elements control whether adsorbed acetic anhydride can reach hydroxyl groups in lignin or not: the higher the uptake, the greater the possibility for acetic anhydride to access more bonded acetyl in the S2 layer of the cell wall, or else the acetyl groups in the middle lamella [11] where lignin is present in larger proportion [7]. Accessibility of lignin in tropical species is also evidenced by the correlation between RI associated to it (1732, 1372 and 1228 cm−1 peaks), which showed high correlation with each other and presented correlation with the WPG as well (Table 2). However, considering the different coefficients of correlation (Table 2, Fig. 3), it is clear that the highest value comes at 1732 cm−1 intensity (associated to acetyl C=O groups), which registers a coefficient of 0.71 (Fig. 3c). Consequently, this RI may be suggested as a good indicator for measuring the degree of acetylation of such tropical hardwood species.
In the acetylation of acetyl groups associated to hemicelluloses and cellulose (1372 and 1592 cm−1, respectively), the biggest variation presented amongst the species (Fig. 2) was associated with the large variety of types and amount of hemicelluloses present in wood [22]. Each one of the species shows different proportions of the various types of monomers conforming wood hemicelluloses [41], resulting in varying degrees of acetylation and different signals per species. Correspondingly, in this work, this behaviour became evident upon observing that the 1372 and 1592 cm−1 peaks of RI did not show any correlation with the WPG (Table 2).
Conclusions
Nine tropical hardwoods, from forest plantations of Costa Rica, were chemically modified with acetic anhydride using three treatment times (1 h, 2.5 h and 4 h), whilst a comprehensive FTIR analysis of the treated samples was carried out. As revealed, tropical woods acetylated in this work presented a high variability in the degree of acetylation, in respect to WPG and RI at 1732, 1592, 1372, 1228 and 1334 cm−1 peaks of the FTIR spectrum. Actually, WPGs varied from 2.2 to 16.8%, with Vochysia ferruginea species exhibiting the highest WPG, whilst Gmelina arborea and Tectona grandis species exhibited the lowest WPG values. It was observed that species with apposite anatomical features, that is, vessels’ diameter (over 120 µm), rays’ width (2 to 10 series of cells or over 252 µm) and rays’ frequency (over 5 radii/mm2) as well as proper types of parenchyma, enabled the flow of acetic anhydride and glacial acetic acid solutions, resulting in higher absorption and WPG values in wood structure. Conversely, those tropical species having less favourable anatomical elements for liquid flow, e.g., smaller and less-frequent radii, plenty of deposits in the vessels, such as gums and tyloses, as well as fewer parenchyma associated to vessels, exhibited much lower WPG values. WPG was scarcely affected by the acetylation times, in most of the species investigated. In fact, the FTIR spectrum showed that the most efficient acetylation time was the period of 2.5 h. Conclusively, it was found that the RI of peaks associated to acetylation (1228, 1372 and 1732 cm−1) can be considered as a reliable indicator of the degree of acetylation for the tropical hardwoods tested. These RIs were increased with an increment in WPG, leaving the IR at 1732 cm−1 (associated to C=O, acetyl groups) as a reliable index for measuring the degree of acetylation in tropical hardwood species.
Availability of data and materials
The data used and/or analysed during the current study are available from the corresponding authors on a reasonable request.
Abbreviations
- WPG:
-
Weight percentage gain
- FTIR:
-
Fourier-Transform Infrared Spectroscopy
- RI:
-
Ratio of intensity from the FTIR spectra
- (CH3CO)2O:
-
Acetic anhydride
- CH3COOH:
-
Glacial acetic acid
- h:
-
Hours
- SD:
-
Standard deviation
- FP:
-
Pore frequency
- LV:
-
Vessel length
- DV:
-
Vessel diameter
- T:
-
Tyloses
- G:
-
Gums
- DIP:
-
Intervascular punctuation diameter
- PA:
-
Apotracheal parenchyma
- PP:
-
Paratracheal parenchyma
- BP:
-
Banded parenchyma
References
Hill CAS (2006) Wood modification. Wiley, Chichester
Rowell RM (2006) Acetylation of wood: journey from analytical technique to commercial reality. For Prod J 56:4–12
Rowell RM (2004) Solid wood processing—chemical modification. Encyclopedia of forest sciences. Elsevier, New York, pp 1269–1274
Adebawo FG, Naithani V, Sadeghifar H, Tilotta D, Lucia LA, Jameel H, Ogunsanwo OY (2016) Morphological and interfacial properties of chemically-modified tropical hardwood. RSC Adv 6:6571–6576
Rowell RM (2006) Chemical modification of wood: a short review. Wood Mater Sci Eng 1:29–33
Giridhar BN, Pandey KK, Prasad BE, Bisht SS, Vagdevi HM (2017) Dimensional stabilization of wood by chemical modification using isopropenyl acetate. Maderas Cienc y Tecnol 19(15–20):2
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood–water interactions. Wood Sci Technol 47:141–161
Willems W (2018) Hygroscopic wood moisture: single and dimerized water molecules at hydroxyl-pair sites? Wood Sci Technol 52:777–791
Hofstetter K, Hinterstoisser B, Salmén L (2006) Moisture uptake in native cellulose—the roles of different hydrogen bonds: a dynamic FT-IR study using Deuterium exchange. Cellulose 13:131–145
Hill CAS, Forster SC, Farahani MRM, Hale MDC, Ormondroyd GA, Williams GR (2005) An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. Int Biodeter Biodegr 55:69–76
Rowell R (2016) Dimensional stability and fungal durability of acetylated wood. Drewno 59:139–150
Lykidis C, Bak M, Mantanis G, Németh R (2016) Biological resistance of pine wood treated with nano-sized zinc oxide and zinc borate against brown-rot fungi. Eur J Wood Wood Prod 74:909–911
Mantanis GI (2017) Chemical modification of wood by acetylation or furfurylation: a review of the present scaled-up technologies. BioResources 12:4478–4489
Taghiyari HR, Bayani S, Militz H, Papadopoulos AN (2020) Heat treatment of pine wood: possible effect of impregnation with silver nanosuspension. Forests 11:466
Papadopoulos AN, Bikiaris DN, Mitropoulos AC, Kyzas GZ (2019) Nanomaterials and chemical modifications for enhanced key wood properties: a review. Nanomaterials 9:607
Papadopoulos AN, Hill CAS (2002) The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz Roh-Werkst 60:329–332
Papadopoulos AN, Hill CAS (2003) The sorption of water vapour by anhydride modified softwood. Wood Sci Technol 37:221–231. https://doi.org/10.1007/s00226-003-0192-6
Papadopoulos AN (2006) Chemical modification of pine wood with propionic anhydride: effect on decay resistance and sorption of water vapour. BioResources 1:67–74
Thybring EE (2013) The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int Biodeter Biodegr 82:87–95
Adebawo F, Sadeghifar H, Tilotta D, Jameel H, Liu Y, Lucia L (2019) Spectroscopic interrogation of the acetylation selectivity of hardwood biopolymers. Starch Stärke 71(11–12):1900086
Ringman R, Beck G, Pilgård A (2019) The importance of moisture for brown rot degradation of modified wood: a critical discussion. Forests 10:522
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Rowell RM (2014) Acetylation of wood—a review. Int J Lignocell Prod 1(1):1–27
Gibson LJ (2012) The hierarchical structure and mechanics of plant materials. J R Soc Interface 9:2749–2766
Moya R, Gaitan-Alvarez J, Berrocal A, Araya F (2020) Effect of CaCO3 in the wood properties of tropical hardwood species from fast-grown plantation in Costa Rica. BioResources 15(3):4802–4822
Larsson Brelid P, Simonson R, Bergman Ö, Nilsson T (2000) Resistance of acetylated wood to biological degradation. Holz Roh-Werkst 58:331–337
Passarini L, Zelinka SL, Glass SV, Hunt CG (2017) Effect of weight percent gain and experimental method on fiber saturation point of acetylated wood determined by differential scanning calorimetry. Wood Sci Technol 51:1291–1305
Huang X, Kocaefe D, Kocaefe Y, Pichette A (2018) Combined effect of acetylation and heat treatment on the physical, mechanical and biological behavior of jack pine (Pinus banksiana) wood. Eur J Wood Wood Prod 76:525–540
Matsunaga M, Hewage DC, Kataoka Y, Ishikawa A, Kobayashi M, Kiguchiet M (2016) Acetylation of wood using supercritical carbon dioxide. J Trop For Sci 28:132–138
Bollmus S, Bongers F, Gellerich A, Lankveld C, Alexander J, Militz H (2015) Acetylation of German Hardwoods. Proc of 8th European conference on wood modification. Helsini, Finland, pp 164–173
Moya R (2018) La producción de madera de especies nativas en plantaciones comerciales: una opción real. Ambientico 267:32–36
Ozmen N (2007) Dimensional stabilisation of fast growing forest species by acetylation. J Appl Sci 7:710–714
Kojima M, Yamamoto H, Okumura K, Ojio O, Yoshida M, Okuyama T, Ona T, Matsune K, Nakamura K, Ide Y, Nugroho S, Hamami M, Hadi YS (2009) Effect of the lateral growth rate on wood properties in fast-growing hardwood species. J Wood Sci 55:417–424
Rowell R (2012) Handbook of wood chemistry and wood composites. CRC Press, New York
Pardo T, Alfaro J (2014) White-rot fungal decay resistance of teak and melina wood treated with acetic anhydride. Int Biodeter Biodegr 88:44–47
Moya R, Salas C, Berrocal A, Valverde JC (2015) Evaluation of chemical compositions, air-dry, preservation and workability of eight fastgrowing plantation species in Costa Rica. Madera Bosques 21:31–47
Tenorio C, Moya R, Salas C, Berrocal A (2016) Evaluation of wood properties from six native species of forest plantations in Costa Rica. Bosque 37:71–84
Ramsden MJ, Blake FSR, Fey NJ (1997) The effect of acetylation on the mechanical properties, hydrophobicity, and dimensional stability of Pinus sylvestris. Wood Sci Technol 31:97–104
Thomas RJ (1976) anatomical features affecting liquid penetrability in three hardwood species. Wood Fiber Sci 4:256–263
Ahmed SA, Chun SK (2009) Observation of liquid permeability related to anatomical characteristics in Samanea saman. Turkish J Agric For 33:155–163
Rowell R, Ibach R (2018) Stable and durable wood products based on molecular modification. J Trop For Sci 30:488–495
Stefke B, Windeisen E, Schwanninger M, Hinterstoisser B (2008) Determination of the weight percentage gain and of the acetyl group content of acetylated wood by means of different infrared spectroscopic methods. Anal Chem 80:1272–1279
Schwanninger M, Stefke B, Hinterstoisser B (2011) Qualitative assessment of acetylated wood with infrared spectroscopic methods. J Near Infrared Spectrosc 19:349–357
Ohkoshi M (2002) FTIR-PAS study of light-induced changes in the surface of acetylated or polyethylene glycol-impregnated wood. J Wood Sci 48:394–401
Acknowledgements
The authors wish to thank Vicerrectoría de Investigación y Extensión, of the Instituto Tecnológico de Costa Rica (ITCR, Cartago, Costa Rica) for the project financial support. This research project was carried out in cooperation with Lab of Wood Science and Technology of the University of Thessaly (Dept. of Forestry, Wood Sciences and Design), Karditsa, Greece. Prof. G.I. Mantanis would like to acknowledge the contribution of program Erasmus + KA107 (International Credit Mobility) for enabling this type of academic and research interaction between ITCR and University of Thessaly. In addition, the authors are grateful to the two anonymous reviewers for their valuable comments. The authors thank María Teresa Vargas L. for translation and edition English.
Funding
Funding was provided by Vicerrectoría de Investigación y Extensión of the Instituto Tecnológico de Costa Rica, Cartago, Costa Rica.
Author information
Authors and Affiliations
Contributions
JG-A contributed with the tree cutting and wood material sampling, determination of wood properties and running the data analysis. AB contributed with the laboratory experiments and the data analysis. GIM contributed with the overall design of the experiment, and the writing of the paper. RM contributed with the designing of the experiment, tree sampling, writing the paper, and coordinating the research project. FA contributed with the designing of the experiment and the laboratory work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gaitán-Alvarez, J., Berrocal, A., Mantanis, G.I. et al. Acetylation of tropical hardwood species from forest plantations in Costa Rica: an FTIR spectroscopic analysis. J Wood Sci 66, 49 (2020). https://doi.org/10.1186/s10086-020-01898-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-020-01898-9