Abstract
Bamboo is readily discolored by mold fungi, which greatly limits its applications. An effective antifungal agent, copper(II) chloride (CuCl2)-grafted silica gel, was prepared by a sol–gel process using tetraethoxysilane (TEOS)/3-aminopropyltriethoxysilane (APTES) mixtures. The elemental composition and the chemical combinations of homogeneous sol mixture (HSM) and bamboo were determined via Fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy with energy-dispersive X-ray spectrometry (SEM–EDS). The mold resistance of bamboo treated with HSM, alkaline copper quat (ACQ), chromated copper arsenate (CCA), and purified water was characterized by an indoor mold test. The micro-morphology of bamboo treated with HSM was investigated using scanning electron microscopy (SEM). HSM penetrated into the bamboo vessels, and formed xerogels, which was able to coordinate copper(II) cations. SEM–EDS investigations suggest that Si–O–Cu linkages may be formed through an exchange reaction between silanol groups and copper complexes. The bamboo samples treated with HSM showed highly efficient mold resistance due to a good penetration of HSM. Furthermore, no fungal hyphae were found in the structure of HSM-treated bamboo after a 5-week mold test. The copper complexes grafted to silica gel developed in this work provide an efficient antifungal agent for a wide range of potential applications in bamboo protection.
Similar content being viewed by others
Introduction
Bamboo is the fastest-growing and most versatile plant on earth, including about 75 genera with approximately 1300 species and varieties covering 25 million hectares worldwide [1]. Bamboo is widely planted in the South of China, including the mountainous regions of Hunan, Hubei, Fujian, Guangdong, Guangxi, Guizhou, Sichuan, Chongqing, Yunnan, and Zhejiang provinces [2]. Bamboo is widely used in building, decorating, paper making, and texturing due to its short rotation, high economic value, and the advantage of sustainable management. However, bamboo is easily degraded by molds, decay fungi, and insects since it contains high amounts of starch and protein [3]. This results in heavy harvest losses, due to decreased physical and mechanical properties, limiting its service life. Generally, untreated bamboo has an average life of less than 1 year [1]. Therefore, to increase its utilization, bamboo must be treated with a preservative.
Copper (Cu) is an essential biocide for bamboo protection, and has been long used as a wood preservative [4]. But the major drawback of copper is unsatisfactory fixation to wood. Efforts to reduce the leaching of the active components of wood preservatives have a long history [5]. Chromated copper arsenate (CCA) has been used for wood preservation for more than 70 years. Chromium (Cr) is a fixation agent for Cu and arsenic, which limits Cu migration [6]. Use of CCA has been banned in the last 10 years in Europe due to concerns about Cr and potential environmental contamination [7, 8]. CCA is problematic from the viewpoint of environmental protection, high toxicity to mammals, and the service life of treated wood [9]. Reducing leachability of Cu-based wood preservatives is still a challenge. Water repellants, polymers, and nanoparticles have been used to lower water uptake of wood surfaces [8, 10,11,12,13]. Thicker coatings or paints have also been applied for this purpose [14, 15]. In general, coating materials reduced Cu leaching efficiently. Microwave pre-treatment, hot air and microwave post impregnation processes also induce Cu fixation to a certain extent [16,17,18].
Modification of wood by sol–gel-derived precursor materials has been shown to limit the release of certain preservatives, such as metals, boric acid and boron compounds, hence increasing resistance against biological degradation by insects and fungi [5, 19,20,21]. Only a few reports are available concerning the leaching resistance of active components embedded into a sol–gel nanomatrix. However, there are few reports on bamboo preservation [22, 23].
The aim of this work was to modify bamboo with homogeneous sol mixtures of tetraethoxysilane (TEOS), 3-aminopropyltriethoxysilane (APTES), and copper(II) salts. The expectation was that mixtures of Cu and Si ions would form a Cu silicate to provide better fixation and reduce leaching. Meanwhile, Alkaline copper quat (ACQ) and CCA-treated bamboo were used as controls. This paper determined the mold-resistance performance of bamboo samples treated with ACQ, CCA homogeneous sol mixtures, and untreated bamboo sample. Furthermore, the micro-morphology of untreated bamboo and homogeneous sol mixture-treated bamboo samples after a mold test was investigated using scanning electron microscopy (SEM).
Materials and methods
Materials
Moso bamboo (Phyllostachys heterocycla var. pubescens) was selected due to its popularity, abundance, and value [24]. China has about 601,000 km2 of bamboo resources, among which over 443,000 km2 is moso bamboo [1]. Moso bamboo samples were collected from Taojiang, Yiyang city, Hunan province, China (111°–112° E, 28° N). Bamboo specimens, few of fungal attack, were air dried, and then the outer and inner bamboo skins were removed. The middle part of bamboo culm walls were retained and cut into 20 × 20 × 5 mm blocks. All specimens were oven dried to constant weight at 105 °C, and conditioned to a constant mass at 20 °C and 65% relative humidity (RH) before further treatment.
Trichoderma viride Pers. ex Fr (Tr), Penicillium citrinum Thom (Pe) and Aspergillus niger V. Tiegh (As), provided by the International Center for Bamboo and Rattan (Beijing, China), were applied in the mold-resistance test. These fungi are common species on bamboo [25].
TEOS was purchased from West Long Chemical Co., Ltd. (China), in Fig. 1. APTES was obtained from Shanghai Luzhong Chemical Reagent Co., Ltd. (China), and Fig. 1 shows the chemical structure. CuCl2 was purchased from Tianjin Guangfu Technology Development Co. Ltd. (China). Chemical formula of CuCl2 was chosen instead of sulphate for its higher solubility in the sol mixture [26]. Ethanol absolute (C2H6O, EtOH) was purchased from Tian Jin Damao Chemical Reagent Co. Ltd. (China). ACQ and CCA were purchased from Guangdong Forest Technology Development Co. Ltd. (China), the active substance ratio were not lower than 15 and 60%, respectively.
Potato dextrose agar (PDA) was used as the culture media for mold fungi. Steam was used to sterilize flasks and PDA, petri dishes and all specimens at 121 °C (0.1 MPa) for 30 min. About 20 ml of molten PDA was poured into each petri dish (100 mm in diameter and 20 mm in height). The fungi were incubated at 28 ± 2 °C and 85% RH.
Preparation of treatment solutions
The copper anchored sol system solution contained TEOS, APTES, EtOH, CuCl2, and purified water in appropriate ratios. The ratio of TEOS, EtOH, purified water, and APTES was 3:5:10:1. The TEOS, EtOH, and purified water were added at a weight ratio of 3:5:10 and heated at 50 °C in a water bath. The solution was mixed for about 30 min; then the pH of the solution was adjusted to 3–4 using hydrochloric acid to form the homogeneous sol. The sol reaction scheme is shown in Fig. 2. Appropriate amount of CuCl2 was added into the homogeneous sol under continuous stirring in a 50 °C water bath. The concentration of CuCl2 was 3.0%. Meanwhile, an appropriate amount of APTES was added into the homogeneous sol, and stirred to enhance reactions to produce homogeneous sol system with anchored copper solution (homogeneous sol mixture). The solution was stored for a few days and maintained under a dry atmosphere before use. Solutions containing 3.0% ACQ or 3.0% CCA were also prepared as controls.
Bamboo samples treatments
Bamboo samples were divided into four groups, each with 20 samples. All the samples were marked and weighed. The samples were put into four 500-ml beakers. About 300-ml homogeneous sol mixture, ACQ, CCA, or purified water were poured into each beaker. The beakers were sealed with waste newspapers. Purified water-treated specimens served as controls. The treatments were steamed at 125 °C for 90 min. In this case, the sol–gel process occured in the bamboo after the impregnation. This process should lead copper to penetrate deeply into bamboo. This process (Fig. 3) generated hybrid inorganic–organic silica xerogels particles penetrating the bamboo cell walls. This system should be able to graft copper(II) through coordinate interactions with the amino groups, avoiding or minimizing leaching. A pictorial view of amino-functionalized silica anchoring copper (II) cations is shown in Fig. 4. After impregnation, the bamboo samples were removed from the treatment solutions, lightly wiped to remove trace of solution from the surface, and dried at 105 °C for 24 h. The samples were weighed and conditioned at atmospheric pressure for a week, which allowed the sol–gel process to take place with the water adsorbed by the bamboo.
Mold-resistance test
Mold-resistance tests were conducted according to ASTM standard D4445 [27] and Chinese Standard GB/T 18261 [28]. The test fungi were inoculated onto test tubes filled with PDA. The molds were incubated for several days at 28 ± 2 °C and 85% RH. About 150-ml purified water was poured into a flask, then sealed and put it into steam sterilizer. Each group of treated samples were wrapped in gauze and heated at 121 °C, 0.1 MPa for 30 min. After sterilization, the mycelium and spores were added to purified water with inoculation loop when the water temperature was about 50 °C. The spore suspension was stirred frequently during inoculation. 1–3 ml spore suspension was streaked into the petri dishes filled with PDA substrate, which was incubated at 28 ± 2 °C and 85% RH. Two sterilized glass rods (3 mm diameter) were placed on the PDA substrate when it was covered with mycelium, and two bamboo specimens were put separately on the glass rod. After inoculation, the dishes were incubated at 28 ± 2 °C and 85% RH for 5 weeks. Mold growth was recorded every week.
Mold growth on each bamboo sample was visually rated using a scale of 0–4 (Table 1) beginning one day after inoculation. The lower the value, the more efficient the chemicals. The overall appraisal of each fungicide was defined as resisting effectiveness (RE) and obtained from the following equation 1:
where RE is the resisting effectiveness of fungicide against the three species of test molds (including Tr, Pe, and As); Dt is the average infection value of the test specimens; and D0 is the average infection value of the controls.
FTIR analysis
For testing the change of chemical bonds, the samples treated by homogeneous sol mixture (HSM) or purified water (untreated, control) were subjected to Fourier transform infrared spectroscopy (FTIR) (IRAffinity-1, Shimadzu Corporation, Japan) characterization. The samples were ground through a 200 mesh screen, and then dried at 60 °C in a muffle oven for 24 h to remove moisture. Finely ground bamboo powder mixed with KBr was pressed into pellets with a 10-mm diameter. FTIR spectra of samples were obtained over a range of 4000–400 cm−1 at a spectral resolution of 4 cm−1 and 30 scans.
SEM and SEM–EDS analyses
A scanning electron microscope (SEM, FEI Quanta 450, USA) was used to examine the micro-morphology of each bamboo test group (treated by homogeneous sol mixture and untreated). The specimens were cut into 5 × 5 mm (long × wide) blocks along the cross section and longitudinal section, and then sputter coated. The samples were dried before sputter coating. Energy-dispersive spectrometer (EDS) micronanalysis was used to investigate on the effectiveness and depth of homogeneous sol mixture penetration inside the bamboo microstructures. The distributions of copper and silicon, which included onto the surfaces and in the insides of the bamboo specimens were determined. The acceleration voltage was 20 kV. Microanalyses were carried out on the surface area of the longitudinal section. Three samples were chosen and three fields in one sample were examined.
Results and discussion
FTIR analysis of untreated bamboo and bamboo treated with HSM
The FTIR spectra of HSM-treated and untreated samples are shown in Fig. 5. The characteristic absorption peaks of HSM-treated bamboo at 1035, 1705, 1724, 2854, and 2922 cm−1 increased compared with the untreated bamboo. The stretching vibrations peak at about 1035 cm−1, mainly due to the C–O–H bonds in the bamboo, was enlarged in the HSM-treated bamboo sample, which was attributed to Si–O linkages of the silica backbone, and the C–O–H of the bamboo cellulose amorphous region for formation of Si–O–Cu linkages [21]. The intense peak at about 1450 cm−1 can be attributed to Si–O stretching in treated bamboo. Carboxylic acid and formation of esters in the cellulose amorphous region lead to assigning the intense peaks at 1705 and 1724 cm−1 to the C=O stretching. A new shoulder appeared for HSM-treated bamboo at about 3300 cm−1, due mainly to the stretching of O–H (hydrogen bonds) in bamboo biopolymers. The differences were observed in bending regions 3500–2800 cm−1 and 1600–1300 cm−1, where contributions of the HSM functional groups prevail (mainly as N–H asymmetric stretching) [4].
Moreover, a shoulder appeared for HSM-treated bamboo at about 940 cm−1 and was assigned to the external Si–OH groups [29]. The peak around at 790 cm−1 could also be attributed to Si–O vibrational modes. On the other hand, no deformation was observed in the O–H and C–H stretching (3300–2800 cm−1) and bending regions (about 1600–1300 cm−1), where contributions of the bamboo functional groups prevail.
Microdistribution of HSM in treated bamboo
The deep interpenetration of the homogeneous sol mixture in the bamboo texture was confirmed by SEM–EDS investigations. Figure 6 shows a representative SEM image of the surface and the relevant X-ray spectrum with the relative atomic percentages, and the elemental composition in vessels (Fig. 6a, the yellow panel) and parenchyma (Fig. 6b, the yellow panel). In the spectrum shown in Fig. 6a, the relative atomic percentages of Cu and Si were 2.31 and 2.43%, respectively. Figure 6b shows that the relative atomic percentages of Cu and Si were 0.47 and 0.30%, respectively. This indicates that the HSM was easily impregnated into the bamboo vessels. The entire tissue of a culm provides the best way to obtain full treatment which is important for the bamboo with its small volume fraction of vessel pathways [1].
The homogeneous sol mixture was expected to contain Cu2+ complexes of APTES mainly in its monomeric form along with intact TEOS molecules [4]. After impregnation into the bamboo samples, the sol–gel process occured mainly onto the bamboo fiber leading to the formation of an inorganic–organic hybrid xerogels. Bond formation in silanol groups on bamboo can be seen in Fig. 7. Bamboo was penetrated by copper via the coordinative interactions with the amino groups of APTES. At the beginning of the sol–gel process, the silanol groups of Si–OH interacted with copper cations and substitute chloride anions by forming Si–O–Cu linkages through a simple exchange reaction schematized: Si–OH + CuCl2 + 3RNH2 → Si–O–Cu(NH2R)2Cl + RNH3+Cl− [21]. The grafting of copper to the functionalized silica gel in the bamboo is shown in Fig. 8. The chlorine deficit was found all over the sections of different bamboo samples.
Accelerated tests against molds
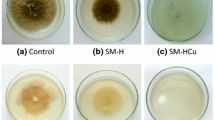
Bamboo in natural conditions is easily attacked by mold fungi [1, 3, 23]. The surface changes in bamboo blocks treated with HSM, CCA, ACQ and purified water followed by exposure to Tr, Pe and As for 5 weeks are shown in Fig. 9 and Table 2.
The infection values of bamboo treated with HSM, CCA, and ACQ were 0, 4, and 8%, respectively. The RE of HSM, ACQ, CCA and control were 100, 96, 92, and 0%, respectively. The HSM treatment raised the bamboo to the “0 resistant class” level and CCA or ACQ to the “1 resistant class” level, as shown in Table 2. HSM, ACQ, and CCA had excellent resistance against all the test fungi (Tr, As and Pe).
Micro-morphologies of untreated and HSM-treated bamboo after mold-resistance test
The microstructures of untreated and HSM-treated bamboo samples were observed continuously and analyzed with the SEM.
The SEM images present a clear micro-morphology of the characteristics of both treated and untreated bamboo (Fig. 9). As shown in Fig. 9a, b, the fungal hyphae were abundant in the vessels of untreated bamboo, and the vessels were slightly damaged Fig. 9b. No hyphae of Tr, As and Pe could be found in the other structures, included sclerenchyma fibres, parenchyma. In contrast, no fungal hyphae were found in the vessels and other structures of bamboo treated with HSM (Fig. 9c, d). Vessels were the main path for mold fungi in untreated bamboo. The HSM effectively prevented attack of the test mold fungi.
Conclusions
The sol–gel method created effective antifungal agents using CuCl2-grafted silica gel. FTIR measurements and SEM–EDS investigations augmented for the interpenetration of HSM inside the bamboo. Grafted copper complexes were covalently bound to the silica gel. This compound penetrated into the bamboo fiber, and then formed xerogels. These xerogels were able to coordinate copper(II) cations and improve mold attack. The infection value of bamboo treated with HSM was 0.00, as the HSM treatment raised the bamboo to the “0 resistant class” level. Meanwhile, no fungal hyphae were found in bamboo treated with HSM. HSM effectively prevented mold fungi attack, creating a wide range of potential applications for bamboo protection and its products.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CuCl2 :
-
copper(II) chloride
- TEOS:
-
tetraethoxysilane
- APTES:
-
3-aminopropyltriethoxysilane
- CCA:
-
copper chromium arsenate
- HSM:
-
homogeneous sol mixture
- FTIR:
-
Fourier transform infrared
- SEM-EDS:
-
scanning electron microscopy quipped with an energy-dispersive X-ray spectrometry
- SEM:
-
scanning electron microscope
- EtOH:
-
ethanol absolute
- RE:
-
resisting effectiveness
- Tr:
-
Trichoderma viride Pers. ex Fr
- Pe:
-
Penicillium citrinum Thom
- As:
-
Aspergillus niger V. Tiegh
References
Liese W, Köhl M (2015) Bamboo—the plant and its uses. Springer International Publishing, USA
Kim YS, Funada R, Singh AP (2016) Secondary xylem biology-origins, functions, and applications. Academic Press, Cambridge
Jiang ZH (2008) Bamboo and rattan in the world. China Forestry Publishing House, Beijing (In Chinese)
Palanti S, Predieri G, Vignali F, Feci E, Casoli A, Conti E (2011) Copper complexes grafted to functionalized silica gel as wood preservatives against the brown rot fungus Coniophora puteana. Wood Sci Technol 45(4):707–718
Mahr MS, Hübert T, Stephan I, Bücker M, Militz H (2013) Reducing copper leaching from treated wood by sol–gel derived TiO2 and SiO2 depositions. Holzforschung 67(4):429–435
Lebow ST, Foster DO, Lebow PK (1999) Release of copper, chromium, and arsenic from treated southern pine exposed in seawater and freshwater. Forest Prod J 49(7):80–89
Temiz A, Yildiz UC, Nilsson T (2006) Comparison of copper emission rates from wood treated with different preservatives to the environment. Build Environ 41(7):910–914
Pankras S, Cooper P, Wylie S (2012) Relationship between copper species in solution and leaching from alkaline copper quat (ACQ) treated wood. Holzforschung 66:505–514
Yu LL, Cao JZ, Cooper PA, Ung YT (2009) Effect of hot air post impregnations on copper leaching resistance in ACQ-D treated Chinese fir. Eur J Wood Wood Prod 67(4):457–463
Cui F, Walcheski P (2000) The effect of water repellent additives on the leaching of CCA from simulated southern yellow pine decks. Document Number IRG/WP 00-50158. International Research Group on Wood Preservation, Stockholm
Mourant D, Yang DQ, Lu X, Riedl B, Roy C (2009) Copper and boron fixation in wood by pyrolytic resins. Bioresour Technol 100:1442–1449
Wu YQ, Jia SS, Yan Q, Luo S, Liu M (2016) A versatile and efficient method to fabricate durable superhydrophobic surfaces on wood, lignocellulosic fiber, glass, and metal substrates. J Mater Chem A 4:14111–14121
Wu YQ, Jia SS, Wang S, Qing Y, Yan N, Wang QH, Meng TT (2017) A facile and novel emulsion for efficient and convenient fabrication of durable superhydrophobic materials. Chem Eng J 328:186–196
Nejad M, Cooper P (2010) Coatings to reduce wood preservative leaching. Environ Sci Technol 44(16):6162–6166
Sun QF, Yu H, Liu Y, Li J, Lu Y, Hunt JF (2010) Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 64:757–761
Yu LL, Cao JZ, Gao W, Su HT (2011) Evaluation of ACQ-D treated chinese fir and mongolian scots pine with different post-treatments after 20 months of exposure. Int Biodeter Biodegr 65(4):585–590
Ramezanpour M, Tarmian A, Taghiyari HR (2015) Improving impregnation properties of fir wood to acid copper chromate (ACC) with microwave pre-treatment. Iforest 8(1):89–94
Ye M, Morrell JJ (2015) Effect of post-treatment processing on copper migration from Douglas-fir lumber treated with ammoniacal copper zinc arsenate. J Environ Manage 152(4):268–272
Mai C, Militz H (2004) Modification of wood with silicon compounds inorganic silicon compounds and sol–gel systems: a review. Wood Sci Technol 37(5):339–348
Kartal SN, Yoshimura T, Imamura Y (2009) Modification of wood with si compounds to limit boron leaching from treated wood and to increase termite and decay resistance. Int Biodeter Biodegr 63(2):187–190
Vignali F, Predieri G, Feci E, Palanti S, Baratto MC, Basosi R, Callone E, Müller K (2011) Interpenetration of wood with NH2 R-functionalized silica xerogels anchoring copper(II) for preservation purposes. J Sol–gel Sci Technol 60(3):445–456
Li G, Wenjing G, Shupin L (2018) Investigation of changes in compressed moso bamboo (Phyllostachys pubescens) after hot-press molding. J Wood Sci 64(5):557–565
Shah DU, Sharma B, Ramage MH (2018) Processing bamboo for structural composites: influence of preservative treatments on surface and interface properties. Int J Adhes Adhes 85:15–22
Huang YH, Fei BH, Wei PL, Zhao C (2016) Mechanical properties of bamboo fiber cell walls during the culm development by nanoindentation. Ind Crop Prod 92:102–108
Wu K, Weng Y (2000) Bamboo mildew-rotting and its relation with environmental condition. Forest Res 13:63–70
Ghosh SC, Militz H, Mai C (2009) The efficacy of commercial silicones against blue stain and mold fungi in wood. Eur J Wood Wood Prod 67(2):159–167
ASTM Standard D4445-10 (2015) Standard test method for fungicides for controlling sapstain and mold on unseasoned lumber (laboratory method), American Society for Testing and Material, West Conshohocken. https://doi.org/10.1520/d445-10r15
Chinese Standard GB/T 18261-2013 (2013) Testing method for anti-mold chemicals in controlling mold and blue stain fungi on wood. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Beijing, China (In Chinese)
Klonkowsky AM, Grobelna B, Widernik T, Jankowska-Frydel A, Mozgawa W (1999) The coordination state anchored and grafted onto the surface of organically modified silicates. Langmuir 15:5814–5819
Acknowledgements
The authors gratefully acknowledge Yan Qing of the Central South University of Forestry & Technology for SEM imaging. And also gratefully acknowledge Major Hunan Provincial Science and Technology Projects (Grant No. 2011FJ1006), Forestry Project of Guizhou Province of China (Grant No. Qian Lin Ke He J Zi [2015]17, Qian Lin Ke He[2019]03, and Qian Lin Ke He[2019]05), and Science and Technology Projects of Guizhou Province of China (Grant No. the Important and Special Project of Qian Ke He [2014]6020 and [2015]6008).
Funding
This work was partly supported by Natural Science Foundation of Guizhou Province of China (Grant No. Base of Qian Ke He[2016]1084), the Project of Guizhou Province Science and Technology Supporting Plan (Grant No. Guizhou supporting plan [2018]2196, [2018]4003, and [2018]5252).
Author information
Authors and Affiliations
Contributions
YW and NJ conceived the idea for the study, AH, YL, and DL conducted the analyses, SY and SL performed research and wrote the paper. All authors discussed the results and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yang, S., Luo, S., Huang, A. et al. Mold resistance of bamboo treated with copper complexes-grafted silica gel and its microdistribution in treated bamboo. J Wood Sci 65, 62 (2019). https://doi.org/10.1186/s10086-019-1839-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-019-1839-8