Abstract
Background
Abnormal activation of NLRP3 inflammasome is related to a series of inflammatory diseases, including type 2 diabetes, gouty arthritis, non-alcoholic steatohepatitis (NASH), and neurodegenerative disorders. Therefore, targeting NLRP3 inflammasome is regarded as a potential therapeutic strategy for many inflammatory diseases. A growing number of studies have identified tanshinone I (Tan I) as a potential anti-inflammatory agent because of its good anti-inflammatory activity. However, its specific anti-inflammatory mechanism and direct target are unclear and need further study.
Methods
IL-1β and caspase-1 were detected by immunoblotting and ELISA, and mtROS levels were measured by flow cytometry. Immunoprecipitation was used to explore the interaction between NLRP3, NEK7 and ASC. In a mouse model of LPS-induced septic shock, IL-1β levels in peritoneal lavage fluid and serum were measured by ELISA. Liver inflammation and fibrosis in the NASH model were analyzed by HE staining and immunohistochemistry.
Results
Tan I inhibited the activation of NLRP3 inflammasome in macrophages, but had no effect on the activation of AIM2 or NLRC4 inflammasome. Mechanistically, Tan I inhibited NLRP3 inflammasome assembly and activation by targeting NLRP3-ASC interaction. Furthermore, Tan I exhibited protective effects in mouse models of NLRP3 inflammasome-mediated diseases, including septic shock and NASH.
Conclusions
Tan I specifically suppresses NLRP3 inflammasome activation by disrupting the association of NLRP3 and ASC, and exhibits protective effects in mouse models of LPS-induced septic shock and NASH. These findings suggest that Tan I is a specific NLRP3 inhibitor and may be a promising candidate for treating NLRP3 inflammasome-related diseases.
Graphical Abstract

Similar content being viewed by others
Introduction
Inflammasome, a cytosolic multi-protein complex, composed of effector molecule caspase-1, the adaptor protein apoptosis-associated speck-like protein (ASC), as well as recognition molecules (Rathinam and Fitzgerald 2016). The recognition molecules include a variety of NOD-like family molecules (NLRs), including NLRP1, NLRP3, NLRC4 (Elliott and Sutterwala 2015), and PYHIN 8 family member absent in melanoma 2 (AIM2) (Lugrin and Martinon 2018). NLPR3 inflammasome can be activated by both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (Kelley et al. 2019). Once activated, NLRP3 forms inflammasome together with ASC and pro-caspase-1, acting as the platform for caspase-1 activation, resulting in the secretion of IL-18 and IL-1β, which mediate the progression of multiple inflammatory diseases (Duez and Pourcet 2021; Youm et al. 2015).
Since NLRP3 can sense pathogens and many stimuli derived from the host, abnormal NLRP3 inflammasome activation is demonstrated to be an essential driver of a series of complex human diseases, including acute kidney injury, atherosclerosis, non-alcoholic steatohepatitis (NASH), and type 2 diabetes (Li et al. 2022a, b; Wang et al. 2021; Yang et al. 2022; Zhao et al. 2021), suggesting that NLPR3 inflammasome may act as a potential therapeutic target for these diseases (Chauhan et al. 2020; Wang and Hauenstein 2020). Therefore, inhibiting NLRP3 inflammasome may be a promising approach for treating NLRP3-mediated inflammatory diseases (Chiazza et al. 2016; Pappritz et al. 2022; Swanson et al. 2019).
Increasing evidence suggests that small molecule compounds from traditional Chinese medicine targeting NLRP3 inflammasome, such as oridonin from Rabdosia rubescens (He et al. 2018), cardamonin from Alpinia katsumadai (Wang et al. 2019b), echinatin from Glycyrrhiza plants (licorice) (Xu et al. 2021), exhibit therapeutic effects on NLRP3-mediated disease in animal models. Tanshinone I (Tan I), the active ingredient in the traditional Chinese medicine Salvia miltiorrhiza (Zhou et al. 2020). Recent reports have demonstrated its multiple pharmacological activities like anti-inflammatory, anti-tumor, and neuroprotective effects, Tan I has also been reported to improve insulin resistance in type 2 diabetes (Jung et al. 2020; Liu and Liu 2020; Wei et al. 2017). Earlier research has shown that Tan I has obvious anti-inflammatory activity and can be used as a potential anti-inflammatory drug (Wang et al. 2015; Yang et al. 2021). However, its anti-inflammatory mechanism and direct target remain to be further clarified.
In this study, we found that Tan I markedly suppressed the activation of NLRP3 inflammasome, but had no impact on NLRC4 or AIM2 inflammasome. Tan I inhibited NLRP3 inflammasome assembly by disrupting the interaction between NLRP3 and ASC and showed a significant protective effect against LPS-induced septic shock and NASH in mice. Collectively, our findings identify Tan I as an attractive inhibitor of NLRP3 inflammasome, providing a potential therapeutic candidate for NLRP3-mediated inflammatory diseases.
Materials and methods
Antibodies and chemicals
Tanshinone I (Tan I, HY-N0134), MCC950 (HY-12815 A), and nigericin (HY-127019) were from MedChemExpress. ATP, SiO2, Pam3CSK4, poly (I:C), poly (dA:dT), and ultrapure lipopolysaccharide (LPS) were from Invivogen. Phorbol 12-myristate 13-acetate (PMA, P8139) and DMSO (D2650) were from Sigma Aldrich. The Starfect high-efficiency transfection reagent (C101-10) was from GenStar. Antibodies were used as follows: anti-mouse IL-1β (1:1000, AF-401-NA, R&D), anti-mouse caspase-1 (1:1000, AG-20B-0042, Adipogen), anti-human cleaved IL-1β (1:2000, 12242, Cell Signaling Technology), anti-human caspase-1 (1:2000, 4199 S, Cell Signaling Technology), anti-NLRP3 (1:2000, AG-20B-0014, Adipogen), anti-ASC (1:1000, sc-22514-R, Santa Cruz), anti-Flag (1:2000, 20543-1-AP, Proteintech), and anti-GAPDH (1:5000, 60004-1-Ig, Proteintech).
Cell culture
Primary mouse bone marrow-derived macrophages (BMDMs) were isolated from the femurs and tibias of C57BL/6, and cultured in DMEM complemented with 10% FBS, 1% penicillin/streptomycin (P/S) and 50 ng/ml mouse macrophage colony stimulating factor (M-CSF, HY-P7085, MedChemExpress), then cultured for 6–7 days. THP-1 cells were cultured in RPMI 1640 medium (10% FBS, 1% P/S). HEK-293T cells were cultured in DMEM (10% FBS, 1% P/S). All cell lines were grown in a 5% CO2 incubator at 37 °C.
Cell viability assay
BMDMs were plated overnight, and then exposed to different concentrations of Tan I for 24 h. CellTiter-Glo® 2.0 Cell Viability Assay (G9241, Promega) was used to analyze the cell viability following the manufacturer’s instructions.
Inflammasome activation assay
BMDMs (1.2 × 106/ml) were plated overnight, culture medium was replaced with fresh media containing 50 ng/ml LPS or 400 ng/ml Pam3CSK4 for 4 h, then culture medium was replaced with Opti-MEM containing Tan I for 1 h before being stimulated with inflammasome agonists: nigericin (10 µM, 45 min), ATP (5 mM, 1 h), SiO2 (250 µg/ml, 6 h), Salmonella (200 ng/ml, 6 h); poly (dA:dT) (2 µg/ml), poly (I:C) (2 µg/ml), or ultra-LPS (1 µg/ml) was transfected into the cells for 6 h.
Immunoblotting
Immunoblotting analyses were conducted as previously described (Huang et al. 2018). Supernatant was added with trichloroacetic acid to precipitate the protein. For cell lysis, cells were lysed in SDS loading buffer. The protein was separated by SDS-PAGE and transferred to PVDF, indicating primary antibodies were used for blots, then incubated with HRP-conjugated species-specific antibodies. Enhanced chemiluminescence was used to visualize the proteins.
ASC oligomerization
The ASC oligomerization was carried out as previously described (Liu et al. 2021). Briefly, samples were lysed with Triton buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% [v/v] Triton X-100) containing protease inhibitor cocktail (C0001, TargetMol) and centrifuged at 4 °C, 6000 g for 10 min. Then, the pellets were washed twice and resuspended in 200 µl ice-cold PBS, and disuccinimidyl suberate (DSS, 2 mM) was added for cross-linking at 37 °C for 30 min, followed by centrifugation at 6000 g for 10 min at 4 °C. The cross-linked pellets were resolved in SDS loading buffer and analyzed by immunoblotting.
Immunoprecipitation assay
HEK-293T cells were plated overnight, followed by transfection with ASC, Flag-NLRP3 plasmids, and then incubated with 20 µM Tan I for 6 h. Subsequently, samples were lysed with NP-40 buffer (0.1% [v/v] Nonidet-P40, 50 mM Tris [pH 7.8], 10% [v/v] glycerol, 50 mM NaCl, and 5 mM EDTA) containing protease inhibitor cocktail, and centrifuged at 4 °C, 12,000 g for 15 min, supernatants were then incubated with anti-Flag M2 beads (A2220, MilporeSigma) for 6 h at 4 °C.
Intracellular K+ measurement
K+ was measured as in the previous description (Shi et al. 2020). Briefly, cells were lysed by ultrapure HNO3, and boiled at 100 °C for 30 min. Intracellular K+ measurements were performed by inductively coupled plasma mass spectrometry.
ROS measurement
The assay for ROS measurement was performed as described previously (Wang et al. 2020b). Samples were harvested and stained with MitoSOX (Invitrogen) for 15 min at 37 °C, washed with HBSS for 3 times, then analyzed by flow cytometry.
Mice
C57BL/6 mice (8–10 weeks, 18–22 g) were obtained from SPF Biotechnology Co., Ltd. (Beijing, China). Mice were maintained under pathogen-free conditions; treatments were randomly allocated. Animal experiments were conducted by protocols approved by the Animal Care and Use Committee of the Fifth Medical Center of the Chinese PLA General Hospital.
LPS-induced septic shock
For the septic shock model, 8-week-old female C57BL/6 mice were pretreated with vehicle, MCC950 (20 mg/kg, i.p.) or Tan I (10 mg/kg, 20 mg/kg, i.p.) (n = 10) for 2 h before injection of 20 mg/kg LPS intraperitoneally, mice were monitored for lethality for 3 days. To detect inflammatory cytokine production, mice were treated with Tan I (20 mg/kg, i.p.) for 2 h, then administered with 20 mg/kg LPS intraperitoneally, 4 h later, mice were euthanized, IL-1β in the plasma or peritoneal lavage fluid was measured by ELISA (R&D).
Methionine-choline-deficient diet model
For induction of NASH, male C57BL/6 mice (8 weeks, 18-22 g) were fed a methionine-choline-deficient (MCD, 518,810, Dyets) diet, whereas the control groups received methionine and choline supplemented (MCS, 518,811, Dyets) diet, under the manufacturer’s instructions. The MCD and MCS fed mice were divided into groups (n = 6) that received vehicle, Tan I (20 mg/kg/d), or MCC950 (20 mg/kg/d) for five days, and then 40 mg/kg via gavage every other day for six weeks (Tan I or MCC950 was given starting when the mice were fed the MCD or MCS diet). Serum and liver were collected after mice were euthanized. Serum levels of AST and ALT (Nanjing jiancheng) were detected, while the pathological changes of the liver were detected by HE staining, Sirius red staining, and Masson staining.
Statistics
For statistical analysis, GraphPad Prism 6 was utilized. For comparison of multiple groups, one-way ANOVA with Dunnett’s post hoc test or Sidak’s post hoc test was used, and unpaired Student’s t-text was used to evaluate differences between the two groups. All of the experimental data were presented as mean ± SEM. The difference was considered statistically significant at * P < 0.05; NS: not significant.
Results
Tan I potently inhibits NLRP3 inflammasome activation
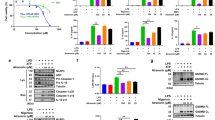
Before exploring the impact of Tan I (Fig. 1A) on NLRP3 inflammasome activation, its effect on cell viability was measured. Bone-marrow derived macrophages (BMDMs) were exposed to different doses of Tan I for 24 h. Data showed that Tan I was not toxic at concentrations below 30 µM (Fig. 1B). NLRP3 inflammasome mediates caspase-1 cleavage and IL-1β maturation, and pyroptosis (Liu et al. 2016). Thus, we tested whether Tan I could inhibit the maturation and secretion of IL-1β, caspase-1 activity, and LDH release (a marker for cell death (Shi et al. 2020), triggered by NLRP3 inflammasome agonists. We illustrated that Tan I inhibited caspase-1 cleavage (Fig. 1C and D), IL-1β maturation (Fig. 1C and E), and LDH release (Fig. 1F) induced by nigericin in a dose-responsive manner. Besides, Tan I also prevented ATP-induced NLRP3 inflammasome activation (Fig. 1, G-J). Moreover, our results suggested that Tan I inhibited caspase-1 cleavage as well as IL-1β maturation triggered by NLRP3 agonist nigericin in THP-1 cells, the human monocyte-like cell line (Supplemental Fig. 1, A-C). In general, our results suggested that Tan I suppressed NLRP3 inflammasome activation.
Tanshinone I (Tan I) suppressed NLRP3 inflammasome activation in BMDMs. (A) Tan I’s structure. (B) Cell viability of BMDMs treated with Tan I. (C-J) BMDMs were primed with LPS, then incubated with Tan I and stimulated with nigericin or ATP, cleaved caspase-1(p20) and mature IL-1β (p17) in supernatant (Sup.) were assessed by immunoblotting (C, G), caspase-1 activity (D, H), IL-1β secretion (E, I), and LDH release (F, J) in the supernatant were detected. Data are shown as mean ± SEM from triplicates, **P < 0.01, ***P < 0.001, NS: not significant (one-way ANOVA with Dunnett’s post hoc test)
Tan I had no impact on NLRC4 or AIM2 inflammasome activation
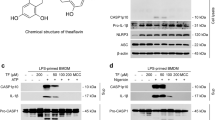
We further studied Tan I’s impact on other stimuli-induced NLRP3 inflammasome activation. LPS-primed BMDMs were treated with Tan I before being stimulated with poly (I: C), SiO2, or ATP. Similarly, we found that Tan I obviously reduced caspase-1 cleavage and IL-1β maturation (Fig. 2, A-C). Additionally, our results showed that Tan I blocked caspase-1 cleavage and IL-1β maturation induced by LPS transfection in Pam3CSK4-primed BMDMs (Fig. 2, D-F), demonstrating the suppression effect of Tan I on non-canonical NLRP3 inflammasome activation. Results showed that Tan I is a potent inhibitor of the NLRP3 inflammasome.
Tan I had no impact on NLRC4 or AIM2 inflammasome activation. (A–C) LPS-primed BMDMs were incubated with or without Tan I (20 µM), followed by the indicated stimuli. Cleaved caspase-1(p20) and mature IL-1β (p17) in supernatant were assessed by immunoblotting (A), activity of caspase-1 (B) and IL-1β secretion (C) in the supernatants were analyzed. (D-F) LPS-primed BMDMs were incubated with or without Tan I (20 µM), followed by treatment with nigericin, poly(dA:dT) or Salmonella, or Pam3CSK4-primed BMDMs were treated with Tan I followed by transfection of LPS, cleaved caspase-1(p20) and mature IL-1β (p17) were assessed by immunoblotting (D), activity of caspase-1 (E) and IL-1β secretion (F) in supernatants were evaluated. Data are shown as mean ± SEM from triplicates, *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significant (unpaired Student’s t test)
AIM2 and NLRC4 inflammasomes also mediate caspase-1 activation (Song et al. 2018). Therefore, we examined whether Tan I could prevent AIM2 or NLRC4 inflammasome activation. After priming, BMDMs were infected with Salmonella typhimurium or transfected with poly (dA: dT) to activate NLRC4 or AIM2 inflammasome, respectively (Bauer and Rauch 2020; Hornung et al. 2009). Of note, our results proved that Tan I blocked neither caspase-1 activation nor IL-1β maturation triggered by Salmonella typhimurium or poly (dA: dT) (Fig. 2, D-F). These data confirmed that Tan I specifically inhibits NLRP3 inflammasome activation, but not AIM2 or NLRC4 inflammasome.
Tan I suppressed ASC oligomerization
Previous research revealed that Tan I inhibits NF-κB activation (Wang et al. 2015; Yang et al. 2021), which mediates the expression of pro-1 L-1β and NLRP3 during LPS priming (Jo et al. 2016). Therefore, we tested whether Tan I influenced the generation of NLRP3 and pro-IL-1β mediated by LPS. BMDMs were exposed to Tan I before or after LPS stimulation. We found that Tan I inhibited the production of pro-IL-1β and TNF-α when pre-treated with Tan I for one hour before LPS stimulation (Fig. 3, A and B), but in the condition that LPS-primed BMDMs were treated with Tan I, Tan I did not affect pro-IL-1β expression. However, it still affected IL-1β secretion induced by NLRP3 agonists (Fig. 2A), indicating that Tan I indeed affects the activation step of NLRP3 inflammasome.
Then, the mechanism underlying Tan I’s effect on NLRP3 inflammasome was studied. ASC oligomerization acts an indispensable role in caspase-1 cleavage during inflammasome activation (Song and Li 2018). We found that Tan I inhibited the oligomerization of ASC induced by nigericin (Fig. 3C) dose-dependently. Further research showed that ASC oligomerization triggered by other NLRP3 agonists was also inhibited by Tan I (Fig. 3, D and E). In addition, Tan I had no influence on ASC oligomerization during NLRC4 or AIM2 inflammasome activation (Fig. 3E).
Tan I suppressed ASC oligomerization. (A and B) BMDMs were cultured with LPS for 4 h, then incubated with DMSO or Tan I for 1 h, or BMDMs were first incubated with Tan I for 1 h, followed by LPS treatment for 4 h, protein level of NLRP3 and pro-IL-1β was determined by immunoblotting (A), ELISA was used to detect TNF-α secretion (B). (C-E) Immunoblotting of cross-linked pellets from LPS-primed BMDMs treated with Tan I before being stimulated by the indicated agonists. Data are shown as mean ± SEM from triplicates, *P < 0.05, **P < 0.01, NS: not significant (one-way ANOVA with Dunnett’s post hoc test)
Tan I restrained NLRP3 inflammasome activation by disrupting NLRP3-ASC interaction
Next, we tested whether Tan I affected the upstream signaling events of NLRP3 inflammasome activation, including K+ efflux, Ca2+ flux, as well as mitochondrial reactive oxygen species (ROS) production (Muñoz-Planillo et al. 2013; Piccini et al. 2008; Zhou et al. 2011). Intracellular potassium was significantly reduced after nigericin treatment, but this effect was not affected by Tan I (Fig. 4A). NLRP3 agonists, such as nigericin and ATP, have been reported to trigger Ca2+ mobilization, blocking Ca2+ signaling would inhibit NLRP3 inflammasome activation (Lee et al. 2012; Murakami et al. 2012). Herein, LPS-primed BMDMs were stimulated with ATP and Ca2+ mobilization was observed, but this mobilization was not affected by Tan I (Fig. 4B). Moreover, mtROS generated by mitochondrial damage is considered to be another upstream signaling event in NLRP3 inflammasome activation (Zhou et al. 2011). Our results demonstrated that Tan I did not influence the mitochondrial ROS production stimulated by nigericin (Fig. 4C).
Tan I inhibited NLRP3 inflammasome by disrupting the association between NLRP3 and ASC. (A) BMDMs were primed with LPS followed by Tan I incubation, and then treated with nigericin, the efflux of potassium was measured. (B) The mobilization of Ca2+ in LPS-primed BMDMs treated with Tan I (or not) followed by ATP for the indicated time was assessed. (C) LPS-primed BMDMs were cultured in the presence or absence of Tan I and then stimulated with nigericin, then stained with MitoSox, mtROS was analyzed by flow cytometry. (D, E) Immunoprecipitation analysis of the interaction among NLRP3, NEK7, ASC in HEK-293T, in the presence of Tan I (20 µM) or not. Data are shown as mean ± SEM from triplicates, *P < 0.05, ***P < 0.001, NS: not significant (one-way ANOVA with Sidak’s post hoc test)
Subsequently, investigations were conducted into Tan I’s impact on NLRP3 inflammasome assembly. NEK7 is a vital regulator of NLRP3 inflammasome and the interaction between NLRP3 and NEK7 is essential for NLRP3 inflammasome activation (He et al. 2016). Once activated, NLRP3 recruits ASC and pro-caspase-1 to form the inflammasome (Shi et al. 2016). So, we further explored whether Tan I could prevent the interaction between the core proteins of NLRP3 inflammasome. Our data suggested that Tan I treatment suppressed the interaction between ASC and NLRP3, but not NLRP3 and NEK7 (Fig. 4, D and E). Thus, our data uncovered that Tan I suppressed NLRP3 inflammasome activation via blocking NLRP3-ASC interaction.
Tan I relieved LPS-induced septic shock
Moreover, the influence of Tan I on NLRP3 inflammasome activation was evaluated in vivo. Previous studies have reported that LPS injected intraperitoneally induces NLRP3-dependent IL-1β secretion and septic shock in mice (Mao et al. 2013). In our study, mice were injected with LPS intraperitoneally after pre-treatment with Tan I or MCC950, a reported inhibitor of NLRP3 (Wu et al. 2020), the survival was observed. In our study, Tan I dose-dependently protected mice from septic shock (Fig. 5A). To test Tan I’s influence on IL-1β secretion in vivo, pretreated mice with Tan I or MCC950, followed by injection with LPS. Four hours later, the samples were collected and the level of IL-1β in peritoneal lavage fluid and serum was determined. The data showed that LPS injection induced the production of IL-1β, which was suppressed by Tan I, and it had a similar effect compared to MCC950 (Fig. 5, B and C). Collectively, Tan I protected mice from septic shock and inhibited NLRP3 inflammasome activation in vivo.
Tan I relieved LPS-induced septic shock and inhibited NLRP3 inflammasome activation in vivo. (A) Female mice (8 weeks) were intraperitoneally (i.p.) injected with vehicle, MCC950 (20 mg/kg), or Tan I (10, 20 mg/kg) respectively, 2 h later, administered with LPS (20 mg/kg, i.p.), then monitored for 72 h (n = 10). (B, C) Mice were injected intraperitoneally with 20 mg/kg Tan I, 20 mg/kg MCC950 for 2 h, followed by an injection of 20 mg/kg LPS for 4 h (n = 6). The levels of IL-1β in peritoneal lavage fluid (B) and serum (C) were analyzed by ELISA. Data are shown as mean ± SEM, ***P < 0.001 (log-rank test (A), one-way ANOVA with Dunnett’s post hoc test (B and C))
Tan I ameliorated non-alcoholic steatohepatitis in mice
NLRP3 inflammasome is reported to play a crucial role in NASH (Mridha et al. 2017). Since 2000, the methionine and choline deficient diet (MCD) model has been widely utilized to produce severe steatohepatitis and fibrosis, that mimics the pathogenesis of human NASH (Ioannou et al. 2013; Leclercq et al. 2000). The histological abnormalities of NASH, a frequent liver ailment, resemble those of alcoholic steatohepatitis. Steatosis, hepatocyte injury, a mixed inflammatory lobular infiltration, and fibrosis are among the histological characteristics. Mice acquired severe steatohepatitis quickly and consistently under the MCD dietary regimen. Steatosis, inflammatory infiltration, hepatic necrosis, and fibrosis are the pathologies that are typical of NASH in humans (Ludwig et al. 1980; Powell et al. 1990). In recent years, a growing number of studies have confirmed the relationship between NLRP3 and NASH, and blockade of NLRP3 inflammasome activation can reduce liver inflammation and fibrosis as well as improve NASH pathology (Calcagno et al. 2022; Mridha et al. 2017). Therefore, we tested the protective effect of Tan I in MCD-induced NASH in mice, and the data suggested that administration of Tan I or MCC950 reversed the liver morphology in the NASH mice, accompanied the reduction of serum AST and ALT induced by MCD diet (Fig. 6, A-C). Furthermore, hepatic histopathological analysis was performed with HE, Sirius red, and Masson staining. Liver fibrosis, fatty vacuoles, and inflammatory cell infiltration were observed in the NASH mice (MCD), while Tan I treatment alleviated these histopathological changes (Fig. 6D, Supplemental Fig. 2, A and B). Moreover, we detected the expression of F4/80 in the liver tissues and found that the expression of F4/80+ was reduced in Tan I and MCC950 treated NASH mice (Supplemental Fig. 2, C and D). To determine whether Tan I blocked the activation of NLRP3 inflammasome in NASH mice, immunoblotting was used to detect the expression of active caspase-1 in mouse liver tissues. Our results showed that caspase-1 activation in the liver of mice fed MCD diets was inhibited by Tan I or MCC950 (Supplemental Fig. 2E). Taken together, these data confirmed that Tan I exhibited a protective effect in the mouse model of NASH.
Tan I ameliorated non-alcoholic steatohepatitis in mice. (A-D) Male C57BL/6 mice (8 weeks, 18-22 g) were fed MCD diet, while the control groups received MCS diet. The MCD and MCS fed mice were divided into groups (n = 6) that received vehicle, Tan I (20 mg/kg), or MCC950 (20 mg/kg) every day for 5 days, and subsequently 40 mg/kg via gavage every other day for six weeks. The liver morphology of mice in different treatment groups was shown, scale bar: 1 cm (A). Serum ALT and AST levels were determined by ELISA (B and C). Liver sections were stained with HE, Sirius Red, and Masson, scale bar: 200 μm (D). Data are shown as mean ± SEM, ***P < 0.001 (one-way ANOVA with Dunnett’s post hoc test)
Discussion
NLRP3 inflammasome mediates the development of many inflammatory diseases, like NASH, acute lung injury, gout, type 2 diabetes, and preeclampsia (Guan et al. 2022; Mangan et al. 2018; Szabo and Petrasek 2015; Zhao et al. 2021; Zhu and Liu 2022). Therefore, targeting NLRP3 inflammasome is considered a prospective strategy. Herein, we report that the main active ingredient of Salvia miltiorrhiza, Tan I specifically inhibits NLRP3 inflammasome activation both in vitro and in vivo, and exhibits an obviously protective effect in mouse models of NLRP3-mediated inflammatory diseases, indicating that Tan I might be a potent therapeutic drug candidate.
In our study, we found that Tan I did not affect the upstream signaling events of NLRP3 inflammasome activation, including K+efflux, Ca2+flux, as well as ROS production. Meanwhile, our results illustrated that Tan I had no impact on NLRP3-NEK7 interaction but inhibited NLRP3-ASC interaction, suggesting Tan I blocked NLRP3 inflammasome activation via disrupting ASC recruitment to NLRP3, but the direct target needs further study. In addition, whether Tan I inhibits NLRP3 interaction with ASC in vivo needs further investigation.
Tan I has been reported to inhibit NF-κB activation (Wang et al. 2015), in our study, we found that Tan I inhibited NF-κB-mediated pro-IL-1β expression in BMDMs when pre-treated with Tan I followed by LPS priming. We noticed that in this contest Tan I had no effect on NLRP3 expression, this may be because pro-IL-1β is more sensitive to the activation of NF-κB, or due to the differential regulatory effect of Tan I on the production of NLRP3 and pro-IL-1β.
Furthermore, we also found that Tan I had a significant beneficial effect on NLRP3 inflammasome-related diseases like septic shock and NASH in mice. Meanwhile, Tan I also suppressed IL-1β secretion in LPS-induced septic shock, suggesting Tan I’s inhibitory effect on NLRP3 inflammasome activation in vivo. NASH has become one of the important causes of liver cirrhosis and liver cancer, but currently there is no effective treatment. In our study, we found that Tan I could alleviate pathologic changes such as hepatic steatosis, inflammatory cell infiltration and liver fibrosis in MCD-induced NASH mice. This suggests that Tan I may be a promising therapeutic candidate for NASH. In addition, previous research reported that Tan I reduced insulin resistance in type 2 diabetes rats (Wei et al. 2017) as well as osteoarthritis in mice (Wang et al. 2019a), NLRP3 inflammasome activation also act as a key role in the progression of type 2 diabetes as well as osteoarthritis. Whether Tan I ameliorates the progression of these diseases via its inhibitory effect on NLRP3 inflammasome requires future studies.
Previous studies revealed that tanshinones, the main component of Salvia miltiorrhiza, have been reported to have anti-inflammatory effects and may be the key component for its anti-inflammatory effects (Jiang et al. 2019; Liu et al. 2022; Wang et al. 2020a). In addition, Tanshinone IIA and Dihydrotanshinone I have been reported to inhibit the activation of NLRP3 inflammasome and protect against NLRP3-related diseases (Gao et al. 2019; Li et al. 2022c; Wei et al. 2021; Xu et al. 2022). A recent study found that 15 tanshinones (isolated from Salvia miltiorrhiza) including Tan I inhibited IL-1β secretion, but did not further demonstrate the specificity of the effect of Tan I on NLRP3 inflammasome activation or the mechanism of action. Whether Tan I is protective against NLRP3-mediated diseases is not yet clarified (Yue et al. 2021). Our study clarified the regulatory effect of Tan I on NLRP3 inflammasome activation both in vitro and in vivo as well as the detailed mechanism, benefiting the understanding of the pharmacological effect of Tan I.
Conclusions
In summary, we demonstrated that Tan I specifically inhibited NLRP3 inflammasome activation via targeting the ASC and NLRP3 complex. In vivo studies have shown that Tan I relieves LPS-induced septic shock and NASH in mice. Altogether, Tan I may be developed as a potent therapeutic drug candidate for the treatment of NLRP3 inflammasome-mediated diseases.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Tan I:
-
tanshinone I
- BMDMs:
-
bone marrow-derived macrophages
- NLRP3:
-
NOD-like receptor thermal protein domain associated protein 3
- ASC:
-
apoptosis-associated speck-like protein
- NASH:
-
non-alcoholic steatohepatitis
- LPS:
-
Lipopolysaccharide
References
Bauer R, Rauch I. The NAIP/NLRC4 inflammasome in infection and pathology. Mol Aspects Med. 2020;76:100863.
Calcagno DM, Chu A, Gaul S, Taghdiri N, Toomu A, Leszczynska A, Kaufmann B, Papouchado B, Wree A, Geisler L, Hoffman HM, Feldstein AE, King KR. NOD-like receptor protein 3 activation causes spontaneous inflammation and fibrosis that mimics human NASH. Hepatology (Baltimore MD). 2022;76(3):727–41.
Chauhan D, Vande Walle L, Lamkanfi M. Therapeutic modulation of inflammasome pathways. Immunol Rev. 2020;297(1):123–38.
Chiazza F, Couturier-Maillard A, Benetti E, Mastrocola R, Nigro D, Cutrin JC, Serpe L, Aragno M, Fantozzi R, Ryffel B. Thiemermann C and Collino M. Targeting the NLRP3 inflammasome to Reduce Diet-Induced metabolic abnormalities in mice. Mol Med. 2016;21(1):1025–37.
Duez H, Pourcet B. Nuclear Receptors in the control of the NLRP3 inflammasome pathway. Front Endocrinol (Lausanne). 2021;12:630536.
Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265(1):35–52.
Gao M, Ou H, Jiang Y, Wang K, Peng Y, Zhang H, Yang M, Xiao X. Tanshinone IIA attenuates sepsis-induced immunosuppression and improves survival rate in a mice peritonitis model. Volume 112. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2019. p. 108609.
Guan X-X, Yang H-H, Zhong W-J, Duan J-X, Zhang C-Y, Jiang H-L, Xiang Y, Zhou Y, Guan C-X. Fn14 exacerbates acute lung injury by activating the NLRP3 inflammasome in mice. Mol Med. 2022;28(1):85.
He Y, Zeng MY, Yang D, Motro B. And Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–7.
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, Zhou R. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9(1):2550.
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–8.
Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, Deng X, Liang G, Zhang H, Jiang W, Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10(4):e8689.
Ioannou GN, Haigh WG, Thorning D, Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54(5):1326–34.
Jiang Z, Gao W, Huang L, Tanshinones. Critical Pharmacological Components in Salvia miltiorrhiza. Front Pharmacol. 2019;10:202.
Jo E-K, Kim JK, Shin D-M, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–59.
Jung DY, Kim JH, Jung MH. Anti-Obesity Effects of Tanshinone I from Salvia miltiorrhiza Bunge in mice Fed a High-Fat Diet through Inhibition of early adipogenesis. Nutrients. 2020;12(5):1242.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328.
Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–75.
Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2 + and cAMP. Nature. 2012;492(7427):123–7.
Li X, Xiao G-Y, Guo T, Song Y-J, Li Q-M. Potential therapeutic role of pyroptosis mediated by the NLRP3 inflammasome in type 2 diabetes and its complications. Front Endocrinol (Lausanne). 2022a;13:986565.
Li X, Zhou X, Liu X, Li X, Jiang X, Shi B, Wang S. Spermidine protects against acute kidney injury by modulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway. Mol Med. 2022b;28(1):103.
Li Y, Fu Y, Sun J, Shen J, Liu F, Ning B, Lu Z, Wei L, Jiang X. Tanshinone IIA alleviates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium tuberculosis-(H37Ra-) infected macrophages by inhibiting endoplasmic reticulum stress. J Ethnopharmacol. 2022c;282:114595.
Liu X, Liu J. Tanshinone I induces cell apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and by suppressing p53/DRAM-mediated autophagy in human hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2020;48(1):488–97.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8.
Liu H, Zhan X, Xu G, Wang Z, Li R, Wang Y, Qin Q, Shi W, Hou X, Yang R, Wang J, Xiao X, Bai Z. Cryptotanshinone specifically suppresses NLRP3 inflammasome activation and protects against inflammasome-mediated diseases. Pharmacol Res. 2021;164:105384.
Liu L, Wang D, Liu M, Yu H, Chen Q, Wu Y, Bao R, Zhang Y, Wang T. The development from hyperuricemia to gout: key mechanisms and natural products for treatment. Acupunct Herb Med. 2022;2(1):25–32.
Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–8.
Lugrin J, Martinon F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. 2018;281(1): 99–114.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discovery. 2018;17(8):588–606.
Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, Li J, Wu X, Wang X, Strober W, Chen C, Meng G, Sun B. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23(2):201–12.
Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NCH, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA. Feldstein AE and Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66(5):1037–46.
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. Kâ+º efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–53.
Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109(28):11282–7.
Pappritz K, Lin J, El-Shafeey M, Fechner H, Kühl U, Alogna A, Spillmann F, Elsanhoury A, Schulz R, Tschöpe C, Van Linthout S. Colchicine prevents disease progression in viral myocarditis via modulating the NLRP3 inflammasome in the cardiosplenic axis. ESC Heart Fail. 2022;9(2):925–41.
Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105(23):8067–72.
Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology (Baltimore MD). 1990;11(1):74–80.
Rathinam VAK, Fitzgerald KA. Inflammasome Complexes: emerging mechanisms and Effector Functions. Cell. 2016;165(4):792–800.
Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, Su L, Pratt D, Bu CH, Hildebrand S, Lyon S, Scott L, Quan J, Sun Q, Russell J, Arnett S, Jurek P, Chen D, Kravchenko VV, Mathison JC, Moresco EMY, Monson NL. Ulevitch RJ and Beutler B. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17(3):250–8.
Shi W, Xu G, Zhan X, Gao Y, Wang Z, Fu S, Qin N, Hou X, Ai Y, Wang C, He T, Liu H, Chen Y, Liu Y, Wang J, Niu M, Guo Y, Xiao X, Bai Z. Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell Death Dis. 2020;11(4):252.
Song N, Li T. Regulation of NLRP3 inflammasome by phosphorylation. Front Immunol. 2018;9:2305.
Song S, Qiu D, Luo F, Wei J, Wu M, Wu H, Du C, Du Y, Ren Y, Chen N, Duan H, Shi Y. Knockdown of NLRP3 alleviates high glucose or TGFB1-induced EMT in human renal tubular cells. J Mol Endocrinol. 2018;61(3):101–13.
Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89.
Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400.
Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889.
Wang S, Jing H, Yang H, Liu Z, Guo H, Chai L, Hu L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J Ethnopharmacol. 2015;164:247–55.
Wang X, Fan J, Ding X, Sun Y, Cui Z, Liu W. Tanshinone I inhibits IL-1β-Induced apoptosis, inflammation and Extracellular Matrix Degradation in Chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Devel Ther. 2019a;13:3559–68.
Wang Z, Xu G, Gao Y, Zhan X, Qin N, Fu S, Li R, Niu M, Wang J, Liu Y, Xiao X, Bai Z. Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B. 2019b;9(4):734–44.
Wang X, Wang Q, Li W, Zhang Q, Jiang Y, Guo D, Sun X, Lu W, Li C, Wang Y. TFEB-NF-κB inflammatory signaling axis: a novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity. J Exp Clin Cancer Res. 2020a;39(1):93.
Wang Z, Xu G, Wang H, Zhan X, Gao Y, Chen N, Li R, Song X, Guo Y, Yang R, Niu M, Wang J, Liu Y, Xiao X, Bai Z. Icariside II, a main compound in Epimedii Folium, induces idiosyncratic hepatotoxicity by enhancing NLRP3 inflammasome activation. Acta Pharm Sin B. 2020b;10(9):1619–33.
Wang P, Ni M, Tian Y, Wang H, Qiu J, You W, Wei S, Shi Y, Zhou J, Cheng F, Rao J, Lu L. Myeloid Nrf2 deficiency aggravates non-alcoholic steatohepatitis progression by regulating YAP-mediated NLRP3 inflammasome signaling. iScience. 2021;24(5):102427.
Wei Y, Gao J, Qin L, Xu Y, Wang D, Shi H, Xu T, Liu T. Tanshinone I alleviates insulin resistance in type 2 diabetes mellitus rats through IRS-1 pathway. Volume 93. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2017. pp. 352–8.
Wei Z, Zhan X, Ding K, Xu G, Shi W, Ren L, Fang Z, Liu T, Hou X, Zhao J, Li H, Li J, Li Z, Li Q, Lin L, Yang Y, Xiao X. Bai Z and Cao J. Dihydrotanshinone I specifically inhibits NLRP3 inflammasome activation and protects against septic shock in vivo. Front Pharmacol. 2021;12:750815.
Wu D, Chen Y, Sun Y, Gao Q, Li H, Yang Z, Wang Y, Jiang X, Yu B. Target of MCC950 in inhibition of NLRP3 inflammasome activation: a literature review. Inflammation. 2020;43(1):17–23.
Xu G, Fu S, Zhan X, Wang Z, Zhang P, Shi W, Qin N, Chen Y, Wang C, Niu M, Guo Y, Wang J, Bai Z, Xiao X. Echinatin effectively protects against NLRP3 inflammasome-driven diseases by targeting HSP90. JCI insight. 2021;6(2):e134601.
Xu L, Liu X, Jia T, Sun Y, Du Y, Wei S, Wang W, Zhang Y, Chen W, Zhang S. Tanshinone IIA ameliorates nonalcoholic steatohepatitis in mice by modulating neutrophil extracellular traps and hepatocyte apoptosis. Evid Based Complement Alternat Med. 2022;2022:5769350.
Yang L, Zhou G, Liu J, Song J, Zhang Z, Huang Q, Wei F. Tanshinone I and Tanshinone IIA/B attenuate LPS-induced mastitis via regulating the NF-κB. Volume 137. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2021. p. 111353.
Yang L, Zhang X, Wang Q. Effects and mechanisms of SGLT2 inhibitors on the NLRP3 inflammasome, with a focus on atherosclerosis. Front Endocrinol (Lausanne). 2022;13:992937.
Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9.
Yue H, Yang Z, Ou Y, Liang S, Deng W, Chen H, Zhang C, Hua L, Hu W, Sun P. Tanshinones inhibit NLRP3 inflammasome activation by alleviating mitochondrial damage to protect against septic and gouty inflammation. Int Immunopharmacol. 2021;97:107819.
Zhao X, Zhang X, Wu Z, Mei J, Li L, Wang Y. Up-regulation of microRNA-135 or silencing of PCSK6 attenuates inflammatory response in preeclampsia by restricting NLRP3 inflammasome. Mol Med. 2021;27(1):82.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5.
Zhou J, Jiang Y-Y, Chen H, Wu Y-C, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739.
Zhu L, Liu L. New Insights into the Interplay among Autophagy, the NLRP3 inflammasome and inflammation in adipose tissue. Front Endocrinol (Lausanne). 2022;13:739882.
Acknowledgements
We thank Prof. Tao Li (National Center for Biomedical Analysis, Beijing, China) for providing Salmonella, ASC and Flag-NLRP3.
Funding
This work has been supported by National Natural Science Foundation of China (82003984), Beijing Natural Science Foundation (7214296, 7232321), National Natural Science Foundation of China (81874368, 81721002), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202005), the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (2017ZX09301022). Provincial basic research program of Guizhou Science and Technology Agency (Qiankehe Foundation – ZK [2023] General 489).
Author information
Authors and Affiliations
Contributions
XYZ, ZFB, XHX, and Jun Z supervised the project. XYZ, ZFB, and XHX acquired funding for the study. XYZ, ZFB, HBL and Jia Z designed the experiments. Jia Z, HBL and ZXH carried out experiments and analyzed the data. GX, WL, WQM, XRH, ZEF, LTR, and TTL helped perform the animal experiments. JCW, WS, and ZYW provided assistance with the investigation. YPY, Jun Z, and WJZ analyzed the data. XYZ, ZFB and Jia Z wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal experiments were conducted by protocols approved by the Animal Care and Use Committee of the Fifth Medical Center of the Chinese PLA General Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Liu, H., Hong, Z. et al. Tanshinone I specifically suppresses NLRP3 inflammasome activation by disrupting the association of NLRP3 and ASC. Mol Med 29, 84 (2023). https://doi.org/10.1186/s10020-023-00671-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-023-00671-0