Abstract
Background
The unique mechanism of diabetic atherosclerosis has been a central research focus. Previous literature has reported that the inflammatory response mediated by dendritic cells (DCs) plays a vital role in the progression of atherosclerosis. The objective of the study was to explore the role of DCs in diabetes mellitus complicated by atherosclerosis.
Methods
ApoE−/− mice and bone marrow-derived DCs were used for in vivo and in vitro experiments, respectively. Masson’s staining and Oil-red-O staining were performed for atherosclerotic lesion assessment. The content of macrophages and DCs in plaque was visualized by immunohistochemistry. The expression of CD83 and CD86 were detected by flow cytometry. The fluctuations in the RNA levels of cytokines, chemokines, chemokine receptors and adhesions were analyzed by quantitative RT-PCR. The concentrations of IFN-γ and TNF-α were calculated using ELISA kits and the proteins were detected using western blot. Coimmunoprecipitation was used to detect protein–protein interactions.
Results
Compared with the ApoE−/− group, the volume of atherosclerotic plaques in the aortic root of diabetic ApoE−/− mice was significantly increased, numbers of macrophages and DCs were increased, and the collagen content in plaques decreased. The expression of CD83 and CD86 were significantly upregulated in splenic CD11c+ DCs derived from mice with hyperglycemia. Increased secretion of cytokines, chemokines, chemokine receptors, intercellular cell adhesion molecule (ICAM), and vascular cell adhesion molecule (VCAM) also were observed. The stimulation of advanced glycation end products plus oxidized low-density lipoprotein, in cultured BMDCs, further activated toll-like receptor 4, protein kinase C and receptor of AGEs, and induced immune maturation of DCs through the RAGE-TLR4-PKCβ1 signaling pathway that was bound together by intrinsic structures on the cell membrane. Administering LY333531 significantly increased the body weight of diabetic ApoE−/− mice, inhibited the immune maturation of spleen DCs, and reduced atherosclerotic plaques in diabetic ApoE−/− mice. Furthermore, the number of DCs and macrophages in atherosclerotic plaques was significantly reduced in the LY333531 group, and the collagen content was increased.
Conclusions
Diabetes mellitus aggravates chronic inflammation, and promotes atherosclerotic plaques in conjunction with hyperlipidemia, which at least in part through inducing the immune maturation of DCs, and its possible mechanism of action is through the RAGE-TLR4-pPKCβ1 signaling pathway.
Similar content being viewed by others
Introduction
The risk of coronary heart disease in individuals with diabetes mellitus (DM) is two to six times greater than for individuals without DM (Sasso et al. 2004). Previous studies have reported that increased secretion of tumor necrosis factor α (TNFα), interleukin (IL)-6, C-reactive protein (CRP), and other substances at low concentrations lead to chronic low-grade inflammatory responses accompanied by the development and progression of diabetes mellitus (Castelblanco et al. 2018). This inflammatory mechanism is currently considered to be a leading mechanism for the development and progression of atherosclerosis (Zhang et al. 2019). The hyperglycemic state of diabetes accelerates atherosclerosis in conjunction with hyperlipidemia, but the mechanism is still not well understood (Wu and Huan 2007). When there is the appearance of atherosclerotic plaque together with diabetes mellitus, atherosclerosis presents several distinctive characteristics, such as microvascular lesions and diffuse lesion plaques, which illustrates that the development of atherosclerosis in DM patients has its own specific mechanism (Coutant et al. 2004).
It is believed that DCs, specialized antigen-presenting, and immune inflammatory-response-initiating cells, plays a role as a "bidirectional immune regulator" in the occurrence and development of atherosclerosis (Daissormont et al. 2011; Kanter et al. 2012). The degree of atherosclerosis that is produced may depend on the balance of immune regulation (Daissormont et al. 2011; Kanter et al. 2012). DCs secrete a range of cytokines, chemokine and chemokine receptors (CCL4, CCR7, CXCR4) after immune maturation to respond to the stimulation of antigens or immune inflammation (Ssemakalu et al. 2015). Cytokines that induce inflammation, such as IL1, IL-6, IL-12, and TNF-α are referred to as pro-inflammatory cytokines (Dinarello 2000). Cytokines such as IL-4 and IL-10 that inhibit the inflammatory processes are called anti-inflammatory cytokines. Previous studies have shown that a high-glucose (Cao et al. 2015) and high-insulin (Lu et al. 2015) environment can promote the differentiation and immune maturation of DCs. Injecting diphtheria toxin into DTR+/+LDLR−/−mice to induce apoptosis of DCs can reduce plaque formation by 55%, and enhance plaque stability (Paulson et al. 2010). These results indicate that reducing DC-mediated inflammation is beneficial in reducing atherosclerosis. Since there is currently no direct way to reduce the number of DCs in clinical practice, regulation of DC maturation, and reduction of the release of inflammatory factors is worthy of attention. Previous studies found that the PKC signaling pathway is not only involved in the formation of atherosclerotic plaques (Durpes et al. 2015), but also plays an essential role in the differentiation and immune maturation of DCs (Stein et al. 2017). However, there is limited data to suggest the role PKCs in atherosclerosis under hyperglycemic condition, and, therefore, the DC maturation elicited in diabetic atherosclerosis.
In this study, we demonstrated that activation of the RAGE-TLR4-PKCβ1 signaling pathway in a high-glucose and high-fat environment was involved in the immune maturation of DCs and the occurrence and development of chronic low-grade inflammation in diabetes, which was closely related to diabetic atherosclerosis.
Material and methods
Animals and splenic CD11c+ DCs separation
All experimental animal procedures were approved by the Animal Care and Use Committee of Fudan University. ApoE−/− mice (Beijing Vital River Laboratory Animal Technology Co., Ltd. male, aged eight weeks and weighing approximately 20 g) were housed in the Animal Administration Center of Fudan University, Shanghai, China. Mice were maintained in cages with a maximum of four mice per cage. The mice were randomly divided into a diabetic group and a control group. Mice in the diabetic group received an intraperitoneal injection of streptozotocin (STZ, 80 mg/kg, Sigma, St Louis, MO, USA) daily for one week. Then the mice were maintained under the same conditions for an additional week before their blood glucose levels were monitored by collecting blood from the tail vein. Mice with fasting blood glucose levels over 13 mM were deemed diabetic and fed a normal diet for eight weeks. Additionally, ApoE−/− mice, aged eight weeks and weighing approximately 20 g, were treated with STZ in the same way as described above, then randomly divided into a diabetic group and an LY333531 group. Each mouse was gavaged once a day with 200μL 10% dimethyl sulfoxide for eight weeks. Half of the mice received 1 mg/kg LY333531 with 10% dimethyl sulfoxide, and half did not. The body weights were measured at the end of eight weeks. At the end of the experiment, the animals were euthanized using CO2 inhalation, the mice eyeballs were extracted and whole blood was taken. The blood lipid profile, such as total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), were measured by the Nanjing Jiancheng Bioengineering Institute. The spleens were removed, crushed, and the CD11c+ DCs were isolated using anti-CD11c+ microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The experiment designer was aware of the group allocation while data collectors and data analysts were not during the outcome assessment and data analysis.
Organ collection and processing
The aorta and heart were carefully perfused with physiological saline to remove the blood. The heart apex was removed, and the aortic arch was separated from aorta distal to the left subclavian artery. The isolated tissues were embedded in optimal cutting temperature compound (OCT compound) and frozen, then stored at -80℃.
Atherosclerotic lesion assessment
The frozen aortic roots were sectioned at a thickness of eight µm using a CM3050S cryostat (Leica Biosystems). Oil-red-O staining was to visualize plaque extension in the aortic root. Frozen sections were fixed, washed, and incubated for 18 min in an Oil-red-O solution, then counterstained with 0.25% brilliant green (Sinopharm Chemical Reagent, China) for 6 min. Oil-red-O staining was used to determine the lipid content of the plaques. Masson’s staining was performed using a Masson kit (Sinopharm Chemical Reagent, China) to stain collagen present in the atherosclerotic lesions. Images were obtained with an optical microscope (Olympus, Japan). Images were analyzed, and quantification was performed using NIH Image J software (Schneider et al. 2012).
Immunohistochemistry
To visualize macrophages in the plaque area, paraformaldehyde-fixed aortic root sections were stained with CD68-FITC antibody (1:50, MA5-16676, Invitrogen) using 4',6-diamidino-2-phenylindole (DAPI) (2ug/ml, D9542, Sigma, USA) as a nuclear counterstain. The presence of DCs in the plaques also was visualized. Paraformaldehyde-fixed sections of aortic root were stained with CD11c antibody (1:100, ab33483, Abcam, USA) and a hamster secondary antibody (PE, 1:100, 12-4112-83, Invitrogen) using DAPI (2ug/ml, D9542, Sigma, USA) as a nuclear counterstain. The co-staining of CD68 and CD14 with CD11c was conducted with CD11c antibody (1:100, Huabio, cat.no. RT1108, lot.no. HL1212, China) and a mouse secondary antibody (1:500, Invitrogen, cat.no. A32723), CD68 antibody (1:100, Servicebio, cat.no. GB11067, lot.no. AC2103065A), CD14 antibody (1:100, Abcam, cat.no. ab221678, lot.no. GR3264380-5), and a rabbit secondary antibody (1:500, Invitrogen, cat.no. A32740). The co-staining of PKC beta isoforms and RAGE with CD11c was conducted with CD11c antibody (1:100, Huabio, cat.no. RT1108, lot.no. HL1212, China) and a mouse secondary antibody (1:500, Invitrogen, cat.no. A32727), PKCβ antibody (1:100, Abclonal, cat.no. A13628, lot.no. 5500002416, China), RAGE antibody (1:50, Abcam, cat.no. ab216329, lot.no. GR3274230-10, USA), and a rabbit secondary antibody (1:500, Invitrogen, cat.no. A32731). Positive-stained areas were selected by an investigator blinded to the treatment, observed using a fluorescence microscope (Olympus, Japan), and quantified using NIH Image J software (Schneider et al. 2012).
Cell culture
Bone marrow-derived DCs (BMDCs) were obtained from C57 BL/6 mice (Beijing Vital River Laboratory Animal Technology Co., Ltd.). After the mice were euthanized using CO2 inhalation, the femurs of both pelvic limbs were removed, and the bone marrow cavity was flushed to obtain bone marrow cells. Bone marrow progenitor cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (R & D Systems, Minneapolis, MN, USA) and 1 ng/ml IL-4 (R & D Systems, Minneapolis, MN, USA). The non-adherent cells were gently washed out after 24 h, and the remaining loosely adherent clusters were cultured. The culture medium was changed every other day. On day six, the cells were stimulated using 50ug/ml oxidized low-density lipoprotein (oxLDL) (Serotec, UK) for 48 h, with or without 200 ug/ml AGEs-BSA (BioVision, Palo Alto, CA, USA) interference to simulate the high-lipid and high-glucose environment of ApoE−/− mice, respectively.
Flow cytometry
Splenic DCs and BMDCs (1 × 106) were harvested and washed, followed by incubation with CD83-PE-conjugated and CD86-FITC-conjugated (BD Pharmingen, San Diego, CA, USA) monoclonal antibodies for 30 min at 4℃. Then the cells were washed and analyzed using a flow cytometer (BD Bioscience). Cells stained with the appropriate isotype-matched immunoglobulin were used as negative controls.
Quantitative RT-PCR
Total RNA was extracted from splenic CD11c + DCs or BMDCs using Trizol reagent (Invitrogen, Carlsbad, CA, USA). We used a SYBR RT-PCR kit (Takara, Dalian, China) for quantitative RT-PCR analyses. The primers for the cytokine genes (IL10, TNFα, IL12a, IL12b, IL1b, IL4, and IL6), chemokine and chemokine receptor genes (CCL4, CCR7, and CXCR4), adhesion genes (ICAM and VCAM), and GAPDH are listed in Additional file 1: Table S1. The relative expression levels of the genes were normalized to those of GAPDH using the 2−ΔΔCt cycle threshold.
Quantification of cytokine production by ELISA
The cells and supernatant of BMDCs were harvested and stored at -80℃ until they were assayed. The concentration of CRP and cytokine (interferon-γ (IFNγ) and TNFα) were analyzed using ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Coimmunoprecipitation
Extracts from mouse BMDCs were prepared in lysis buffer (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% v/v Sigma Protease and Phosphatase Inhibitor Cocktail) and diluted to a final volume of 500μL (500 μg). Protein A:protein G beads (1:50; Sigma) were added, then polyclonal TLR4 antibody (2 μg; cat.no. 19811–1-AP, ProteinTech company) and polyclonal phospho-PKCβ1 antibody (2 μg, cat.no. PA5-105,463, Invitrogen) also were added, respectively. After 4-h of continuous gentle agitation at 4℃, the beads were collected by pulse spin, washed three times in lysis buffer, and resuspended in PBS.
Western blot
BMDCs were harvested for the extraction of total proteins with RIPA buffer. The protein concentrations were determined using a BCA protein assay (Beyotime, Shanghai, China). Antibodies of toll-like receptor 4 (TLR4) (1:1000, cat.no. 14358), RAGE (1:1000, cat.no. 55222), interleukin receptor-associated kinase 4 (IRAK4) (1:1000, cat.no. 4363), phosphor-IRAK4 (1:1000, cat.no. 11927), phospho-NF-κB (1:1000, cat.no. 3033), NF-κB (1:1000, cat.no. 8242), phospho-IκB (1:1000, cat.no. 9246), IκB (1:1000, cat.no. 9242) were all purchased from Cell Signal Technology, USA. Antibodies purchased from Santa Cruz included phospho-PKCα (1:300, cat.no. SC-377565), phospho-PKCβ2 (1:300, cat.no. SC-365463). Antibody of phospho-PKCβ1 was purchased from Invitrogen (1:500, cat.no. PA5-105463). Antibodies purchased from Abcam included phospho-PKCγ (1:1000, cat.no. ab5796), PKCα (1:1000, cat.no. ab32376), PKCβ1 (1:1000, cat.no. ab195039), PKCβ2 (1:1000, cat.no. ab32026), PKCγ (1:1000, cat.no. ab71558). The optical densities were analyzed using ImagePro 5.0 (Media Cybernetics, Inc., Silver Spring, MD, USA) and normalized to the protein level of β-actin or GAPDH.
Statistical analysis
The results were presented as means ± S.E.M. and analyzed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons using the SPSS software package, version 16.0. P < 0.05 was considered to be statistically significant.
Results
Diabetic ApoE−/− mice increased aortic atherosclerosis with chronic low-grade inflammation and dendritic cell activation
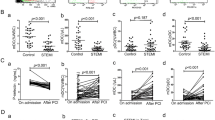
The fasting blood glucose levels in the diabetic ApoE−/− group were significantly increased, while the blood lipid levels were not significantly different (Additional file 1: Fig. S1). The thickness of the atheromatous plaque fibrous cap is reported to be proportional to the collagen content in it (Naghavi et al. 2003a, 2003b), and thicker fiber caps suggest more stable plaques. On the other hand, excessive aggregation of inflammatory cells such as macrophages and DCs indicates that plaques are more likely to rupture. The size of the atherosclerotic plaques in the aortic roots of the diabetic ApoE−/− mice was significantly increased compared with the control group. The plaque collagen content was decreased, while the numbers of macrophages and DCs were increased (Fig. 1a–d). These results indicated that diabetes aggravated atherosclerosis independently of blood lipid levels and promoted inflammation within the plaque, which might decrease plaque stability. The expression of the co-stimulatory molecules, CD83 and CD86, in splenic DCs was significantly increased, and the expression of IL12b, IL4, IL6, and chemokine receptors such as CCR7 and CXCR4 also were significantly up-regulated in the diabetic mice (Fig. 1e–g). These results reveal that the atherosclerotic microenvironment associated with diabetes significantly promoted the immune maturation of DCs. The concentrations of TNFα, IFNγ, and CRP were significantly increased in peripheral blood, and the expression of ICAM and VCAM in the aorta also were up-regulated in the diabetic mice (Fig. 1h–j). These observations point to the existence of a chronic low-grade inflammatory response in the diabetic mice, which influenced the aortic adhesion molecule expression.
Diabetic ApoE−/− mice increased aortic atherosclerosis with chronic low-grade inflammation and dendritic cell activation. Compared with the control group, the size of the atherosclerotic plaques (a) in the aortic roots of the diabetic ApoE−/− mice was increased, and the plaque collagen content (b) was decreased, while the numbers of macrophages c and DCs d were increased. The expression of CD83 and CD86 e in DCs isolated from spleens was increased, as well as the expression of IL12b, IL4, IL6 (f), and chemokine receptors such as CCR7 and CXCR4 g also were up-regulated in the diabetic mice. The expression of ICAM and VCAM (h) in the aorta were up-regulated in the diabetic ApoE−/− mice, and the concentrations of TNFα, IFNγ, and CRP (i, j) were also significantly increased in peripheral blood. Values, mean ± SED; n = 8; *p < 0.05 vs. DM group, scale bar, 250 μm; DM Diabetes mellitus, AS Atherosclerosis, DAPI 4,6-diamino-2-phenyl indole, IL Interleukin, ICAM Intercellular adhesion molecule, VCAM Vascular cell adhesion molecule, TNFα Tumor necrosis factor alpha, IFNγ IFN gamma. CRP C-reactive protein
AGEs plus oxLDL further induced the immune maturation of dendritic cells and activated the typical PKC signaling pathway
After induction of differentiation, the number of CD11c+ cells detected by flow cytometry accounted for 78.4% of the total cells (Additional file 1: Fig. S2). Before using oxLDL, this study ruled out the presence of endotoxin contamination by limulus amebocyte lysate assay. In conjunction with stimulation by oxLDL, AGEs significantly induced expression of the maturation marker, CD83, and the co-stimulating molecule, CD86, in BMDCs. ELISA results also demonstrated significantly up-regulated expression of IFNγ and TNFα (Fig. 2a, b), suggesting that AGEs plus oxLDL promoted the release of inflammatory cytokines in BMDCs.
AGEs plus oxLDL further induced the maturation of DCs and activated certain signaling pathways. The oxLDL plus AGEs treatment increased the expression of CD83 and CD86 (a), in BMDCs, and also up-regulated expression of IFNγ and TNFα (b), which was demonstrated by ELISA. In conjunction with stimulation by oxLDL, AGEs induced a greater degree of the phosphorylation of IκB, NF-κB (c), IRAK4 (d), PKCα/β1/β2 (f), and the expression of RAGE (e). Values, mean ± SED; n = 3, *p < 0.05 vs. oxLDL group; oxLDL oxidized low density lipoprotein, AGEs advanced glycation end-products, TNFα Tumor necrosis factor alpha, IFNγ IFN gamma, NF-κB nuclear factor-κB, RAGE Receptor for advanced glycation end products, pPKC phosphorylated protein kinase C, TLR4 Toll-like receptor 4, IRAK4 Interleukin receptor associated kinase 4
In addition, we found that oxLDL plus AGEs induced the phosphorylation of IκB and NF-κB (Fig. 2c) to a greater degree than oxLDL alone, and stimulated the nuclear transcription process. TLR4 is an important signal pathway for activating NF-κB, and it also is closely associated with DCs. AGEs plus oxLDL did not up-regulate the expression of TLR4. However, the phosphorylation of IRAK4, an important kinase downstream of TLR4 that is recruited by TLR4/MyD88, was significantly up-regulated (Fig. 2d), which would further phosphorylate IRAK1 and activate NF-κB. Previous studies found that various members of the PKC family are known to activate NF-κB (Oeckinghaus et al. 2011). This study focused on the typical PKCs instead of novel or atypical PKCs. In addition, as shown in this study, AGEs plus oxLDL can up-regulate the expression of RAGE, and activate PKCα and PKCβ1/β2 signaling pathways (Fig. 2e, f). Furthermore, the co-staining of PKC beta and RAGE with CD11c confirmed their co-localization in the aortic plaque (Additional file 1: Fig. S3a, b).
PKC, RAGE, and TLR4 were involved in the immune maturation process of DCs with AGEs plus oxLDL intervention
In this study, RAGE neutralizing antibody, TLR4 inhibitors, and three PKC inhibitors (CGP53353, Sigma-Aldrich, USA, 410 nM for PKCβII vs 3.8 μM for PKCβI vs 25uM for pKCα) that targeted corresponding PKC subtypes, were added to BMDCs with oxLDL plus AGEs. After pretreatment with RAGE neutralizing antibody, the expression of CD83 and CD86 were significantly decreased, and accompanied by a significant down-regulation of secretion of the inflammatory cytokines and IκB/NF-κB phosphorylation (Fig. 3a–c). These results suggested that the combination of AGEs and RAGE activated the immune maturation of BMDCs through the NF-κB signaling pathway.
RAGE antibodies and TLR4 inhibitors inhibit the immune maturation of DCs. After pretreatment with RAGE neutralizing antibody, the expression of CD83 and CD86 were decreased, and accompanied by a significant down-regulation of secretion of the inflammatory cytokines and IκB/NF-κB phosphorylation (a–c). In the TLR4 inhibitor group, CD83 and CD86 were down-regulated, and the ability of BMDCs to release inflammatory cytokines also was decreased (d, e). Combined with the decreased expression of p-NF-κB and p-IκB (f). Values, mean ± SED; n = 3, *p < 0.05 vs. oxLDL + AGEs group; oxLDL oxidized low density lipoprotein, AGEs advanced glycation end-products; RAGE Receptor for advanced glycation end products, NF-κB nuclear factor-κB, IFNγ IFN gamma; TLR4 Toll-like receptor 4, TNFα Tumor necrosis factor alpha
In the TLR4 inhibitor group, CD83 and CD86 were significantly down-regulated, and the ability of BMDCs to release inflammatory cytokines also was decreased. Combined with the decreased expression of p-NF-κB and p-IκB (Fig. 3d–f), these observations indicated that the TLR4 signaling pathway was involved with oxLDL plus AGEs to induce the BMDCs immune maturation process.
PKCβ1 inhibitors significantly down-regulated the expression of CD83 and CD86, as well as the inflammatory cytokines, TNFα and IFNγ, which are secreted by BMDCs. However, PKCα inhibitors and PKCβ2 inhibitors did not have similar effects (Fig. 4a, b). PMA, a PKC agonist, up-regulated the expression of CD83 and CD86 in BMDCs and promoted the secretion of the inflammatory cytokines, TNFα and IFNγ, while PKCβ1 inhibitors inhibited these effects (Fig. 4c, d). Additionally, PKCβ1 inhibitors inhibited the PMA- and oxLDL plus AGEs-induced activation of the NF-κB signaling pathway (Fig. 4e, f). This study repeated related experiments with PKCβ1 siRNA and found that the knockdown of PKCβ1 also inhibited the activation of NF-κB signaling pathway and the immune maturation of BMDCs (Additional file 1: Fig. S4), which is similar to the effect of PKC beta isoform 1 inhibitor. These results demonstrated that the PKCβ1 activity might have an essential role in the immune maturation of BMDCs.
PKCβ1 inhibitors significantly inhibited the immune maturation of DCs. PKCβ1 inhibitors significantly down-regulated the expression of CD83 and CD86 in BMDCs, as well as the inflammatory cytokines, TNFα and IFNγ, tested by ELISA. However, PKCα inhibitors and PKCβ2 inhibitors did not have similar effects (a, b, *p < 0.05 vs. oxLDL + AGEs group). PMA, a PKC agonist, up-regulated the expression of CD83 and CD86 in BMDCs and promoted the secretion of the inflammatory cytokines, TNFα and IFNγ, while PKCβ1 inhibitors inhibited these effects (c, d, *p < 0.05 vs. control group; #p < 0.05 vs. PMA group). PKCβ1 inhibitors inhibited the PMA− and oxLDL plus AGEs-induced activation of the NF-κB signaling pathway (e, f, *p < 0.05 vs. oxLDL + AGEs group). Values, mean ± SED; n = 3, oxLDL oxidized low density lipoprotein, AGEs advanced glycation end-products, PKC protein kinase C, IFNγ IFN gamma, TNFα Tumor necrosis factor alpha, PMA phorbol ester, NF-κB nuclear factor-κB
The relationship among RAGE, TLR4, and phospho-PKCβ1
The phosphorylation of PKCβ1, which reflects the activity of PKCβ1, is induced by oxLDL plus AGEs, and it was significantly down-regulated by the RAGE neutralization antibody (Fig. 5a). However, after the inhibition of PKCβ1, there was no significant difference in the change in RAGE (Fig. 5d). This observation suggested that RAGE activation played an important role in the phosphorylation of PKCβ1, and PKCβ1 acted downstream of RAGE. The phosphorylation of PKCβ1 was significantly decreased in the TLR4 inhibitor group (Fig. 5c), while RAGE expression was not affected (Fig. 5c), which suggested that TLR4 is intermediate in the RAGE and PKCβ1 signal pathway. TLR4 expression in the PKCβ1 inhibitor intervention group showed no significant differences (Fig. 5e), suggesting that PKCβ1 might act downstream of TLR4. The involvement of TLR4 was required for the activation of PKCβ1 by RAGE. After the application of the PKCβ1 inhibitor or RAGE neutralizing antibody, phosphorylation of IRAK4 was significantly inhibited (Fig. 5b, e), which means that the TLR4/NF-κB signaling pathway was blocked and that PKCβ1 phosphorylation played a key role in the phosphorylation of IRAK4 induced by oxLDL plus AGEs. These results indicated that AGEs combined with RAGE and then activated the TLR4-PKCβ1 signaling pathway. Coimmunoprecipitation showed that TLR4 was bound to RAGE and phospho-PKCβ1 (Fig. 5f), while phospho-PKCβ1 was bound to RAGE (Fig. 5g), indicating that RAGE, TLR4, and phospho-PKCβ1 were bound together structurally on the cell membrane.
The relationship among RAGE, TLR4, and phospho-PKCβ1. The phosphorylation of PKCβ1, induced by oxLDL plus AGEs, was down-regulated by the RAGE neutralization antibody, as well as the phosphorylation of IRAK4, but there was no significant difference in the change in TLR4 (a, b). The phosphorylation of PKCβ1 was decreased in the TLR4 inhibitor group, while RAGE expression was not affected (c). TLR4 and RAGE expression in the PKCβ1 inhibitor intervention group showed no significant differences, but the phosphorylation of IRAK4 was significantly inhibited (d, e). Coimmunoprecipitation showed that TLR4 was bound to RAGE and phospho-PKCβ1 (f), while phospho-PKCβ1 was bound to RAGE (g). Values, mean ± SED; n = 3, *p < 0.05 vs. oxLDL + AGEs group, oxLDL oxidized low density lipoprotein, AGEs advanced glycation end-products, RAGE Receptor for advanced glycation end products, PKC protein kinase C, TLR4 Toll-like receptor 4, IRAK4 Interleukin receptor associated kinase 4
In summary, the results of these cell experiments suggested that the RAGE-TLR4-PKCβ1 signal pathway might play an important role in the immune maturation of BMDCs induced by oxLDL plus AGEs. Also, RAGE, TLR4, and phospho-PKCβ1 were bound together structurally, on the cell membrane.
LY333531 inhibited the activation of dendritic cells in diabetic ApoE−/− mice and reversed the progression of atherosclerosis
LY333531 is a specific protein kinase C beta inhibitor, which can competitively and reversibly inhibit PKCβ1. The level of peripheral blood glucose was not significantly decreased in the LY333531 group, but the body weight of the mice was increased significantly (Additional file 1: Fig. S5a, b). LY333531 did not affect the blood lipid levels in the diabetic ApoE−/− mice (Additional file 1: Fig. S5c–f), suggesting that LY333531 might have improved the condition of diabetic mice through ways other than glycemic control or decreasing blood lipid levels.
The expression of CD83 and CD86 in DCs isolated from spleens was inhibited significantly in the LY333531 group (Fig. 6a). The qPCR results also indicated that the expressions of TNFα, IL12b, IL6, CCL4, CCR7, and CXCR4 were significantly inhibited in DCs, while the expression of IL10 was increased (Fig. 6b, c). These results indicated that systemic PKCβ inhibition significantly suppressed the immune maturation of splenic DCs. In the LY333531 group, not only were the aortic adhesion molecules, ICAM1 and VCAM1, inhibited (Fig. 6d), but inflammatory markers such as TNFα, IFNγ, and CRP also were inhibited significantly in peripheral blood (Fig. 6e, f). These results indicated that the systemic administration of PKCβ inhibitor reduced the chronic low-grade systemic inflammation of diabetes mellitus.
LY333531 inhibited the immune maturation of DCs and alleviated atherosclerosis in diabetic ApoE−/− mice. The expression of the co-stimulatory molecules, CD83 and CD86, in splenic DCs was inhibited in the LY333531 group (a). The qPCR results also indicated that the expressions of TNFα, IL12b, IL6, CCL4, CCR7, and CXCR4 were inhibited in DCs, while the expression of IL10 was increased (b, c). In the LY333531 group, not only were the aortic adhesion molecules, ICAM1 and VCAM1, inhibited (d), but inflammatory markers such as TNFα, IFNγ, and CRP also were inhibited in peripheral blood (e, f). Systemic administration of LY333531 delayed the formation of aortic root plaques in mice with diabetic atherosclerosis when compared with the control group (g). Masson staining revealed that the collagen fiber composition in the plaques increased (h). Staining of cells in the plaque indicated that the distribution of inflammatory cells was decreased after LY333531 administration. Co-staining of CD68 and CD14 with CD11c showed that after excluding the influence of macrophages and monocytes, CD11c-labeled DCs had a significant reduction in plaques (i, j). Values, mean ± SED; n = 8; *p < 0.05 vs. ApoE + DM group; DM Diabetes mellitus, IL Interleukin, ICAM Intercellular adhesion molecule, VCAM Vascular cell adhesion molecule, TNFα Tumor necrosis factor alpha, IFNγ IFN gamma, AS Atherosclerosis, DAPI 4,6-diamino-2-phenyl indole
Systemic administration of LY333531 significantly delayed the formation of aortic root plaques in mice with diabetic atherosclerosis when compared with the control group (Fig. 6g).
Masson staining revealed that the collagen fiber composition in the plaques increased (Fig. 6h). Staining of cells in the plaque indicated that the distribution of inflammatory cells was decreased after LY333531 administration. Since CD11c can be expressed on activated macrophages and monocytes, this study used CD68 and CD14 as markers for macrophages and monocytes, respectively, for co-staining with CD11c. The results showed that after excluding the influence of macrophages and monocytes, CD11c-labeled DCs had a significant reduction in plaques (Fig. 6i, j), suggesting that the overall stability of the plaque was enhanced.
Discussion
Low-grade inflammation (Castelblanco et al. 2018; Zhang et al. 2019), which is critical in atherosclerosis development, has been reported to be an important feature in diabetes. DCs are antigen-presenting cells that play an important role in the inflammatory response. A previous study reported that a lack of DCs in mice could significantly reverse the progression of atherosclerotic plaques (Durpes et al. 2015). In further exploration of the subsets of DCs (Sun et al. 2020), it was found that CD11b+DC (Stoneman et al. 2007; Busch et al. 2014; Gao et al. 2016; Rombouts et al. 2016) and CCL17+DC (Rader and Daugherty, 2008; Weber et al. 2011) subsets have the effect of promoting atherosclerosis, and further exploration under more experimental conditions will provide multi-dimensional evidence. Whether CD103+DCs (Choi et al. 2011; Li et al. 2017; Clement et al. 2018) and plasmacytoid DCs (Daissormont et al. 2011; Macritchie et al. 2012) play pro-atherosclerotic or anti-atherosclerotic roles is controversial, which may depend on animal strains, different pathological models and other conditions. PKC is activated in diabetes (Liu et al. 2018) and is involved in a range of diabetic complications such as diabetic nephropathy and retinopathy (Chistiakov et al. 2019). Therefore, this study investigated whether the PKC signaling pathway was involved in the immune maturation of DCs in diabetic hyperlipidemia mice and the development of diabetic atherosclerosis.
The current study found that in diabetic ApoE−/− mice, exposure to high glucose induced a DC-mediated chronic low-grade inflammatory response, which was related to the size and stability of the atherosclerotic plaques. Furthermore, AGEs induced the immune maturity of DCs in conjunction with stimulation by oxLDL. The possible mechanism involved the RAGE-TLR4-pPKCβ1 signaling pathway (Fig. 7). In addition, with PKCβ as the intervention target, LY333531 inhibited the DCs immune maturation that was induced by diabetic atherosclerosis, reduced the systemic chronic low-grade inflammatory response of diabetes mellitus, and stabilized and reduced the atherosclerotic plaques.
RAGE-TLR4-pPKCβ1 signal pathway diagram. After AGEs was combined with RAGE, the expression of RAGE was up-regulated and PKCβ1 was activated together with TLR4, phosphorylated PKCβ1 was transferred from the cytoplasm to the membrane, and formed RAGE-TLR4-pPKCβ1 complex, then activated the TLR4 signaling pathway through the phosphorylation of IRAK4, which promoted the phosphorylation of NF-κB, and further promoted the immune maturation of dendritic cells and the expression of inflammatory factors
Paulson et al. found that DCs are activated and transformed in blood vessel intima into foam cells by phagocytosis of oxLDL, which promoted the development of atherosclerosis (Durpes et al. 2015). Meanwhile, in vivo studies (Blank et al. 2012) have found that both fluctuating and stable hyperglycemia can promote and enhance the differentiation and immune maturation of DCs. Furthermore, another in vitro experiment (Ge et al. 2005) confirmed that AGEs could induce the maturation of DCs and enhance immunity of DCs through increasing the expressions of scavenger receptor A and RAGE, which were mediated by Jnk pathway. Similarly, our study demonstrated that the expression of CD83 and CD86, which represent DC maturation, was up-regulated in the spleens of diabetic ApoE−/− mice. Also, the expression of inflammatory cytokines (IL12b, IL4, and IL6), chemokine and chemokine receptors (CCL4, CCR7, and CXCR4) were significantly increased, indicating that diabetes plus hyperlipidemia could further induce immune maturation of DCs, and probably induced chronic low-grade inflammation that was mediated by DCs.
In the process of advanced atherosclerosis, DCs invade the atherosclerotic plaque from the adventitia along with vascular nourishment-related neovascularization, which leads to plaque instability and ulceration (Yilmaz et al. 2004). A previous study found that injection of diphtheria toxin into DTR+/+LDL−/− mice induced apoptosis of DCs and reduced plaque formation by 55%, which enhanced plaque stability (Durpes et al. 2015). Therefore, it is hypothesized that the immune maturity of the DCs is related to the instability of the plaque. In the present study, the number of DCs and their immune maturation were significantly increased in diabetic ApoE−/− mice, while the stability of the plaques decreased. When LY333531 was used to inhibit the DCs' immune maturation, the number of macrophages in the plaque significantly decreased, and the overall plaque stability was enhanced. These results extended the correlation between PKC and simple atherosclerosis to the occurrence and development of diabetic atherosclerosis.
The TLR-NF-κB signaling pathway is the primary inflammatory signaling pathway, and TLR activation also is required in the process of immune maturation of DCs (Alloatti et al. 2015). TLR4 is a TLR-associated protein that recognizes a range of endogenous ligands and activates the NF-κB signaling pathway, which promotes increased inflammatory cytokine gene expression in the atherosclerotic inflammatory process (Hayashi et al. 2012), while DC maturation via the NF-κB signaling pathway is well- known (Jung et al. 2020). In addition, TLR4 knockdown significantly down-regulates early atherosclerosis in diabetic ApoE−/− mice (Lu et al. 2013), which may be related to monocyte activation (Bielinski et al. 2011) that is mediated by the TLR4 signaling pathway. In the present study, we found that the use of TLR4 inhibitors significantly down-regulated immune maturation of DCs and secretion of inflammatory cytokines induced by oxLDL plus AGEs. In addition, phosphorylation of IκB and NF-κB was significantly inhibited, which suggested that TLR4 was involved in the immune maturation of DCs.
Previous studies (Lin et al. 2011) indicated that selective inhibition of PKCα or PKCβ could inhibit the differentiation of CD14+ monocytes into macrophages or DCs in vitro and further inhibit their antigen-presenting function. Cejas et al. found that PKCβ was continuously activated during the differentiation of CD34+ bone marrow stem cells into DCs (Cejas et al. 2005). This effect could be enhanced by the PKC agonist, phorbol, and inhibited by PKC inhibitors (Davis et al. 1998; Cejas et al. 2005), suggesting that the PKC signaling pathway played an important role in the differentiation and immune maturation of DCs. However, the specific PKC subtype that is the main subtype affecting the differentiation and maturation of DCs is still controversial. Previous research reported that PKCα/δ/ε activation played a role in the activation of inflammatory cells through the TLR4 signaling pathway. The classical PKC signaling pathway in our study also was involved in the immune maturation process of DCs. Inhibition of PKCβ1 significantly down-regulated the expression of CD83, CD86, TNFα, and IFNγ induced by AGEs plus oxLDL or PMA. PKCα inhibitors and PKCβ2 inhibitors did not have the same effect, suggesting that the specific PKCβ1 subtype phosphorylation played an important role in the DCs immune maturation. Linghua et al. confirmed that the activation of PKCβ accelerated the process of diabetic atherosclerosis (Kong et al. 2013), and this study further confirmed the role of PKCβ1 and DC immune maturation in diabetic atherosclerosis.
AGEs have been shown to play a causative role in diabetic vascular disease, including atherosclerosis (Zhang et al. 2003). RAGE is a specific receptor for AGEs, which is expressed in low amounts in normal tissues but exhibits high levels of expression in AGEs-enriched regions of diabetic blood vessels (Yamagishi et al. 2003). RAGE plays a critical role in diabetic atherosclerosis through perpetuation of chronic vascular inflammation and through impairment of cholesterol metabolism (Bucciarelli et al. 2002; Wendt et al. 2006; Senatus et al. 2020). Blockade of RAGE stabilizes atherosclerotic lesion area in diabetic atherosclerosis mice (Bucciarelli et al. 2002), and deletion of Ager (the gene encoding RAGE) accelerates regression of diabetic atherosclerosis, at least in part through IRF7, which is verified in BMDMs (Senatus et al. 2020). RAGE recognizes AGEs and activates NF-κB (Ohtsu et al. 2017), but the intracellular domain of RAGE has only 43 amino acids, and there is no TIR-like homologous molecule for signal recognition. In this study, we confirmed that RAGE-TLR4-pPKCβ1 was a tripartite structure on the cell membrane. Ying Ju et al. demonstrated an NF-κB activation signaling pathway that was triggered by TLR4 and RAGE-regulated p38MAPK/JNK-activated PPARγ down-regulation in human osteoarthritis (OA) chondrocytes (Chen et al. 2013). This result explained the activation of the signal pathway of NF-κB in human OA chondrocytes by RAGE (Chen et al. 2013). Based on these results, this study proposed the hypothesis that when AGEs were bound to RAGE, this up-regulated the expression of RAGE and interacted with TLR4, which promoted PKCβ1 phosphorylation and formed a complex with RAGE-TLR4. After receptor ligation, TLR4 recruited MyD88 molecules. MyD88 further recruited IRAK4 and IRAK1 through its N-terminal death domain (DD). IRAK4 exerted kinase activity through autophosphorylation to phosphorylate IRAK1, which resulted in the activation of the latter and hyper-autophosphorylation. IRAK1 then dissociated from the complex and interacted with TRAF6 to finally activate the NF-κB signaling pathway. (Fig. 7) Thus, the expression of immune maturation and inflammatory factors in DCs were promoted, which might partially explain the mechanism by which hyperglycemia promotes the immune maturation of DCs. However, this possible mechanism needs further exploration.
There are several limitations associated with this study. First, specific inhibitors of PKCβ1 were not utilized in the in vivo experiments, which reduced the robustness of the experimental animal evidence. Second, repeated incubation failures occurred that resulted in our inability to include DTR+LDLR−/− or DTR−LDLR−/− transgenic mice in the current study. The unavailability of these transgenic mice led to a lack of direct in vivo evidence to support the relationship between DCs and atherosclerotic plaques. Third, our experimental design was based on type 1 diabetes, and it is necessary to conduct further research on type 2 diabetes. Fourth, the mechanism by which RAGE-TLR4-PKCβ1 bind together requires further exploration. Finally, this study only explored the typical PKC signaling pathways, but not other types of PKC signaling pathways.
Conclusions
The present study demonstrated that diabetes mellitus aggravated chronic inflammation, and promoted atherosclerotic plaques in conjunction with hyperlipidemia, which at least in part through inducing the immune maturity of DCs. Compared with oxLDL, AGEs plus oxLDL further induced the immune maturation of DCs, and possibly through the RAGE-TLR4-pPKCβ1 signaling pathway. These results provided new insights for clinical prevention and treatment of diabetic atherosclerosis.
Availability of data and materials
The datasets during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- AS:
-
Atherosclerosis
- DAPI:
-
4,6-Diamino-2-phenyl indole
- IL:
-
Interleukin
- ICAM:
-
Intercellular adhesion molecule
- VCAM:
-
Vascular cell adhesion molecule
- TNFα:
-
Tumor necrosis factor alpha
- IFNγ:
-
IFN gamma. CRP: C-reactive protein
- oxLDL:
-
Oxidized low density lipoprotein
- AGEs:
-
Advanced glycation end-products
- NF-κB:
-
Nuclear factor-κB
- RAGE:
-
Receptor for advanced glycation end products
- pPKC:
-
Phosphorylated protein kinase C
- TLR4:
-
Toll-like receptor 4
- IRAK4:
-
Interleukin receptor associated kinase 4
- PKC:
-
Protein kinase C
- PMA:
-
Phorbol ester
- TG:
-
Total triglyceride
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- DCs:
-
Dendritic cells
- BMDCs:
-
Bone marrow-derived DCs
- OCT:
-
Optimal cutting temperature
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- OA:
-
Osteoarthritis
- DD:
-
Death domain
References
Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U, Joannas L, Vivar OI, Lennon-Dumenil AM, Savina A, Gevaert K, Beyaert R, Hoffmann E, Amigorena S. Toll-like receptor 4 engagement on dendritic cells restrains phago-lysosome fusion and promotes cross-presentation of antigens. Immunity. 2015;43:1087–100.
Bielinski SJ, Hall JL, Pankow JS, Boerwinkle E, Matijevic-Aleksic N, He M, Chambless L, Folsom AR. Genetic variants in TLR2 and TLR4 are associated with markers of monocyte activation: the Atherosclerosis Risk in Communities MRI Study. Hum Genet. 2011;129:655–62.
Blank SE, Johnson EC, Weeks DK, Wysham CH. Circulating dendritic cell number and intracellular TNF-alpha production in women with type 2 diabetes. Acta Diabetol. 2012;49(Suppl 1):S25-32.
Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–35.
Busch M, Westhofen TC, Koch M, Lutz MB, Zernecke A. Dendritic cell subset distributions in the aorta in healthy and atherosclerotic mice. PLoS ONE. 2014;9:e88452.
Cao W, Chen J, Chen Y, Chen S, Chen X, Huang H, Liu P. Advanced glycation end products induced immune maturation of dendritic cells controls heart failure through NF-kappaB signaling pathway. Arch Biochem Biophys. 2015;580:112–20.
Castelblanco E, Hernandez M, Castelblanco A, Gratacos M, Esquerda A, Mollo A, Ramirez-Morros A, Real J, Franch-Nadal J, Fernandez-Real JM, Mauricio D. Low-grade inflammatory marker profile may help to differentiate patients with LADA, classic adult-onset type 1 diabetes, and type 2 diabetes. Diabetes Care. 2018;41:862–8.
Cejas PJ, Carlson LM, Zhang J, Padmanabhan S, Kolonias D, Lindner I, Haley S, Boise LH, Lee KP. Protein kinase C betaII plays an essential role in dendritic cell differentiation and autoregulates its own expression. J Biol Chem. 2005;280:28412–23.
Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS ONE. 2013;8:e66611.
Chistiakov DA, Kashirskikh DA, Khotina VA, Grechko AV, Orekhov AN. Immune-inflammatory responses in atherosclerosis: the role of myeloid cells. J Clin Med. 2019. https://doi.org/10.3390/jcm8111798.
Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–31.
Clement M, Haddad Y, Raffort J, Lareyre F, Newland SA, Master L, Harrison J, Ozsvar-Kozma M, Bruneval P, Binder CJ, Taleb S, Mallat Z. Deletion of IRF8 (Interferon Regulatory Factor 8)-dependent dendritic cells abrogates proatherogenic adaptive immunity. Circ Res. 2018;122:813–20.
Coutant F, Agaugue S, Perrin-Cocon L, Andre P, Lotteau V. Sensing environmental lipids by dendritic cell modulates its function. J Immunol. 2004;172:54–60.
Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–95.
Davis TA, Saini AA, Blair PJ, Levine BL, Craighead N, Harlan DM, June CH, Lee KP. Phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J Immunol. 1998;160:3689–97.
Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8.
Durpes MC, Morin C, Paquin-Veillet J, Beland R, Pare M, Guimond MO, Rekhter M, King GL, Geraldes P. PKC-beta activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc Res. 2015;106:303–13.
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J Cell Mol Med. 2016;20:2318–27.
Ge J, Jia Q, Liang C, Luo Y, Huang D, Sun A, Wang K, Zou Y, Chen H. Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells. Arterioscler Thromb Vasc Biol. 2005;25:2157–63.
Hayashi C, Papadopoulos G, Gudino CV, Weinberg EO, Barth KR, Madrigal AG, Chen Y, Ning H, LaValley M, Gibson FC 3rd, Hamilton JA, Genco CA. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J Immunol. 2012;189:3681–8.
Jung HJ, Park SH, Cho KM, Jung KI, Cho D, Kim TS. Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-kappaB Pathway. Front Immunol. 2020;11:571959.
Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, Braun KR, Potter-Perigo S, Sanda S, Wight TN, Pennathur S, Serhan CN, Heinecke JW, Coleman RA, Bornfeldt KE. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–24.
Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS, Yan SF. PKCbeta promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol. 2013;33:1779–87.
Li Y, Liu X, Duan W, Tian H, Zhu G, He H, Yao S, Yi S, Song W, Tang H. Batf3-dependent CD8alpha (+) Dendritic Cells Aggravates Atherosclerosis via Th1 Cell Induction and Enhanced CCL5 Expression in Plaque Macrophages. EBioMedicine. 2017;18:188–98.
Lin YF, Leu SJ, Huang HM, Tsai YH. Selective activation of specific PKC isoforms dictating the fate of CD14 (+) monocytes towards differentiation or apoptosis. J Cell Physiol. 2011;226:122–31.
Liu CH, Hua N, Fu X, Pan YL, Li B, Li XD. Metformin regulates atrial SK2 and SK3 expression through inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats. BMC Cardiovasc Disord. 2018;18:236.
Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol. 2013;216:61–71.
Lu H, Huang D, Yao K, Li C, Chang S, Dai Y, Sun A, Zou Y, Qian J, Ge J. Insulin enhances dendritic cell maturation and scavenger receptor-mediated uptake of oxidised low-density lipoprotein. J Diabetes Complications. 2015;29:465–71.
Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2569–79.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003a;108:1772–8.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003b;108:1664–72.
Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708.
Ohtsu A, Shibutani Y, Seno K, Iwata H, Kuwayama T, Shirasuna K. Advanced glycation end products and lipopolysaccharides stimulate interleukin-6 secretion via the RAGE/TLR4-NF-kappaB-ROS pathways and resveratrol attenuates these inflammatory responses in mouse macrophages. Exp Ther Med. 2017;14:4363–70.
Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–90.
Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–13.
Rombouts M, Ammi R, Van Brussel I, Roth L, De Winter BY, Vercauteren SR, Hendriks JM, Lauwers P, Van Schil PE, De Meyer GR, Fransen E, Cools N, Schrijvers DM. Linking CD11b (+) Dendritic Cells and Natural Killer T Cells to Plaque Inflammation in Atherosclerosis. Mediators Inflamm. 2016;2016:6467375.
Sasso FC, Carbonara O, Nasti R, Campana B, Marfella R, Torella M, Nappi G, Torella R, Cozzolino D. Glucose metabolism and coronary heart disease in patients with normal glucose tolerance. JAMA. 2004;291:1857–63.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Senatus L, Lopez-Diez R, Egana-Gorrono L, Liu J, Hu J, Daffu G, Li Q, Rahman K, Vengrenyuk Y, Barrett TJ, Dewan MZ, Guo L, Fuller D, Finn AV, Virmani R, Li H, Friedman RA, Fisher EA, Ramasamy R, Schmidt AM. RAGE impairs murine diabetic atherosclerosis regression and implicates IRF7 in macrophage inflammation and cholesterol metabolism. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.137289.
Ssemakalu CC, Ubomba-Jaswa E, Motaung KS, Pillay M. The effect of solar irradiated vibrio cholerae on the secretion of pro-inflammatory cytokines and chemokines by the JAWS II dendritic cell line in vitro. PLoS ONE. 2015;10:e0130190.
Stein J, Steven S, Bros M, Sudowe S, Hausding M, Oelze M, Munzel T, Grabbe S, Reske-Kunz A, Daiber A. Role of protein kinase C and Nox2-derived reactive oxygen species formation in the activation and maturation of dendritic cells by phorbol ester and lipopolysaccharide. Oxid Med Cell Longev. 2017;2017:4157213.
Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–93.
Sun L, Zhang W, Zhao Y, Wang F, Liu S, Liu L, Zhao L, Lu W, Li M, Xu Y. Dendritic cells and T cells, partners in atherogenesis and the translating road ahead. Front Immunol. 2020;11:1456.
Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MA, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Forster I, Zenke M, Hieronymus T, Junt T, Zernecke A. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–910.
Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, Jenkins DG, Stein G, Schmidt AM, Yan SF. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–7.
Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–9.
Yamagishi S, Takeuchi M, Inagaki Y, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmacol Res. 2003;23:129–34.
Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–10.
Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8.
Zhang ZY, Miao LF, Qian LL, Wang N, Qi MM, Zhang YM, Dang SP, Wu Y, Wang RX. Molecular mechanisms of glucose fluctuations on diabetic complications. Front Endocrinol (lausanne). 2019;10:640.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82070408) and Zhejiang Natural Science Fund (LY18H020007). We are very grateful to Dr. Chenxia Wu, Zhejiang Chinese Medical University, for her help in searching for antibodies. We are grateful for the language polish service provided by EditSprings.
Funding
This work was supported by National Natural Science Foundation of China (82070408) and Zhejiang Natural Science Fund (LY18H020007).
Author information
Authors and Affiliations
Contributions
WBZ, ZYC and LDZ designed research. LDZ, YL and TX performed molecular biology experiment: LDZ, QBL, XKB and XLL performed animal experiments. GSF, YZZ and JBG analyzed and interpreted data. LDZ and YL drafted the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Fasting blood glucose and blood lipids level in mice were tested. Figure S2. Detection of CD11c+ cells purity by flow cytometry. Figure S3. The co-staining of PKC beta isoforms and RAGE with CD11c. Figure S4. Knockdown of PKCβ1 significantly inhibited the immune maturation of DCs. Figure S5. Plasma glucose, body weight and blood lipids were measured. Table S1. List of primers used in quantitative RT-PCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Li, Y., Xu, T. et al. Dendritic cell-mediated chronic low-grade inflammation is regulated by the RAGE-TLR4-PKCβ1 signaling pathway in diabetic atherosclerosis. Mol Med 28, 4 (2022). https://doi.org/10.1186/s10020-022-00431-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-022-00431-6