Abstract
Introduction
Patients with alcohol use disorders (AUD) are at increased risk of developing sepsis and have higher mortality. AUD are associated with higher cortisol and anti-inflammatory cytokine profile. Higher cortisol increases risk of death in septic patients. The relationship between AUD and cortisol in septic patients is unknown. We aimed to study this relationship and postulated that AUD would be associated with higher cortisol and anti-inflammatory cytokine profile.
Methods
This was a prospective cohort study of 40 medical intensive care unit (ICU) patients admitted with sepsis. Cortisol, anti-inflammatory interleukin (IL) 10, and pro-inflammatory IL1β, IL6, tumor necrosis factor (TNF) α were measured.
Results
Thirteen (32%) out of 40 patients had AUD. AUD patients had higher cortisol by univariate (39 microg/dl versus 24, P = 0.04) and multivariable analyses (44 microg/dl versus 23, P = 0.004). By univariate analyses, AUD patients had higher IL10 (198 picog/dl versus 47, P = 0.02) and IL6 (527 picog/ml versus 156, P = 0.048), but similar IL1β and TNFα. By multivariable analyses, AUD patients had higher IL10 (182 picog/dl versus 23, P = 0.049) but similar IL1β, IL6, and TNFα. AUD patients had lower IL1β/IL10 (univariate 0.01 versus 0.10, P = 0.04; multivariable 0.01 versus 0.03, P = 0.04), lower TNFα/IL10 (univariate 0.15 versus 0.52, P = 0.03; multivariable 0.11 versus 0.63, P = 0.01), but similar IL6/IL10.
Conclusions
AUD are common diagnoses among medical ICU patients with sepsis. Patients with AUD have higher cortisol concentrations and have differences in cytokine expression. Future studies should seek to determine if these differences may explain the higher severity of illness seen in patients with sepsis and AUD.
Trial registration
ClinicalTrials.gov: NCT00615862
Similar content being viewed by others
Introduction
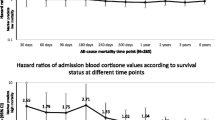
Patients with sepsis who have increased cortisol concentrations or poorer response to adrenocorticotropin hormone (ACTH) stimulation have higher mortality than those with normal cortisol and normal response to ACTH [1–3]. Annane et al. found that cortisol >34 microg/dl was associated with a 2.4 higher odds of death compared to ≤34 microg/dl [1]. Lipiner-Friedman et al. also found that patients who died of sepsis had higher cortisol compared to those who survived (29 microg/dl versus 24 microg/dl); patients who died also had a lower incremental increase in cortisol in response to ACTH administration (6 microg/dl versus 11 microg/dl) [3]. It is unknown if impaired hypothalamic-pituitary-adrenal (HPA) axis is a marker for increased risk of death or the cause of increased mortality [2].
Alcohol use disorders (AUD) are common problems worldwide [4]. In the United States, 7% of the population has AUD, and among hospitalized patients, the rate of AUD is estimated to be 21 to 42% [5–8]. Patients with AUD are predisposed to developing sepsis, are more likely to require mechanical ventilation, and have a higher risk of death [9–12]. A number of studies have demonstrated higher cortisol in surgical patients with AUD, but not all studies support this finding [13–16]. Individuals with AUD who present for elective outpatient detoxification also have higher cortisol compared to individuals without AUD [17, 18].
The reasons for the increased sepsis mortality in patients with AUD may partly be explained by the effects of alcohol on cytokine production. Patients with AUD are known to have altered expression of pro-inflammatory cytokines, including interleukin (IL) 6, tumor necrosis factor (TNF) α, and IL1β [14, 16, 19, 20]. Similarly, anti-inflammatory cytokine (IL10) production has been found to be either elevated or decreased in surgical patients with AUD [15, 19]. Patients with AUD have been shown to have a decreased ratio of pro-inflammatory to anti-inflammatory cytokines, a finding that has been linked to the development of nosocomial sepsis [14, 19].
Recent studies also demonstrate a link between cortisol and immune function [21, 22]. Activation of the HPA axis is associated with immunosuppression, while down-regulation of the axis improves immune function [21, 22]. We hypothesized that septic patients with AUD compared to those without AUD might have differences in cortisol and cytokine expression.
The relationship between cortisol and co-existing AUD in critically ill patients with sepsis has not been examined. We conducted an observational pilot study to determine if septic patients with AUD would have higher cortisol compared to septic patients without AUD. We also hypothesized that septic patients with AUD would have more depressed immune function as measured by higher anti-inflammatory cytokine IL10 and lower ratio of pro-inflammatory cytokines (that is, IL1β, IL6, and TNFα) to anti-inflammatory cytokine (that is, IL10). The results of this study have been published in abstract format [23].
Materials and methods
Inclusion and exclusion criteria
All patients admitted to the medical ICU from the Emergency Department were evaluated for study eligibility if they met criteria for sepsis as established by the American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference [24]. Exclusion criteria were: age <18, pregnancy, prisoners, no cortisol measured within 24 hours after Emergency Department presentation, etomidate administration prior to cortisol measurement, steroid administration prior to measurement of cortisol, and inability to obtain consent. Of note, no patient was diagnosed with alcohol withdrawal during hospitalization; diagnosis of alcohol withdrawal is based on clinical diagnosis and through monitoring for withdrawal using the Clinical Institute Withdrawal Assessment (CIWA-Ar) [25].
The Virginia Commonwealth University Human Investigation Review Committee approved the study (HM11399) and written informed consent was obtained from patients or legally authorized representatives. To protect patients from adverse consequences related to AUD diagnoses, we also obtained a Certificate of Confidentiality from the National Institutes of Health. The study was registered with ClinicalTrials.gov (NCT00615862). The study was conducted in accordance with the ethical standards of the Declaration of Helsinki.
Definition of AUD
AUD was ascertained by administering a validated questionnaire to patients or legally authorized representatives (in case patients were unable to respond). The Short Michigan Alcohol Screening Test is a 13-item questionnaire that queries about adverse consequences of alcohol consumption and has been successfully used by other ICU investigators [10, 26]. Patients who responded affirmatively to ≥3 questions were considered to have AUD (Table 1). Patients were classified into those with AUD and those without AUD.
Cortisol concentrations
Cortisol as measured by the Virginia Commonwealth University Medical Center Department of Pathology was recorded. Quantification of cortisol before and one hour after administration of 250 microg of ACTH was completed within the first 24 hours after Emergency Department presentation. Delta cortisol (Δcortisol) was defined as the incremental increase in cortisol in response to ACTH administration. We defined cortisol as the baseline cortisol (prior to ACTH administration). Cortisols were tested in samples within one hour after collection using the ADVIA Centaur cortisol assay (Bayer, Tarrytown, NY, USA), which is a competitive immunoassay using direct chemiluminescent technology.
Cytokine quantification
Investigators measured IL10, IL1β, IL6, and TNFα on the serum sample from which the baseline cortisol was measured. If insufficient volume of serum remained, cytokines were not measured. Patients did not have blood drawn exclusively for study purposes. Samples were stored at -80 degrees Celsius. Cytokines were measured using Milliplex AP Assay which is based on the Luminex xMAP technology (Millipore Corporation, Billierica, MA, USA) and measures cytokines using antibody techniques.
Other variables
Demographics, infection site and type, mechanical ventilation characteristics, length of stay, and mortality were also recorded. Severity of illness as measured by Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) were computed [27, 28].
Data analysis
The primary endpoint of the study was the comparison in baseline cortisol between the two groups of patients. Because we expected different demographics between the two groups (that is, demographics such as age, and co-morbidities such as cirrhosis), we also adjusted for between group characteristics when the P ≤ 0.25. Multivariable analysis using the standard least square was performed and included all possible two-way interactions. We set α = 0.05, and we did not adjust for multiple comparisons in this pilot study.
Secondary outcomes were between group comparison of Δcortisol, cytokines (IL10, IL1β, IL6, and TNFα), and pro-inflammatory to anti-inflammatory cytokine ratios (IL1β/IL10, IL6/IL10, and TNFα/IL10).
A two-sided t-test was used when the outcome variable was continuous after appropriate logarithmic conversion when variables were not normally distributed. Homogeneity of variance was found to be present using the Brown-Forsythe test. Chi Square Test or Fisher's Exact Test was used when the outcome variable was categorical. A log-rank test was used to compute mechanical ventilation duration and ICU and hospital lengths of stay.
Normally distributed data are reported as mean and 95% confidence interval (CI). Non-normally distributed data are reported as median and interquartile range.
Sample size calculation
Spies et al. reported cortisol concentrations in surgical patients with AUD [16]. Based on their data, the AUD group had a mean cortisol one day after surgery of 750 nmol/l. We estimated that the group of patients without AUD had a mean 250 nmol/l. We assumed the data were lognormal since the mean was not symmetric within the interquartile range, and we concluded that a 10% increase in cortisol between the patients without and those with AUD would be clinically relevant. Conservatively, the interquartile range was ± standard deviation. Thus the standard deviation was approximately half the interquartile range. Assuming at least a 10% increase in the mean cortisol in the group of patients with AUD compared to the group without AUD, we determined that a sample size of 40 patients in total would be associated with a 90% power at an α = 0.05. Sample size calculation was performed using nQuery Advisor (version 7.0, Statistical Solutions Ltd., Cork, Ireland).
Results
Between July 2008, and May 2009, 137 patients were admitted with a diagnosis of sepsis from the Emergency Department. Thirty-four patients did not have cortisol measured, 26 received etomidate, 21 received steroids (either stress dose or were on chronic steroids), 10 were prisoners, and 6 declined study participation.
Forty patients were enrolled, and 13 were diagnosed with AUD (32%). All patients with AUD were actively drinking at the time of hospital admission, and no patient was diagnosed with alcohol withdrawal during their hospitalization (that is, CIWA-Ar <10 in all cases). The demographics of the cohort are detailed in Table 2. Patients with AUD tended to be younger, tended to have lower glucose concentrations, tended to have cirrhosis more frequently, and tended to require vasopressor support more frequently; these four variables were included in multivariable analyses. The lung was the most common site of infection. There was no difference between the two groups in the need for and duration of mechanical ventilation, lengths of stay, and mortality (Table 2).
By univariate analysis, patients with AUD had significantly higher cortisol levels (Table 3). Multivariable analysis also demonstrated AUD was an independent predictor of higher cortisol.
A total of 28 patients underwent quantification of cytokines, 10 with AUD and 18 without AUD. By univariate analyses, patients with AUD had higher IL10 and IL6, but lower IL1β/IL10 and TNFα/IL10 (Table 3). Multivariable analyses revealed that patients with AUD had higher IL10, but lower IL1β/IL10 and TNFα/IL10.
The study was started after publication of the CORTICUS trial, and only 15 patients underwent administration of ACTH, 5 in the group with AUD and 10 in the group without AUD [29]. Because of this small number of patients, the data are not reported in table format. By univariate analysis, patients with AUD had a smaller Δcortisol compared to the patients without AUD: 6 microg/dl, 95% CI (3.1; 10.5) versus 12 microg/dl, 95% CI (8.0; 19.0), P = 0.04. Multivariable analysis demonstrated that AUD was an independent predictor for lower Δcortisol: Patients with AUD had a Δcortisol of 3 microg/dl, 95% CI (1.3; 6.0) versus 7 microg/dl, 95% CI (3.8; 12.4), P = 0.01. When using a Δcortisol ≤9 microg/dl as a diagnostic cutoff of relative adrenal insufficiency, the two groups had similar rates of relative adrenal insufficiency by univariate analysis (4 out 5 patients with AUD versus 3 out of 10 patients without AUD, P = 0.06) and multivariable analysis (P = 0.08).
Discussion
In this prospective observational pilot study, we found that a high proportion of patients with community acquired sepsis have AUD (32%), and that co-diagnoses of AUD are associated with higher cortisol concentrations. In secondary analyses, we found that patients with and without AUD had differences in cytokine expression. Patients with AUD had higher levels of the anti-inflammatory cytokine IL10 but there was no difference in pro-inflammatory cytokines IL1β, IL6, and TNFα. Patients with AUD had an anti-inflammatory cytokine profile, as measured by depressed ratios of IL1β/IL10 and TNFα/IL10; however, the ratio of IL6/IL10 was similar.
AUD have been associated with HPA dysfunction in ambulatory individuals [17, 18, 30]. A majority of the literature finds that surgical ICU patients with AUD have higher cortisol concentrations [13–16]. In our current study, we similarly determined that septic patients with co-diagnoses of AUD had higher cortisol: AUD was associated with a 1.9-fold higher concentration by multivariable analysis. We do not believe the increased cortisol concentrations in patients with AUD were caused by the development of alcohol withdrawal syndrome as no patient was diagnosed with this complication during hospitalization (as measured every four hours by CIWA-Ar which is our standard of care).
Patients with AUD had similar pro-inflammatory cytokines concentrations (IL1β, IL6, and TNFα) but had higher levels of anti-inflammatory cytokine IL10 compared to patients without AUD (Table 3). These findings are supported by other studies. Anti-inflammatory IL10 is elevated in the immediate post-operative period in patients with AUD [15, 19]. Studies examining surgical patients have found conflicting results on the levels of pro-inflammatory cytokine TNFα and IL1β [14]. IL6 levels in patients with AUD have been found to be similar, higher, or lower than patients without AUD [14, 15, 19]. The increased IL10 concentration in patients with AUD resulted in a lower ratio of IL1β/IL10 and TNFα/IL10 but a similar ratio of IL6/IL10, findings supported by other studies [14, 19]. The differences observed between our study results and other studies may be explained by the timing of cytokine measurement (within 24 hours of admission) and the patient population studied. Other studies have examined post-operative patients while our study evaluated medical ICU patients admitted with sepsis.
The implications of this pilot study are that septic medical ICU patients with AUD exhibit heightened stress response and less robust immune response in the setting of life threatening sepsis.
Our study has several limitations. Because we started our study after publication of the CORTICUS study, only 15 out of 40 patients underwent stimulation with ACTH, and the small number of patients in whom Δcortisol could be computed limits generalizability [3]. We also did not find a difference in mortality between patients with and without AUD, but we did not power our study to detect this difference. A larger study powered to detect mortality differences needs to be conducted. In addition, we found an association between AUD and cortisol and immune function, but the observational nature of our study does not permit determination of cause and effect. Cortisol and cytokine levels were determined on one occasion and were not evaluated longitudinally over time. In addition, we measured cytokine concentrations only if sufficient serum volume remained, leading to potential bias of test results: Two-thirds of patients without AUD had cytokines measured while three-quarter of patients with AUD had cytokines quantified. In the acute phase of sepsis, cytokines change over time. Our measurement at one point in time does not fully reflect the interactions between AUD and systemic inflammation. In this exploratory study, we also did not adjust α for multiple analyses, and future studies enrolling with adequate power and enrolling larger number of patients need to be conducted. Finally, only 29% of septic patients were enrolled in the study, and we do not have demographics on non-enrolled patients. It is possible that the enrolled patients were qualitatively different from those not enrolled, and that these difference may have impacted our results.
Conclusions
In conclusion, AUD are common co-diagnoses among patients with sepsis, affecting approximately one-third of patients. AUD are associated with higher cortisol concentrations and a different cytokine composition. Anti-inflammatory cytokine IL10 is increased and the ratios of IL1β/IL10 and TNFα/IL10 are lower in patients with AUD, suggesting that AUD may be associated with immunosuppression. Future studies should aim to determine if these differences may be the cause of higher morbidity and mortality experienced by patients with AUD.
Key messages
-
In septic medical ICU patients, patients with co-existing AUD have higher cortisol concentrations compared to patients without AUD.
-
Septic patients with AUD have differences in cytokine composition compared to septic patients without AUD.
Abbreviations
- ACTH:
-
adrenocorticotropin hormone
- AUD:
-
alcohol use disorders
- CI:
-
confidence interval
- CIWA-Ar:
-
Clinical Institute Withdrawal Assessment
- Δcortisol:
-
delta cortisol
- HPA:
-
hypothalamic-pituitary-adrenal
- ICU:
-
intensive care unit
- IL:
-
interleukin
- TNF:
-
tumor necrosis factor.
References
Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E: A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000, 283: 1038-1045. 10.1001/jama.283.8.1038.
Marik PE, Zaloga GP: Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002, 122: 1784-1796. 10.1378/chest.122.5.1784.
Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, Briegel J, Keh D, Singer M, Moreno R, Bellissant E, Annane D, Corticus Study Group: Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007, 35: 1012-1018. 10.1097/01.CCM.0000259465.92018.6E.
World Health Organization, Department of Mental Health and Substance Abuse: Global status report on alcohol 2004. 2004, Geneva
Department of Health and Human Services, Substance Abuse and Mental Health Services Administration: Results from the 2005 national survey on drug use and health: national findings. 2006, Rockville, MD: Office of Applied Studies, DHHS Publication No. SMA 06-4194
Gerke P, Hapke U, Rumpf HJ, John U: Alcohol-related diseases in general hospital patients. Alcohol and Alcoholism. 1997, 32: 179-184.
Smothers BA, Yahr HT, Ruhl CE: Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004, 164: 749-756. 10.1001/archinte.164.7.749.
Lau K, Freyer-Adam J, Coder B, Riedel J, Rumpf HJ, John U, Hapke U: Dose-response relation between volume of drinking and alcohol-related diseases in male general hospital inpatients. Alcohol and Alcoholism. 2008, 43: 34-38. 10.1093/alcalc/agm154.
Saitz R, Ghali WA, Moskowitz MA: The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997, 157: 1446-1452. 10.1001/archinte.157.13.1446.
Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA: Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003, 31: 869-877. 10.1097/01.CCM.0000055389.64497.11.
O'Brien JM, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS: Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007, 35: 345-350.
de Wit M, Best AM, Gennings C, Burnham EL, Moss M: Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007, 31: 1224-1230. 10.1111/j.1530-0277.2007.00421.x.
Tonnesen H, Petersen KR, Hojgaard L, Stokholm KH, Nielsen HJ, Knigge U, Kehlet H: Postoperative morbidity among symptom-free alcohol misusers. Lancet. 1992, 340: 334-337. 10.1016/0140-6736(92)91405-W.
Spies CD, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schroder T, Hoflich C, Sinha P, Paschen C, Mirsalim P, Brunsch R, Hopf J, Marks C, Wernecke KD, Pragst F, Ehrenreich H, Muller C, Tonnesen H, Oelkers W, Rohde W, Stein C, Kox WJ: Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology. 2004, 100: 1088-1100. 10.1097/00000542-200405000-00010.
Sander M, von Heymann C, Neumann T, Braun JP, Kastrup M, Beholz S, Konertz W, Spies CD: Increased interleukin-10 and cortisol in long-term alcoholics after cardiopulmonary bypass: a hint to the increased postoperative infection rate?. Alcohol Clin Exp Res. 2005, 29: 1677-1684. 10.1097/01.alc.0000179365.58403.b2.
Spies C, Eggers V, Szabo G, Lau A, von Dossow V, Schoenfeld H, Althoff H, Hegenscheid K, Bohm B, Schroeder T, Pfeiffer S, Ziemer S, Paschen C, Klein M, Marks C, Miller P, Sander M, Wernecke KD, Achterberg E, Kaisers U, Volk HD: Intervention at the level of the neuroendocrine-immune axis and postoperative pneumonia rate in long-term alcoholics. Am J Respir Crit Care Med. 2006, 174: 408-414. 10.1164/rccm.200506-907OC.
Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S: Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997, 21: 1285-1293.
Gianoulakis C, Dai X, Brown T: Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003, 27: 410-423. 10.1097/01.ALC.0000056614.96137.B8.
Sander M, Irwin M, Sinha P, Naumann E, Kox WJ, Spies CD: Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Intensive Care Med. 2002, 28: 285-292. 10.1007/s00134-001-1199-9.
Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G: Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation?. Alcohol Clin Exp Res. 2002, 26: 1609-1614.
Tracey KJ: The inflammatory reflex. Nature. 2002, 420: 853-859. 10.1038/nature01321.
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES: The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000, 52: 595-638.
de Wit M, Wiaterek GK, Gray ND, Goulet KE, Gennings C, Clore JN, Sweeney LB: Relationship between alcohol use disorders, cortisol concentrations, and cytokine levels in medical intensive care unit patients. Am J Respir Crit Care Med. 2010, 181: A6132-
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992, 20: 864-874. 10.1097/00003246-199206000-00025.
Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM: Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989, 84: 1353-1357. 10.1111/j.1360-0443.1989.tb00737.x.
Selzer ML, Vinokur A, van Rooijen L: A self-administered Short Michigan Alcoholism Screening Test (SMAST). J Stud Alcohol. 1975, 36: 117-126.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med. 1985, 13: 818-829. 10.1097/00003246-198510000-00009.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22: 707-710. 10.1007/BF01709751.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J, CORTICUS Study Group: Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008, 358: 111-124. 10.1056/NEJMoa071366.
Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ: Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003, 27: 1420-1427. 10.1097/01.ALC.0000087581.13912.64.
Acknowledgements
The authors wish to acknowledge the editorial assistance of Ellen Burnham, MD, MS, Assistant Professor of Medicine, Pulmonary Sciences and Critical Care Medicine,
University of Colorado Denver School of Medicine, Aurora, Colorado. The authors also wish to acknowledge Shirley L.T. Helm, MS, Core Laboratory Manager, General Clinical Research Center, Virginia Commonwealth University for her assistance in measurement of cytokine concentrations. Finally, the authors wish to thank Linda S. Douglas, Administrative Assistant, Division of Pulmonary Disease and Critical Care Medicine, Department of Internal Medicine, Virginia Commonwealth University for her assistance in preparing this manuscript.
Funding: NIH-M01-RR00065.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MdW participated in study design, data collection, data analysis and interpretation, and manuscript preparation. GKW, NDG and KEG participated in data collection, data analysis, and manuscript preparation. LBS and JNC participated in study design, data analysis and manuscript preparation.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
de Wit, M., Wiaterek, G.K., Gray, N.D. et al. Relationship between alcohol use disorders, cortisol concentrations, and cytokine levels in patients with sepsis. Crit Care 14, R230 (2010). https://doi.org/10.1186/cc9385
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc9385