Abstract
Introduction
Partial assist ventilation reduces work of breathing in patients with bronchospasm; however, it is not clear which components of the ventilatory cycle contribute to this process. Theoretically, expiratory positive airway pressure (EPAP), by reducing expiratory breaking, may be as important as inspiratory positive airway pressure (IPAP) in reducing work of breathing during acute bronchospasm.
Method
We compared the effects of 10 cmH2O of IPAP, EPAP, and continuous positive airwaypressure (CPAP) on inspiratory work of breathing and end-expiratory lung volume (EELV) in a canine model of methacholine-induced bronchospasm.
Results
Methacholine infusion increased airway resistance and work of breathing. During bronchospasm IPAP and CPAP reduced work of breathing primarily through reductions in transdiaphragmatic pressure per tidal volume (from 69.4 ± 10.8 cmH2O/l to 45.6 ± 5.9 cmH2O/l and to 36.9 ± 4.6 cmH2O/l, respectively; P < 0.05) and in diaphragmatic pressure–time product (from 306 ± 31 to 268 ± 25 and to 224 ± 23, respectively; P < 0.05). Pleural pressure indices of work of breathing were not reduced by IPAP and CPAP. EPAP significantly increased all pleural and transdiaphragmatic work of breathing indices. CPAP and EPAP similarly increased EELV above control by 93 ± 16 ml and 69 ± 12 ml, respectively. The increase in EELV by IPAP of 48 ± 8 ml (P < 0.01) was significantly less than that by CPAP and EPAP.
Conclusion
The reduction in work of breathing during bronchospasm is primarily induced by the IPAP component, and that for the same reduction in work of breathing by CPAP, EELV increases more.
Similar content being viewed by others
Introduction
Applying positive airway pressure during ventilation is an established means of augmenting ventilation and oxygenation in patients with airflow obstruction. Expiratory positive airway pressure (EPAP), as a means of counteracting airway collapse, evolved from the observation that patients with airflow limitation spontaneously expire through 'pursed' lips [1–3]. In patients, Barach and Swenson [1] demonstrated bronchographically that bronchoconstriction was reduced by 4–8 cmH2O positive airway pressure. Presumably, the increased airway pressure reflects backward, thereby increasing intraluminal bronchial pressure and preventing dynamic airway collapse during exhalation. Brochard and coworkers [4] documented that noninvasive application of positive airway pressure could support individuals with chronic airflow obstruction without the need for intubation of the trachea and mechanical ventilation. Presumably, work of breathing is reduced by positive airway pressure.
In support of this concept, studies using either continuous positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP) during airflow limitation have shown a reduction in the inspiratory work of breathing [5–8]. These studies have further suggested that the use of CPAP or PEEP may benefit mechanically ventilated patients with chronic obstructive pulmonary disease (COPD) by facilitating weaning from positive pressure ventilation [6, 7]. Furthermore, CPAP delivered by mask may also be useful in avoiding the need for intubation and mechanical ventilation in patients with COPD with acute hypercapnic respiratory failure [7]. Finally, CPAP may also be useful in improving exercise tolerance in patients with COPD [9, 10].
CPAP may potentially be useful in the management of acute bronchial asthma, which increases inspiratory work of breathing and predisposes patients to air trapping, dynamic hyperinflation, and auto-PEEP. Martin and coworkers [5] showed that CPAP applied by mask to patients with histamine-induced bronchospasm reduced pulmonary resistance and inspiratory work of breathing. Shivaram and colleagues [11] applied graded levels of CPAP by mask to patients with acute asthma and observed an improved sensation of comfort, reduced inspiratory work of breathing, and increased flow in the late phase of tidal expiration. In a prospective, single-blind study [12], patients with acute bronchial asthma who received 5 and 7.5 cmH2O CPAP by nasal mask showed a significant improvement in dyspnea and a reduction in respiratory rate without any deleterious effects on hemodynamics, gas exchange, or expiratory airflow.
Conceptually, CPAP can be partitioned into two components: inspiratory positive airway pressure (IPAP) and EPAP. IPAP may decrease the work of breathing by supplementing inspiratory airway pressure and decreasing inspiratory muscle work. EPAP may counteract the inspiratory threshold imposed by auto-PEEP and increase intraluminal pressure, which 'pneumatically' holds the airway open, thus preventing dynamic airway collapse. Furthermore, EPAP may recruit expiratory muscles, which can defend end-expiratory lung volume (EELV) and aid in unloading inspiratory muscles, further decreasing the inspiratory effort [5, 13]. Separating the effects of EPAP from those of IPAP is relevant clinically because of the common use of bilevel positive airway pressure in the management of patients with airflow obstruction. However, neither the relative contribution of either EPAP or IPAP, nor the mechanisms by which they exert their effect on work of breathing are currently understood.
Thus, we compared the independent effects of IPAP, EPAP, and CPAP on the inspiratory work of breathing and EELV changes using a canine model of methacholine-induced bronchospasm. Our data suggest that both with and without hyperinflation the IPAP component of CPAP is responsible for the reduction in the work of breathing during acute bronchoconstriction. Furthermore, the blunted increases in EELV occurring after positive expiratory pressure (EPAP and CPAP) during bronchoconstriction appear to result from expiratory muscle recruitment.
Method
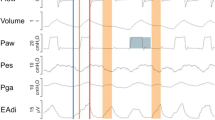
Eighteen fasting mongrel dogs (19.6 ± 0.5 kg) were anesthetized with pentobarbital (30 mg/kg), intubated (9.0 mm ID), and ventilated (10–15 ml/kg, fractional inspired oxygen 0.4–0.5, frequency adjusted to maintain the end-tidal partial carbon dioxide tension in the range 35–40 mmHg). Anesthesia was maintained with pentobarbital (1 mg/kg per hour). Temperature was kept between 36–38°C by external heat. Airway pressure (Paw) was measured 5 cm from the distal end of the endotracheal tube. Heart rate was derived from the electrocardiogram, arterial pressure from a peripheral arterial catheter, and pulmonary arterial pressure and pulmonary arterial occlusion pressure from a balloon-tip pulmonary arterial catheter. Infusion of 0.9 N NaCl was done to keep the end-expiratory pulmonary arterial occlusion pressure between 7 and 10 mmHg. A 5 cm air-filled balloon catheter placed in the mid-chest via 5 cm laporotomy was used to measure pleural pressure (Ppl). Accuracy of Ppl was validated as previously described [14]. Another balloon catheter was placed below the left hemidiaphragm to measure abdominal pressure (Pabd). Respiratory inductive plethysmography bands (Respitrace 900SC, NIMS, North Bay Village, FL, USA) were placed around the rib cage and abdomen (Fig. 1). The animals were then placed prone and allowed to stabilize for 30 min.
Animal model instrumentation. AB, abdomen signal; BiPAP™, apparatus delivering inspiratory positive airway pressure and continuous positive airway pressure (Respironics, Monroeville, PA, USA); Pabd, abdominal pressure; Part, arterial pressure; Paw, airway pressure; Ppl, pleural pressure; RC, rib cage signal; SUM, summed signal from rib cage and abdomen.
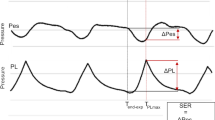
Proximal endotracheal tube flow was measured using a pneumotachograph. The dynamic characteristics of the lungs, chest wall, and total respiratory system during tidal breathing were calculated as the ratio of tidal changes in transpulmonary pressure, Ppl, and Paw to tidal volume (Vt), respectively. Pressure–time products of Ppl and transdiaphragmatic pressure (Pdi) were calculated as the area of the inspiratory portion of the pressure curve (Fig. 2). The durations of one breath, inspiration, and expiration were measured from the airflow tracing. Pulmonary resistance was measured using the multiple airflow interrupter method [15] after airway secretions were removed by suction and three double Vts were administered.
Representative strip-chart recorder tracing describing the methods used for measuring tidal swings in the pleural pressure (Ppl) and transdiaphragmatic pressure (Pdi) tracings. The pressure–time integrals of the diaphragm (∫Ppldt) and pleural pressure (∫Pdidt) were computer-calculated as the area of the pressure tracing defined by the inspiratory portion of the airflow tracing. EXP, expiratory; INSP, inspiratory.
The respiratory inductive plethysmographically derived Vt was calibrated using the isovolume maneuver and validated against the pneumotachograph-derived volume [16] and a super syringe. The absolute lung volume change measured in duplicate with initiation and removal of positive airway pressure was used to estimate changes in EELV (DC-coupled mode; Fig. 3). Auto-PEEP was measured using the inspiratory flow-initiation method [17].
On the left is a tracing of the summed signal from respiratory inductive plethysmography used for calculating changes in end-expiratory lung volume (EELV). The arrow labeled 'ON' depicts the switch from spontaneous breathing to the initiation of 10 cmH2O of inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP), or continuous positive airway pressure (CPAP). The arrow labeled 'OFF' depicts the removal of positive airway pressure and return to spontaneous breathing. Changes in EELV were quantified by measuring changes in the baseline portion of the tracing. The changes in EELV induced by the addition (ON) and removal (OFF) of positive airway pressure were averaged, and the values are reported in Fig. 7. The increase in height of the summed signal represents changes in tidal volume (Vt), as measured using plethysmography, but these values were not measured for these experiments. On the right is a graph comparing plethysmographically derived summed signal measurements of changes in EELV with spirometer-derived values in three pharmacologically paralyzed animals during both inflation and deflation of the lungs.
IPAP and CPAP were created using the BiPAP™ (Respironics, Monroeville, PA, USA). IPAP has a mandatory minimum EPAP level of 2 cmH2O. EPAP was generated using a non-rebreathing T-piece valve with a threshold spring-loaded PEEP valve attached to the expiratory limb.
All animals received no positive airway support, and 10 cmH2O IPAP, EPAP, and CPAP before and during methacholine-induced bronchospasm. The order of IPAP, EPAP, and CPAP was varied using a Latin Square design. Measurements were taken after 5 min spontaneous breathing, with or without positive airway pressure breathing. Bronchospasm was induced by methacholine infusion (0.1 to 0.3 mg/kg per min) until tearing, hypersalivation, and diarrhea were noted. Afterward, 20–30 min elapsed to achieve maximal bronchoconstriction [18]. The animals were then given pentobarbital boluses and killed by bolus KCl infusion. Placement of catheters was confirmed at necropsy.
Three to six breaths were analyzed per step. All off positive airway pressure data were pooled. Analysis of variance for repeated measures with a Newman–Keuls correction was applied if a statistical difference was detected (CRUNCH, Oakland, CA, USA).
Results
Effects of methacholine-induced bronchospasm on control variables
Pooled control respiratory variables before and after induction of methacholine-induced bronchospasm are summarized in Table 1. Methacholine infusion resulted in an increase in respiratory frequency (P < 0.002) along with a less significant drop in Vt (P < 0.03), which resulted in an overall increase in minute ventilation (P < 0.05). Peak and mean airflow rates were unchanged during both inspiratory and expiratory phases of respiration after the administration of methacholine. Resistance increased and the dynamic compliance of the lung, chest wall, and entire respiratory system decreased significantly (Table 1, Fig. 4). Respiratory system mechanics remained constant throughout the entire experimental time period. The inspiratory effort increased significantly during methacholine infusion, as estimated by changes in tidal Ppl, Pdi, and their respective pressure–time products. No measurable auto-PEEP was detected using the inspiratory flow initiation method in any animal. In further support of this lack of hyperinflation, end-expiratory transpulmonary pressure, which is used to estimate absolute end-expiratory lung volume, exhibited no change during methacholine infusion (Fig. 5). End-expiratory Pabd, which is used to estimate abdominal expiratory muscle activity, approximately doubled (P < 0.01) during methacholine infusion (Fig. 5). However, end-expiratory Ppl did not increase during methacholine infusion. The static compliance of the respiratory system was unchanged during methacholine infusion, as measured in three paralyzed animals with stepwise inflation and deflation maneuvers.
Lung mechanics before and after intravenous methacholine infusion. Resistance of the respiratory system (Rrs) was significantly increased and dynamic compliance (Cdyn) was significantly decreased, as measured in all animals (n = 18). The static compliance (Cst) was unchanged after methacholine administration, as measured in three pharmacologically paralyzed animals. *P < 0.05.
Effects of intravenous methacholine infusion on auto-positive end-expiratory pressure (auto-PEEP), end-expiratory transpulmonary pressure (Ptp, exp), and end-expiratory abdominal pressure (Pabd, exp). Methacholine induced no significant hyperinflation according to the auto-PEEP and Ptp, exp measurements. However, expiratory muscle activity significantly increased after methacholine administration, as demonstrated by the increase in Pabd, exp. *P < 0.01.
Effects of inspiratory, expiratory, and continuous positive airway pressure
Stability of animal preparation
The animals remained stable throughout the experimental time period, as indicated by no detectable changes in all measured variables, either before or after methacholine infusion, during control periods off positive pressure support. The constancy of the control data also supports the assumption that the effects of 5 min IPAP, EPAP, and CPAP did not persist after the discontinuation of positive airway pressure.
Breathing pattern
During methacholine infusion, applications of all forms of positive airway pressure were associated with similar increases in Vt (P < 0.0002) and a corresponding decrease in respiratory rate (P < 0.01) with a constant minute ventilation. Peak inspiratory flow rate increased with IPAP (P < 0.05) and EPAP (P < 0.05), whereas the ratio of the duration of inspiration to that of one full breath was unchanged. During exhalation, peak expiratory flow, mean expiratory flow, and the ratio of the duration of expiration to that of one full breath were unaltered by any form of positive airway pressure (Table 1).
Inspiratory work of breathing
As summarized in Fig. 6, IPAP and CPAP reduced the work of breathing primarily through changes in diaphragmatic work, as estimated by changes in Pdi tidal swings (P < 0.05) and the diaphragmatic pressure–time product (P < 0.05). Although CPAP tended to reduce further the work of breathing over IPAP alone, this additional reduction was not statistically different. The use of EPAP alone increased the Ppl and Pdi work of breathing values. IPAP and CPAP similarly reduced Ppl work, but this difference did not achieve statistical significance.
Changes in pleural (left) and transdiaphragmatic (right) work indicesafter the application of 10 cmH2O inspiratory positive airway pressure(IPAP), expiratory positive airway pressure (EPAP), and continuous positive airway pressure (CPAP). IPAP and CPAP significantly reduced transdiaphragmatic pressure (P < 0.05) but not pleural pressure work indices compared with control conditions. EPAP significantly increased all indices of respiratory work (*P < 0.05).
End-expiratory lung volume changes
As summarized in Fig. 7, IPAP, EPAP, and CPAP all increased EELV but to different degrees. Before methacholine administration CPAP resulted in the greatest increase in EELV, followed by both EPAP and IPAP (P < 0.01). During methacholine infusion, CPAP and EPAP increased EELV similarly but to a greater extent than did IPAP (P < 0.01). IPAP was associated with a small but similar increase in EELV before and during methacholine infusion. However, the increases in EELV after EPAP and CPAP were consistently greater before methacholine infusion (P < 0.005).
Change in end-expiratory lung volume (EELV) after 10 cmH2O inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP), and continuous positive airway pressure (CPAP) before and after methacholine infusion. For the same positive airway pressure condition, ΔEELV was significantly less (*P < 0.05) after methacholine.
Expiratory muscle activity
Following the initial increase in end-expiratory Pabd during methacholine infusion, there was no significant change with IPAP, EPAP, and CPAP. However, end-expiratory Ppl did increase significantly following IPAP (P < 0.05), EPAP (P < 0.01), and CPAP (P < 0.01), suggesting that the expiratory muscles of the rib cage were activated during these ventilatory changes (Table 1).
Lung mechanics
The dynamic characteristics of the total respiratory system (lungs plus chest wall) were unaffected by IPAP and CPAP but were reduced with the use of EPAP (P < 0.05), whereas measures of dynamic chest wall mechanics were unaltered by any form of positive airway pressure (Table 1).
Isovolume expiratory airflow
In three animals the corrected expiratory isovolume–flow curves were analyzed after IPAP, EPAP, and CPAP. In all cases the positions of all positive airway pressure curves were shifted to the left (increased EELV) but the shape of the flow decays were similar, indicating simply a parallel shift in the flow–volume relation without change in expiratory airway resistance (Fig. 8).
Airflow–volume tidal expiratory curves shifted to allow airflow comparisons under isovolumic conditions in three randomly selected animals. Inspiratory positive airway pressure (IPAP) and continuous positive airway pressure (CPAP) produced a leftward parallel shift in the expiratory curves, which is indicative of increases in end-expiratory lung volume (EELV) without changes in expiratory resistance compared with control conditions. Functional residual capacity (FRC) was defined as the EELV during spontaneous breathing (no positive airway pressure). Relative volume represents the increases in EELV above FRC, as defined above.
Discussion
Positive airway pressure is often used to support spontaneously breathing patients with increased airways resistance. Our data demonstrate, in an animal model of methacholine-induced bronchospasm, that the inspiratory work of breathing was reduced with 10 cmH2O IPAP and CPAP, whereas this work was significantly increased with 10 cmH2O EPAP. These data suggest that during bronchoconstriction, with or without hyperinflation, the presence of inspiratory pressure support primarily reduces the inspiratory work of breathing.
As the respiratory workload is increased, whether by changes in resistance or in compliance, inspiratory muscle work increases to meet the demands of generating a greater transpulmonary inflating pressure to effect a similar volume change. During airflow limitation, the inspiratory work of breathing may be increased by a variety of mechanisms, including increased airway resistance, an inefficient diaphragm length–tension relation from hyperinflation, or an inspiratory threshold load imposed by auto-PEEP. Inspiratory positive airway pressure support, such as that delivered by either IPAP or CPAP, can partially or completely satisfy these increased inspiratory muscle demands during spontaneous breathing [5–7, 19].
Assuming that Ppl measurements represent the rib cage musculature and that Pdi represents diaphragmatic pressure work [20], we noted quantitatively different responses in the distribution of work after IPAP and CPAP. Although both Ppl and Pdi indices of inspiratory work decreased after IPAP and CPAP, only the reduction in Pdi work achieved statistical significance. This was true for both absolute tidal pressure swings and the pressure–time products for both Ppl and Pdi. Other studies have similarly shown a significant reduction in tidal Pdi swings but not esophageal pressure swings with CPAP in patients with airflow limitation [6, 10]. A possible explanation may be a redistribution of respiratory work away from the diaphragm, with the rib cage musculature receiving a greater proportion of the workload. This hypothesis is supported by studies showing that increasing levels of pressure support to patients who are chronically mechanically ventilated resulted in a decrease work of breathing and increased Vt [21]. Alternatively, CPAP has been shown to hyperinflate preferentially the rib cage compartment over the abdominal compartment in patients with COPD and acute histamine-induced bronchospasm [5, 22], which may limit the CPAP-induced reduction in Ppl workload. Because we did not individually examine the rib cage and abdominal plethysmographic EELV component changes, we cannot determine whether this observation was also true in our model.
EPAP-induced hyperinflation increased all indices of respiratory work. This could not be explained by an increase in EELV alone because a similar amount of hyperinflation occurred with CPAP but CPAP reduced respiratory work. Although this form of expiratory Paw most resembles the spontaneous 'pursed lip' breathing noted in patients with obstructive airway diseases [1–3], it does not appear to reduce the inspiratory work of breathing. Other investigators have demonstrated that EPAP alone increases the work of breathing, whereas CPAP reduces this work in an unselected patient population with various etiologies of respiratory failure [23]. O'Donnell and coworkers [9] also found that 4–5 cmH2O EPAP significantly increased the subjective level of dyspnea during submaximal exercise in patients without COPD but it had an inconsistent response in those with COPD. Thus, hyperinflation with EPAP, if inspiration is not simultaneously pressure supported, does not appear to reduce inspiratory work.
For equal reductions in inspiratory work with IPAP and CPAP, CPAP resulted in a greater increase in EELV (93 ± 69 ml versus 48 ± 31 ml). This hyperinflation potentially is mechanically disadvantageous to the diaphragm. This paradox between decreases in inspiratory work despite increases in EELV with CPAP has been seen in other studies using CPAP in patients with obstructive airway disease [6, 7]. Petrof and coworkers [6] applied increasing levels of CPAP (5, 10, and 15 cmH2O) to mechanically ventilated patients and showed a progressive reduction in work of breathing despite a simultaneous increase in EELV. This was explained by regional inhomogeneities of the mechanical properties of the diseased lungs [6]. Lung units with the longest time constants or highest auto-PEEP levels require higher levels of CPAP to produce reductions in the inflation work in these diseased areas, whereas lung regions with shorter time constants or less auto-PEEP become overinflated at higher CPAP levels. Although the net effect is a reduction in respiratory workload, CPAP-induced hyperinflation may lead to adverse effects including impairment in gas exchange, barotrauma, and hemodynamic deterioration.
This CPAP-induced paradoxic decrease in inspiratory work of breathing and increase in lung volume has made it difficult to define the 'optimal' CPAP levels for ventilatory support in airflow limitation. When auto-PEEP is present, the amount of CPAP or PEEP needed on exhalation to counteract the elastic recoil of the respiratory system has been suggested to be somewhere at or near the measured auto-PEEP level [7]. However, this approach is oversimplified and invalid for a number of reasons. First, as Petrof and coworkers [6] showed, the work of breathing continued to decline even when CPAP levels exceeded the measured auto-PEEP levels in patients with less than predicted increases in EELV. However, Gay and coworkers [24] demonstrated that applying external PEEP to airflow-limited patients, even at airway pressure levels substantially below auto-PEEP, can reduce isovolume expiratory flow rates and significantly increase EELV. Finally, the methodology used to measure auto-PEEP can influence the values obtained in the same individual.
Because all regions of the lungs are not affected to the same degree, certain lung units may have longer time constants and therefore be more predisposed to hyperinflation than other areas. The end-expiratory technique first described by Pepe and Marini [25] accounts for all lung units in communication with the airway opening where pressure is being measured, and it therefore represents the 'average' auto-PEEP value of an inhomogeneous respiratory system. The inspiratory flow-initiation method used in this study and initially described by Rossi and coworkers [17] measures only the 'least' auto-PEEP, because the inspiratory flow begins as soon the lowest auto-PEEP threshold is exceeded. In one study in which the two techniques were compared in the same individuals [6], the end-expiratory occlusion auto-PEEP values were always higher than those obtained with the inspiratory flow technique. Thus, at any given level of positive expiratory pressure with CPAP, EPAP, or PEEP, it is likely that regional lung unit volume can increase, decrease, or remain unchanged depending on regional mechanical properties and regional auto-PEEP levels. Lung volume could theoretically be decreased if the backpressure provided by CPAP or EPAP pneumatically splinted open flow-limiting airway segments, thereby reducing expiratory airway resistance and increasing expiratory airflow. Alternatively, through expiratory muscle recruitment, CPAP or EPAP may also protect or even decrease EELV [13, 26].
Ranieri and coworkers [27] studied the effects of progressive levels of PEEP from 5 to 15 cmH2O in pharmacologically paralyzed, mechanically ventilated patients with severe obstructive airway disease and found that hyperinflation did not occur until the external PEEP exceeded 85% of the auto-PEEP levels present. However, because the degree of hyperinflation depends on several factors such as available duration of expiration, respiratory frequency, and lung mechanics, this simple guideline cannot necessarily be extrapolated to nonparalyzed, spontaneously breathing patients, in whom breathing patterns may vary considerably.
We noted that, for similar levels of IPAP, EPAP, and CPAP, EELV increased to a much lesser degree during methacholine infusion. Several possible explanations may exist. First, if auto-PEEP is present, then EPAP or CPAP should not significantly increase EELV until the auto-PEEP level is exceeded [5, 7, 28]. However, this cannot be generalized to a multicompartment, inhomogeneous system because alveolar driving pressure varies and can be regionally reduced at expiratory airway pressures below the auto-PEEP levels as described above [24, 29]. In the present study, despite significant methacholine-induced bronchoconstriction and rapid respiratory frequencies, we observed no measurable auto-PEEP using the inspiratory flow-initiation technique. The capacity of this technique to detect auto-PEEP, if present in our model, was subsequently validated in two animals in which the progressive addition of external PEEP was associated with respiratory inductive plethysmographic increases in EELV, as described by Hoffman and coworkers [28]. Thus, our model exhibited no significant dynamic hyperinflation. Hyperinflation cannot explain the blunted increases in EELV during methacholine infusion. Another possible explanation is that methacholine might have significantly decreased the static compliance properties of the respiratory system, such that for the same pressure there would be a correspondingly smaller volume change. However, in three paralyzed animals, progressive inflation and deflation curves revealed no methacholine-induced change in static lung or chest wall compliance.
Finally, the explanation we favor is the influence of significant increases in expiratory muscle activity during methacholine-induced bronchospasm, as noted by changes in end-expiratory Pabd and Ppl. Expiratory muscle activity was increased with methacholine infusion and remained elevated during the entire methacholine stage of the experiment. This persistent tonicity of the expiratory muscles would decrease the compliance of the chest and abdominal wall, and reduce the EELV changes of EPAP and CPAP. There was no significant relation between Pabd and increases in lung volume (r = 0.34, P = 0.07). However, the change in end-expiratory Ppl and EELV had a curvilinear relation, indicating that EELV changes were dependent on the degree of expiratory rib cage activity. The more positive end-expiratory Ppl values had the lowest increase in EELV after EPAP and CPAP (r = 0.42, P < 0.05). This expiratory muscle activity would be obliterated with neuromuscular paralysis, which is consistent with our observations. Interestingly, Lessard and coworkers [30] noted that increasing IPAP increased both auto-PEEP and expiratory efforts in individuals with chronic airflow obstruction receiving pressure support ventilation. Although EELV was not increased, stop flow measured airway pressure increased, presumably due to increased expiratory muscle activity. Mancebo and coworkers [31] documented that when extrinsic PEEP was increased up to the auto-PEEP value, respiratory drive, estimated by the pressure at the mouth 0.1 s into an obstructed spontaneous breath (P0.1), also decreased, with P0.1 and work of breathing decreasing in parallel. These data collectively suggest that mechanical factors other than increasing EELV induce increased respiratory effort, but that respiratory drive matches ventilator manipulated changes in work of breathing.
As others have suggested, and as our data demonstrate, the application of positive airway pressure during exhalation, as with CPAP and EPAP, may recruit expiratory muscles of respiration [5, 6, 13, 26]. Although this will increase the work performed by the expiratory muscles, the effort of the inspiratory muscle can be reduced through a variety of mechanisms, including cephalad displacement of the diaphragm to a more efficient length–tension position, passive outward recoil of the rib cage after relaxation of the expiratory muscles, and diaphragmatic descent by reduction in intra-abdominal pressure after muscular relaxation [6, 13, 32, 33].
However, the recruitment patterns of expiratory muscles are incompletely understood during conditions of increased respiratory workload or in response to expiratory airway pressure as with EPAP, PEEP, or CPAP. We noted a difference in the expiratory activity of the rib cage and abdominal components after application of various forms of Paw. After IPAP, EPAP, and CPAP no changes occurred in end-expiratory Pabd compared with unsupported breathing, indicating that no form of positive Paw recruited abdominal expiratory muscles. However, all forms of Paw increased end-expiratory Ppl, which is consistent with activation of the expiratory rib cage musculature. Thus, our data suggesting that IPAP and CPAP preferentially unload the diaphragm over the rib cage musculature during spontaneous inspiration may be attributed to the redistribution of workload and selective recruitment of the expiratory rib cage muscles to assist with respiratory work. Further work using electromyography would be useful in confirming our interpretations of the muscular recruitment and derecruitment patterns after the application of positive Paw during acute bronchospasm.
In our attempt to elucidate whether the decreased inspiratory work of breathing by IPAP and CPAP were due only to inspiratory pressure assistance, we examined the isovolume flow curves in three animals after methacholine infusion. We found that the position of the expiratory flow decay was shifted to the left, which is consistent with increases in EELV, but the shape of the expiratory flow–volume segment was similar during IPAP and CPAP. This flow–volume behavior indicates that, although our animals had a significant degree of bronchospasm, airflow limitation was absent. Furthermore, although previous studies have postulated that CPAP may also reduce airway resistance through stimulation of tracheal stretch receptors [5], this was not apparent by analysis of the isovolume flow curves. Thus, it does not appear that the CPAP reduction in the inspiratory work of breathing was due to changes in expiratory flow resistance.
Conclusion
Our data are consistent with an inspiratory effect of positive airway pressure during acute bronchospasm as a factor minimizing the increased work of breathing. The diaphragm appears to be preferentially unloaded during inspiration compared with the thoracic cage inspiratory musculature. Furthermore, generalized increases in expiratory muscle tone, predominantly in the rib cage compartment, appear to reduce the positive expiratory airway pressure-induced increases in EELV.
Key messages
-
The IPAP component of CPAP reduces the work of breathing during bronchospasm
-
The EPAP component minimally alters work of breathing but increases lung volume
Abbreviations
- COPD:
-
chronic obstructive pulmonary disease
- CPAP:
-
continuous positive airway pressure
- EELV:
-
end-expiratory lung volume
- EPAP:
-
expiratory positive airway pressure
- IPAP:
-
inspiratory positive airway pressure
- Pabd:
-
abdominal pressure
- Paw:
-
airway pressure
- Pdi:
-
transdiaphragmatic pressure
- PEEP:
-
positive end-expiratory pressure
- Ppl:
-
pleural pressure
- Vt:
-
tidal ventilation.
References
Barach AL, Swenson P: Effect of breathing gases under positive pressure on lumens of small and medium-sized bronchi.Arch Intern Med 1939, 63:946–948.
Ingram RH Jr, Schilder DP: Effect of pursed lips expiration on the pressure-flow relationship in obstructive lung disease.Am Rev Respir Dis 1967, 96:381–388.
Thoman RL, Stoker GL, Ross JC: The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease.Am Rev Respir Dis 1966, 93:100–106.
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, Isabey D, Harf A: Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease.N Engl J Med 1995, 333:817–822.
Martin JG, Shore S, Engel LA: Effect of continuous positive airway pressure on respiratory mechanics and pattern of breathing in induced asthma.Am Rev Respir Dis 1982, 126:812–817.
Petrof BJ, Legare M, Goldberg P, Milic-Emili J, Gottfried SB: Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive airway disease.Am Rev Respir Dis 1990, 141:281–289.
Smith TC, Marini JJ: Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction.J Appl Physiol 1988, 65:1488–1499.
Miro AM, Shivaram U, Hertig I: Continuous positive airway pressure in patients with chronic obstructive pulmonary disease in acute hypercapnic respiratory failure.Chest 1993, 103:266–268.
O'Donnell DE, Sanii R, Anthonisen NR, Younes M: Effect of dynamic airway compression on breathing pattern and respiratory sensation in severe chronic obstructive pulmonary disease.Am Rev Respir Dis 1987, 135:912–918.
Petrof BJ, Calderini E, Gottfried SB: Effect of CPAP on respiratory effort and dyspnea during exercise in severe COPD.J Appl Physiol 1990, 69:179–188.
Shivaram U, Donath J, Khan FA, Juliano J: Effects of continuous positive airway pressure in acute asthma.Respiration 1987, 52:157–162.
Shivaram U, Miro AM, Cash ME, Heurich AE, Finch PJP, Kamholz SL: Nasal CPAP therapy in the treatment of acute bronchial asthma.J Crit Care 1993, 8:87–92.
Road JD, Leevers AM: Inspiratory and expiratory muscle function during continuous positive airway pressure in dogs.J Appl Physiol 1990, 68:1092–1100.
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J: A simple method for assessing the validity of the esophageal balloon technique.Am Rev Respir Dis 1982, 126:788–791.
Gottfried SB, Rossi A, Calverley PM, Zocchi L, Milic-Emili J: Interrupter technique for measurement of respiratory mechanics in anesthetized cats.J Appl Physiol 1984, 56:681–690.
Stradling JR, Chadwick GA, Quirk C, Phillips T: Respiratory inductance plethysmography: calibration techniques, their validation and the effects of posture.Bull Eur Physiopathol Respir 1985, 21:317–324.
Rossi A, Gottfried SB, Zocchi L, Higgs BD, Lennox S, Calverley PM, Begin P, Grassino A, Milic-Emili J: Measurement of static compliance of the total respiratory system in patients with acute respiratory failure during mechanical ventilation. The effect of intrinsic positive end-expiratory pressure.Am Rev Respir Dis 1985, 131:672–677.
Ramsdell JW, Georghiou PF: Prolonged methacholine-induced bronchoconstriction in dogs.J Appl Physiol 1979, 47:418–424.
Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi AA, Brun-Buisson C, Rauss A, Lemaire F, Harf A: Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a facemask.N Engl J Med 1990, 323:1523–1530.
De Troyer A, Estenne M: Limitations of measurement of transdiaphragmatic pressure in detecting diaphragmatic weakness.Thorax 1981, 36:169–174.
Banner MJ, Kirby RR, Blanch PB, Layon AJ: Decreasing imposed work of the breathing apparatus to zero using pressure-support ventilation.Crit Care Med 1993, 21:1333–1338.
Lennox S, Mengeot PM, Martin JG: The contributions of rib cage and abdominal displacements to the hyperinflation of acute bronchospasm.Am Rev Respir Dis 1985, 132:679–684.
Schlobohm RM, Falltrick RT, Quan SF, Katz JA: Lung volumes, mechanics and oxygenation during spontaneous positive-pressure ventilation: the advantage of CPAP over EPAP.Anesthesiology 1981, 55:416–422.
Gay PC, Rodarte JR, Hubmayr RD: The effects of positive expiratory pressure on isovolume flow and dynamic hyperinflation in patients receiving mechanical ventilation.Am Rev Respir Dis 1989, 139:621–626.
Pepe PE, Marini JJ: Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect.Am Rev Respir Dis 1982, 126:166–170.
Farkas GA, Schroeder MA: Mechanical role of expiratory muscles during breathing in prone anesthetized dogs.J Appl Physiol 1990, 69:2137–2142.
Ranieri VM, Giuliani R, Cinnella G, Pesce C, Brienza N, Ippolito EL, Pomo V, Fiore T, Gottfried SB, Brienza A: Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation.Am Rev Respir Dis 1993, 147:5–13.
Hoffman RA, Ershowsky P, Kreiger BP: Determination of auto-PEEP during spontaneous and controlled ventilation by monitoring changes in end-expiratory thoracic gas volume.Chest 1989, 96:613–616.
Tuxen DV: Detrimental effects of positive end-expiratory pressure during controlled mechanical ventilation of patients with severe airflow obstruction.Am Rev Respir Dis 1989, 140:5–9.
Lessard MR, Lofaso F, Brochard L: Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients.Am J Respir Crit Care Med 1995, 151:562–569.
Mancebo J, Albaladejo P, Touchard D, Bak E, Subirana M, Lemaire F, Harf A, Brochard L: Airway occlusion pressure to titrate positive end-expiratory pressure in patients with dynamic hyperinflation.Anesthesiology 2000, 93:81–90.
Hubmayr RD, Sprung J, Nelson S: Determinants of transdiaphragmatic pressure in dogs.J Appl Physiol 1990, 69:2050–2056.
Pengelly LD, Alderson AM, Milic-Emili J: Mechanics of the diaphragm.J Appl Physiol 1971, 30:797–805.
Acknowledgements
This study was funded in part by grants from the Laerdal Foundation for Acute Medicine (Norway) and the Veterans Administration. The authors wish to thank Anthony Lanier and Brian Ondulick for their technical assistance and Nancy Arora for her critical scientific editorial review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Miro, A.M., Pinsky, M.R. & Rogers, P.L. Effects of the components of positive airway pressure on work of breathing during bronchospasm. Crit Care 8, R72 (2004). https://doi.org/10.1186/cc2461
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc2461