Abstract
The epithelial–mesenchymal transition (EMT) is a developmental mechanism of crucial importance in establishing the body plan in many multicellular organisms. Several transduction pathways controlling the various steps of the morphological transition have been identified by molecular analyses of this process in cell lines and in vivo. The newly formed mesenchymal cells can exhibit locomotory and invasive phenotypes, suggesting that EMTs contribute to the progression of carcinoma. Diverse evidence indicates that EMT subprograms are involved in the appearance of different breast carcinoma types. Several normal and malignant breast cell lines are currently being analyzed to define key steps in EMT and to identify candidate genes. DNA profiling technology is also being applied to uncover pathways that lead to a metastatic phenotype.
Similar content being viewed by others
Introduction
The epithelial–mesenchymal transition (EMT) was originally defined by developmental biologists as a morphological conversion occurring at specific sites in embryonic epithelia to give rise to individual migratory cells [1]. EMT is a fundamental process in the development of most metazoans and is primarily involved in the shaping of embryos. In mammals, EMT has been associated with the formation of the parietal endoderm [2]. It is also directly involved in the formation of the mesoderm and definitive endoderm at the primitive streak during gastrulation [3]. Neural crest cells emerge from the dorsal neural epithelium through EMT before undergoing extensive migration and differentiation into many derivatives [4]. EMT has also been implicated in the ontogeny of other structures including somites and heart endocardium [5].
Although EMT was recognized in the late nineteenth century, it is only recently that some of the molecular mechanisms in the developing embryo have been revealed. Most current studies are performed in vitro with epithelial cell lines, which can be converted into fibroblast-like cells under specific culture conditions. However, not all normal or malignant cell lines share all characteristics with embryonic epithelia. In many instances, epithelial cell lines can be refractory to EMT, perhaps owing to the inaccessibility of scatter factors or to intrinsic inhibitory mechanisms. Conversely, culture conditions do not always allow epithelial cells to achieve full polarity and can facilitate dispersion. The definition of an EMT, and the requirements to execute one, in vitro are at variance with those in vivo and therefore cannot exactly recapitulate these events. It is therefore not surprising to find studies differing in their stringency for the various criteria for defining an EMT.
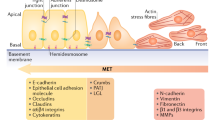
The current criteria for defining an EMT in vitro [1, 6] involve the loss of epithelial cell polarity, the separation into individual cells and subsequent dispersion after the acquisition of cell motility. EMT presumably involves the disassembly of tight junctions, adherens junctions and desmosomes as well as the reorganization of cell substrate adhesion complexes. After the loss of cell polarity, the cytoskeleton is significantly remodeled. A shift from cytokeratin intermediate filaments to vimentin is considered to be an important criterion for EMT, although vimentin is not necessarily a reliable marker of mesenchymal cells (transcription of the vimentin gene is particularly sensitive to serum components). The epithelial and mesenchymal phenotypes also show particular transcription profiles including cytoskeletal components and extracellular matrix components. It is likely that several other types of protein will be found to be associated with EMT in only one of the two states. This minireview addresses the question of whether EMT can be involved in breast cancer progression and whether these tumor types can benefit from a molecular understanding of EMT [7].
EMT signal transduction pathways
EMT can be induced in vitro in several epithelial cell lines by growth factors activating tyrosine kinase surface receptors. These factors include scatter factor/hepatocyte growth factor, fibroblast growth factors, epithelial growth factor (EGF) family members, and insulin-like growth factors 1 and 2 [7]. For instance the human 184 breast cell line can respond to EGF [8]. In most cases the Ras/mitogen-activated protein kinase (MAPK) pathway has a crucial role in inducing EMT, but in some cell lines the transient activation of Src, phosphoinositide 3-kinase (PI3K) and Rac has an effect on particular aspects of EMT. At least two normal breast cell lines (NMuMG and EpH4: two normal murine mammary cell lines) and other lines respond specifically to transforming growth factor-β (TGF-β), although one of them (the EpH4 line) undergoes EMT when expressing the oncogenic H-Ras [9–11]. MAPK and PI3K have also been implicated in TGF-β signaling and more direct signalling, both through the conventional Smad pathways and other as yet unknown pathways [12]. These signaling pathways, and particularly the cooperativity between Ras and TGF-β signalling, are also observed in the EMT process in squamous carcinoma of the skin, which can progressively acquire a fibroblast-like morphology in later stages of the disease [13]. The classical TGF-β signaling pathway leading to inhibition of cell growth or even the induction of apoptosis is abrogated by activated Ras in part through Raf/MAPK or PI3K activation. In addition, it has been clearly shown in the skin carcinogenesis model that nuclear accumulation of Smad2 by oncogenic Ras is required for progression towards the spindle cell carcinoma stage.
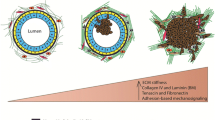
ECM components, including collagens and laminin 5, can also induce EMT in some cell lines [14]. Current research is aimed at identifying gene targets within these pathways. One class of potential targets is the metalloproteases, which are important in morphogenesis. Their function in mammary gland branching morphogenesis [15, 16] and tumor formation has been described recently [17]. Interestingly, only the expression of stromelysin 1 (matrix metalloprotease [MMP]-3) in the Scp2 mammary cell line is sufficient to induce EMT in vitro and tumorigenesis in vivo [18]. The targeted expression of MMP3 in the mammary gland can induce premalignant and malignant lesions, forming poorly differentiated tumors with a fibroblast-like morphology in a proportion of the carcinoma cells [19].
These data suggest that EMT is involved in early or later steps of breast malignancy development. However, breast malignancies are a heterogeneous group of different morphological entities. Pathological characteristics of breast carcinomas need to be defined so that the phenotype can be correlated with the molecular alterations observed in vitro or in animal models.
Pathological characteristics of breast malignancies
Epithelial tumors (carcinomas) are the most frequent type of breast tumor. The first step in the histological study of breast carcinomas is to determine whether the tumor is confined to the glandular component of the organ (in situ carcinoma) or whether it invades the stroma (invasive carcinoma). Then, the histological subtype within each category is determined and prognostic indicators such as stage, grade and the presence of vascular invasion are assessed. The stage of the tumor is evaluated according to its size and the presence of axillary lymph node metastases. The grade of a carcinoma is an estimation of its differentiation.
The grading of breast carcinomas is based solely on the invasive portion of the tumor. The grading procedure involves a three-tiered system for describing the tumor structure. The first criterion is the evaluation of tubule formation. The absence of tubules with only cord sheets and isolated cells might reflect an incomplete EMT process. The second criterion is the nuclear grade assessed by comparison with the sizes of nuclei in normal cell and between carcinoma cells. The third criterion is the mitotic count expressed as the number of mitoses per 10 high-magnification fields [20].
The term invasive carcinoma encompasses numerous entities differing from each other by morphological characteristics related to the degree of differentiation and the organization of the cells.
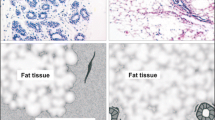
The most common form (75% of cases) is ductal invasive carcinoma. The cells can grow in irregular or rounded sheets, or nests or cords of solid clusters of cells, frequently interspersed with isolated cells. The presence of gland lumens, poorly or well formed, reflects the degree of differentiation of the tumor. Those glands lacking myoepithelial cells are not delimitated by a basement membrane. The amount of stroma and in particular the amount of the inflammatory infiltrate varies considerably between individual ductal invasive carcinomas. An association with in situ carcinoma is observed in almost 70% of cases [21–23].
The second large category of invasive carcinoma is the group of lobular carcinomas, which account for 10–15%. These tumors are composed of carcinoma cells, isolated or organized in single file or narrow cords, usually with an abundant surrounding stroma. Gland formation is not a feature of classical infiltrative lobular carcinoma. An in situ component is associated with this form in nearly 90% of cases [24]. Additional patterns of infiltrative lobular carcinomas have been recognized: they have different architectural and cytological patterns but cohesive cells. These patterns include the solid pattern, composed of large nests of closely packed but non-cohesive cells separated by thin vascular channels, the alveolar pattern characterized by clusters of 20 cells arranged in round nests separated by small amounts of stroma, and the pleomorphic pattern composed of cells showing a higher degree of nuclear atypia and more mitotic figures [23].
In addition to the ductal and the lobular types of breast carcinoma, other rare types have been described, each accounting for less than 5% of the total. The group of metaplastic carcinomas is of particular interest with regard to EMT. This is because these carcinomas are believed to be of epithelial origin. There are two main categories: one composed of intermixed cells with epithelial morphology but exhibiting a glandular differentiation associated with a squamous metaplasia, and the second composed of epithelial cells with glandular differentiation tightly mixed with non-epithelial cells. The non-epithelial cells are composed of spindle shape cells, bone and cartilaginous cells or both. The spindle cells often seem to be merged with epithelial cells, yet they represent the majority cell type characterized by morphological heterogeneity. Cytokeratin and more specifically high-molecular-mass cytokeratins and vimentin are observed within both the spindle cell and epithelial cell components [23].
Some other rare types of breast carcinoma are associated with a more favorable clinical outcome. These are generally well-differentiated tumors such as tubular carcinoma composed of angular glands lined by a single layer of cylindrical cells; mucinous carcinoma, a tumor that produces large amounts of extracellular mucus; and cribriform carcinoma composed of large clusters of cells with glandular differentiation [25]. Some other lesions show an undifferentiated morphology. An example is the medullary carcinoma characterized by large syncytial sheets of large cells with atypical nuclei and high rates of mitosis, mixed with an abundant inflammatory stroma [26].
This non-exhaustive overview of the pathology of breast carcinomas illustrates the broad diversity of the morphological aspects of breast tumors. This diversity is in part related to the differentiation state of the carcinoma cells. The prognosis is evaluated from the pathological type, the stage and the grade of its tumor and this is used to determine the individual therapeutic scheme. Unfortunately, the evaluation of prognosis is still inaccurate. A better knowledge of the EMT pathways and of the genes involved in breast carcinomas might be of great value in improving our understanding of these tumors and consequently allowing a more reliable prognosis for patients.
Involvement of EMT in breast carcinoma
Loss of heterozygosity at 16q22.1 is relatively frequent in breast carcinoma, implicating E-cadherin as a tumor suppressor gene. E-cadherin, the prototype type 1 epithelial cadherin, has been studied extensively in EMT. In vivo, E-cadherin is downregulated specifically at sites of EMT such as gastrulation in Drosophila and in several vertebrates including the mouse. Numerous studies have described a partial or complete loss of E-cadherin during carcinoma progression, which is correlated with an unfavorable prognosis [27, 28] and confirming that E-cadherin is a caretaker of the epithelial state. Several distinct mechanisms of E-cadherin downregulation have been described. Mutations have been found in the E-cadherin gene in about 50% of lobular carcinomas of the breast [29]. Most mutations lead to non-functional proteins. In accordance with the Knudson two-hit hypothesis, most of these mutations are found in tumors with loss of heterozygosity (LOH) of the E-cadherin wild-type locus. A recent study examined in depth whether the two-hit hypothesis applies to sporadic ductal invasive carcinoma of the breast [30]. No mutations were found in this series, confirming previous studies. However, LOH in the E-cadherin locus was not significantly associated with hypermethylation in the other allele, suggesting the existence of other mechanisms for E-cadherin gene extinction.
Genetic screening in Drosophila led to the identification of snail, a gene involved in the control of gastrulation [31]. snail transcripts are specifically expressed in invaginating mesodermal cells just before their EMT. Snail and a closely related member of this zinc finger transcriptional repressor, named Slug, have also been found in vertebrates. There is convincing evidence that Slug is required for gastrulation and neural crest emigration in Xenopus and in the chick embryo, and that Snail has a similar role in the mouse [32]. The important discovery was made that Snail can downregulate transcription of the E-cadherin gene through its interaction with E boxes in the proximal region of the promoter [33]. Slug can also bind to the same region of the promoter, although with lower affinity (A Cano, personal communication). Other transcription factors also inhibit the transcription of E-cadherin genes: an example is the zinc finger protein SIP1, a downstream target gene in the TGF-β-mediated induction of EMT in the NMuMG cell line [34]. Snail expression has been analyzed by in situ hybridization in breast carcinomas and compared with that of E-cadherin. Snail is expressed mostly in dedifferentiated tumors and is correlated with grading. In heterogeneous tumors, Snail is expressed in carcinoma cell islands devoid of E-cadherin. It is found in all ductal invasive carcinomas with lymph node involvement. However, Snail was not found in the small number of lobular carcinomas investigated by Blanco et al. [35]. Another study reports Snail expression in tumors in which the E-cadherin promoter is hypermethylated rather than in tumors with LOH in the E-cadherin locus [30]. Another recent study analyzed the roles of the various E boxes in the control of E-cadherin transcription: the findings stress the importance of E-box C and thus are at variance with previous studies. In addition, in breast cell lines, Slug expression is more tightly correlated than Snail with a lack of E-cadherin expression [36].
Clearly there is a need for more extensive analysis of breast tumor samples, but significant progress has already been made toward understanding one key aspect of EMT in breast cancers. The phenotype of breast cancer micrometastases in lymph nodes and in the bone marrow suggests that EMT occurs within the primary tumors [37]. Furthermore, if these cells are at the origin of secondary tumors, it would indicate that there is a potential reversal of the phenotype because E-cadherin can be re-expressed in the metastatic lesion, which has a generally more differentiated phenotype than the primary tumor [38, 39]. This is indeed consistent with the notion that E-cadherin is regulated mostly by epigenetic mechanisms. It also suggests that LOH at one locus, together with a mutation or a definitive extinction of transcription at the other locus, explains only a small fraction of all breast carcinomas.
There are several breast carcinoma fibroblast-like lines that express the classical type 1 N-cadherin [40] and possibly some type 2 cadherins. The expression of N-cadherin de novo in breast carcinoma cells induces an EMT [41]. The mechanism by which N-cadherin can overcome the maintenance of the epithelial state by E-cadherin is not known, although a domain in N-cadherin essential for this effect has been identified [42]. E-cadherin can be down-regulated by other mechanisms including the recently described mechanism of ubiquitination and endocytosis of E-cadherin in epithelial cells stimulated by EMT-inducing growth factors [43].
Concluding remarks
Epithelial cell plasticity is a major feature of embryonic development. Epithelial cell intercalation during the convergence–extension movement, or during the process of cavitation and branching morphogenesis, implies local phenotypic changes in the cells undergoing these processes. The development of the mammary gland probably employs these mechanisms. EMT is one of the most drastic aspects of epithelial cell plasticity. Some of the molecular programs of EMT might be involved in the development of the mammary gland, particularly at terminal end buds or possibly in lateral branching [44]. However, EMT is also likely to be important in tumor progression. One of the best markers of EMT associated with breast cancer is the loss of E-cadherin, and this is controlled in part by Snail family members, as is EMT associated with development. Other transduction pathways might be found in the mammary tumors in which TGF-β or tyrosine kinase surface receptor ligands are produced.
Recent DNA chip profiling has already proved powerful for tumor classification: a subset of breast carcinomas display molecular markers of the basal cell phenotype [45] associated with myoepithelial cell differentiation [46]. This subgroup has the poorest prognosis of all the groups studied as assessed by hierarchical clustering [45]. These findings do not preclude the possibility that these tumors contain myoepithelial cells rather than carcinoma cells with basal characteristics. A recent study shows that murine HC11 clones with basal cell characteristics acquire a motile phenotype in vitro and invasive properties in vivo when exposed to EGF [47]. The plasticity of human breast carcinoma has also been investigated in vitro by establishing cell lines and assaying them for their tumorigenic properties. At least one particular line has a myofibroblastic phenotype, suggesting the intriguing possibility that in some cases the myofibroblasts in the stroma of breast tumors might be derived from the carcinoma cells [48].
A subset of genes, rather than a particular single gene, can be used as a prognostic marker, and this approach shows great promise for predicting metastatic outcome [49]. Various laboratories are currently trying to identify genes more specifically associated with EMT, tumor invasion and tumor metastasis, either with cell line models or with tumors of diverse grades and stages. It is expected that new candidate genes will be validated in the near future and that we will learn significantly more about the effects of EMT on the progression of breast carcinomas.
Abbreviations
- EGF:
-
= epidermal growth factor
- EMT:
-
= epithelial–mesenchymal transition
- LOH:
-
= loss of heterozygosity
- MAPK:
-
= mitogen-activated protein kinase
- MMP-3:
-
= matrix metalloprotease-3
- PI3K:
-
= phosphoinositide 3-kinase
- TGF:
-
= transforming growth factor.
References
Hay ED: An overview of epithelio-mesenchymal transformation. Acta Anat. 1995, 154: 8-20.
Veltmaat JM, Orelio CC, Ward-Van Oostwaard D, Van Rooijen MA, Mummery CL, Defize LH: Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. Int J Dev Biol. 2000, 44: 297-307.
Ciruna B, Rossant J: FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001, 1: 37-49.
Nieto MA: The early steps of neural crest development. Mech Dev. 2001, 105: 27-35. 10.1016/S0925-4773(01)00394-X.
Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y: Epithelial-mesenchymal transformations in early avian heart development. Acta Anat. 1996, 156: 173-186.
Valles AM, Thiery JP, Boyer B: In vitro studies of epithelium-to-mesenchyme transitions. In: Cell Biology: A Laboratory Handbook. Edited by: Celis J. 1994, New York: Academic Press, 232-242.
Thiery JP: Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002, 2: 442-454. 10.1038/nrc822.
Matthay MA, Thiery JP, Lafont F, Stampfer F, Boyer B: Transient effect of epidermal growth factor on the motility of an immortalized mammary epithelial cell line. J Cell Sci. 1993, 106: 869-878.
Miettinen PJ, Ebner R, Lopez AR, Derynck R: TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994, 127: 2021-2036.
Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E: TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996, 10: 2462-2477.
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S: Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002, 156: 299-313. 10.1083/jcb.200109037.
Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP: Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA. 2001, 98: 6686-6691. 10.1073/pnas.111614398.
Oft M, Akhurst RJ, Balmain A: Metastasis is driven by sequential elevation of H-Ras and Smad2 levels. Nat Cell Biol. 2002, 4: 487-494. 10.1038/ncb807.
Grassi M, Moens G, Rousselle P, Thiery JP, Jouanneau J: The SFL activity secreted by metastatic carcinoma cells is related to laminin 5 and mediates cell scattering in an integrin-independent manner. J Cell Sci. 1999, 112: 2511-2520.
Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ: The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001, 128: 3117-3131.
Wiseman BS, Werb Z: Stromal effects on mammary gland development and breast cancer. Science. 2002, 296: 1046-1049. 10.1126/science.1067431.
Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002, 2: 161-174. 10.1038/nrc745.
Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z: The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999, 98: 137-146.
Sternlicht MD, Bissell MJ, Werb Z: The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000, 19: 1102-1113. 10.1038/sj.onc.1203347.
Elston C, Ellis I: Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991, 19: 403-410.
Tavassoli F: Infiltrating carcinoma: common and familiar special types. In: Pathology of the Breast. 1999, New York: McGraw-Hill, 401-481. 4
Rosen P: Invasive ductal carcinoma. In: Tumors of the Mammary Gland. 1992, Washington DC: Armed Forces Institute of Pathology, 157-168.
Rosen P: Invasive duct carcinoma: assessment of prognosis, morphologic pronostic markers and tumor growth rate. In: Rosen's Breast Pathology. 2001, Philadelphia: Lippincott, Williams and Wilkins, 1004-
Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, Durand JC, Fourquet A, Pouillart P: Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996, 77: 113-120. 10.1002/(SICI)1097-0142(19960101)77:1<113::AID-CNCR19>3.0.CO;2-8.
Ellis I, Galea M, Broughton N, Locker A, Blamey R, Elston C: Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology. 1992, 20: 479-489.
Rapin V, Contesso G, Mouriesse H, Bertin F, Lacombe MJ, Piekarski JD, Travagli JP, Gadenne C, Friedman S: Medullary breast carcinoma. A reevaluation of 95 cases of breast cancer with inflammatory stroma. Cancer. 1988, 61: 2503-2510.
Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994, 1198: 11-26. 10.1016/0304-419X(94)90003-5.
Wheelock MJ, Soler AP, Knudsen KA: Cadherin junctions in mammary tumors. J Mammary Gland Biol Neoplasia. 2001, 6: 275-285. 10.1023/A:1011319507155.
Berx G, Becker KF, Hofler H, van Roy F: Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998, 12: 226-237. 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D.
Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW, Shen CY: Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene. 2001, 20: 3814-3823. 10.1038/sj.onc.1204505.
Leptin M: twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991, 5: 1568-1576.
Nieto MA: The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002, 3: 155-166. 10.1038/nrm757.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA: The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000, 2: 76-83. 10.1038/35000025.
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F: The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001, 7: 1267-1278. 10.1016/S1097-2765(01)00260-X.
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA: Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002, 21: 3241-3246. 10.1038/sj.onc.1205416.
Hajra KM, Chen DY, Fearon ER: The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002, 62: 1613-1618.
Braun S, Pantel K: Biological characteristics of micrometastatic cancer cells in bone marrow. Cancer Metastasis Rev. 1999, 18: 75-90. 10.1023/A:1006212403983.
Ilyas M: Adhesion molecule expression in breast cancer: the phoenix in tumour metastasis?. J Pathol. 2000, 190: 3-5. 10.1002/(SICI)1096-9896(200001)190:1<3::AID-PATH490>3.0.CO;2-5.
Bukholm IK, Nesland JM, Borresen-Dale AL: Re-expression of E-cadherin, α-catenin and β-catenin, but not of γ-catenin, in metastatic tissue from breast cancer patients. J Pathol. 2000, 190: 15-19. 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.3.CO;2-C.
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA: Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000, 148: 779-790. 10.1083/jcb.148.4.779.
Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, Birchmeier W: Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998, 143: 533-545. 10.1083/jcb.143.2.533.
Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR: N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol. 2000, 151: 1193-1206. 10.1083/jcb.151.6.1193.
Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W: Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002, 4: 222-231. 10.1038/ncb758.
O'Brien LE, Zegers MM, Mostov KE: Opinion. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002, 3: 531-537. 10.1038/nrm859.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein-Lonning P, Borresen-Dale AL: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001, 98: 10869-10874. 10.1073/pnas.191367098.
Deugnier MA, Teuliere J, Faraldo MM, Thiery JP, Glukhova M: The importance of being a myoepithelial cell. Breast Cancer Res. 2002, 4: 224-230. 10.1186/bcr459.
Deugnier MA, Faraldo MM, Janji B, Rousselle P, Thiery JP, Glukhova MA: EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J Cell Biol. 2002, 159: 453-463. 10.1083/jcb.200207138.
Petersen OW, Lind Nielsen H, Gudjonsson T, Villadsen R, Ronnov-Jessen L, Bissell MJ: The plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast Cancer Res. 2001, 3: 213-217. 10.1186/bcr298.
van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH: Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002, 415: 530-536. 10.1038/415530a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
This article is the third in a review series on Host microenvironment in breast cancer development, edited by Gloria Heppner. Other articles in the series can be found at http://breast-cancer-research.com/articles/series.asp?rqs=Heppner
Rights and permissions
About this article
Cite this article
Vincent-Salomon, A., Thiery, J.P. Host microenvironment in breast cancer development: Epithelial–mesenchymal transition in breast cancer development. Breast Cancer Res 5, 101 (2003). https://doi.org/10.1186/bcr578
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr578