Abstract
The existence of psoriatic arthritis as a distinct clinical entity remains a topic of debate; some authors propose that it is simply the co-occurrence of psoriasis and inflammatory arthritis. However, a distinct entity is likely to have distinct susceptibility factors in addition to those that contribute to psoriasis and inflammatory arthritis alone. These aetiological factors may be genetic and/or environmental, and in this review, the evidence for distinct psoriatic arthritis genetic susceptibility factors is considered.
Similar content being viewed by others
Introduction
Psoriasis is a T-cell-mediated inflammatory disease that affects the skin and occurs in one to two per cent of the general population. In 1964, an association between inflammatory arthritis (IA) and psoriasis was formally recognised, although it was probably first described by Alibert in 1818 [1]. The existence of psoriatic arthritis (PsA) as a distinct clinical entity, however, remains a topic of debate, with some clinicians arguing that it simply represents the coincidental occurrence of psoriasis and inflammatory arthritides such as rheumatoid arthritis (RA) or ankylosing spondylitis [2]. A review of the investigation of PsA genetic susceptibility factors is, therefore, both controversial and complex.
Community surveys have confirmed the association between IA and psoriasis. For example, a cross sectional survey in primary care from North East England has reported a prevalence of psoriasis of 1.7%, and of psoriasis associated with IA of 0.3% [3]. Community surveys have reported incidences of psoriasis in 4.5–5.3% of patients presenting with IA [4, 5], but the incidence may be even higher for patients whom are seronegative for rheumatoid factor [6]. Conversely, patients with psoriasis are more likely to have IA [5]. This increased association supports the existence of a distinct disease, psoriatic arthritis (PsA).
Relationship between psoriasis and PsA
The evidence from these recent community-based studies supports the association of IA and psoriasis, and there are several explanations for how this increased association occurs. One theory is that psoriasis and PsA may be two completely separate diseases with different susceptibility factors; the skin manifestation may simply be the final common pathway. There is no difference in type or distribution of psoriasis, however, between those with or without arthritis. Another explanation is that psoriasis and PsA may share susceptibility factors, which may be genetic, environmental or both. Nearly all cases of PsA develop in patients with psoriasis or a family history of psoriasis, so it could be interpreted that psoriasis susceptibility factors are necessary for, but not sufficient to cause, PsA. The development of PsA may require additional factors, either genetic or environmental. A third explanation is that since synovial joint inflammation is common to both RA and PsA, susceptibility factors may be shared between these two conditions, but the presence of psoriasis may modify the expression in joints and the pattern of joint involvement. It has been proposed, however, that PsA is primarily a disease of enthesitis with secondary synovial inflammation, and that arthritis per se, in the presence of psoriasis, does not represent PsA [7].
Whatever the mechanism of association, many clinicians support the idea that a distinct clinical entity of PsA does exist. Studies to prove the existence of PsA are limited, however, by the lack of a good disease definition. PsA has been broadly defined as "an inflammatory arthritis associated with psoriasis which is usually negative for rheumatoid factor" [1]. This definition has been widely criticised and no internationally agreed criteria for the diagnosis of PsA have ever been successfully developed and applied (reviewed in [8]). Recently, classification criteria have been proposed based on analysis of 260 PsA patients, but these require validation [9]. Currently, however, most clinicians make the diagnosis based on the presence of IA in a patient with psoriasis after exclusion of other causes.
The controversy over the distinctiveness of PsA is important to bear in mind when investigating potential susceptibility factors. A distinct entity is likely to have a distinct aetiology in addition to that of related inflammatory arthropathies that may simply coincide with psoriasis [10, 11]. These aetiological factors may be genetic and/or environmental; this review concentrates on the former. Demonstration of a genetic association with PsA over and above the expected effects of a combination of psoriasis and IA would support the concept of PsA as a distinct disease.
Evidence for genetic susceptibility to PsA
Twin and family studies are the classic way of investigating genetic contribution to a disease but twin studies have not, to my knowledge, yet been undertaken in PsA. Family studies do, however, suggest that first-degree relatives are at increased risk of developing PsA [1]. The sibling recurrence risk (λs) measures the excess risk to a sibling over the general population risk and is estimated to be approximately four for psoriasis [12]. Moll and Wright's original family studies suggest that the λs for PsA may be significantly higher, indicating an even stronger genetic contribution to its aetiology [1].
Studies of linkage and association to the human leukocyte-associated antigen region
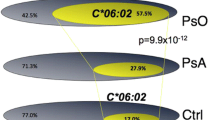
Linkage to the human leukocyte-associated antigen C gene (HLA C) has been reported consistently in psoriasis sibling pair families, and association studies have revealed that the HLA Cw*0602 allele is most commonly associated [13–20]. Recently the association has been refined to a 100 kb telomeric region of the HLA C locus, suggesting that the true susceptibility locus it is not HLA C, but another gene in linkage disequilibrium (LD) with it [21]. Although several studies have reported a higher frequency of HLA Cw6 in PsA patients compared to controls [22–33], only a few have compared allele frequencies between PsA, psoriasis and controls [22, 24, 26, 31]. These studies suggest that the primary association to the gene is with psoriasis rather than PsA. Case control investigations of HLA have found that, although B13, DR7, and B57 are all associated with PsA, the association may not be independent of psoriasis and may be due to LD across the region with Cw6[22, 24, 25]. One study investigating linkage in affected sibling pair families with psoriasis has suggested that linkage to the HLA gene region is stronger in those families without joint involvement, although no attempt was made to make a diagnosis of PsA [18]. This study raises the hypothesis, however, that HLA may not contribute significant additional susceptibility to PsA over psoriasis alone. Conversely, there have been reports of association with PsA to the HLA B locus, particularly with B7 and B27, independent of psoriasis [22, 31, 34]. The association with B27 is particularly strong in PsA patients with sacroiliitis, but it has also been detected in patients with distal interphalangeal joint involvement, suggesting that the HLA-B27 association may not simply reflect the co-incidence of ankylosing spondylitis with psoriasis [31]. HLA-DR4 has been associated with a peripheral symmetrical arthritis suggesting an overlap with RA susceptibility loci [24]. HLA B38 and B39 have both been associated with PsA, and in one study the association of B38 was independent of psoriasis [26], but neither of these is in LD with Cw6[23, 24, 26, 28, 30]. They may, however, be in LD with a susceptibility gene mapping close by.
Non-HLA genes mapping to the MHC region
Other investigators have examined the possibility that a non-HLA gene, mapping to the MHC region of chromosome 6p, may be a PsA susceptibility gene. MHC chain-related gene A (MICA) is considered to be a candidate gene because it is in LD with HLA B alleles and may, therefore, explain the association to these alleles. An association of MICA to PsA has been reported to occur independently of psoriasis [35] and a higher frequency of the trinucleotide repeat MICA-A9 polymorphism in PsA patients compared to controls has been replicated in a separate population [36]. Even though the sample sizes in these studies were small, this replication of findings suggests that the association may be real. The tumour necrosis factor-α gene (TNF-α) also maps close to the HLA B locus and is a strong candidate gene because levels are known to be increased in patients with psoriasis [37]. An association has been found between haplotypes of microsatellite markers mapping close to the TNF-α gene and PsA, independently of psoriasis and independently of HLA Class I associations. An association with a promoter polymorphism (-308), independent of the microsatellite haplotype association, was also detected, leading the authors to speculate that more than one susceptibility locus maps to the region [38]. The -238 TNF-α promoter polymorphism has previously been associated with both juvenile-onset psoriasis and PsA [39]. Two other studies, however, have failed to replicate these findings. One was a Japanese study of 20 PsA patients and 87 population controls [40] and the other included 52 Jewish PsA patients and 73 controls [36]. Both studies were, therefore, considerably underpowered to exclude an association. No association between low molecular protein (LMP) 2 or 7 polymorphisms [41] and PsA has been detected, whilst the transporter associated with antigen processing 1 (TAP1) *0101 allele has been associated with psoriasis but not with PsA [42].
Non-HLA genes mapping outside the MHC region
No evidence for association of T-cell receptor gene polymorphism and either psoriasis or PsA [25] has been reported. Studies investigating association with the immunoglobulin gene heavy chain have yielded conflicting results: in a study of English patients an association was found with PsA but not with psoriasis [43], but in Italian patients the converse was reported [44]. The explanation for the apparent contradiction is likely to relate to the small numbers of patients investigated. This is a common problem in most of the case control studies reported to date. Small sample sizes result in low power to detect an association and an increased chance of detecting spurious associations due to type I error. PsA is a particularly difficult disease to study because the clinical manifestations are so heterogenous. Large sample sizes with sufficient numbers to allow stratification of patients into more homogenous subsets, whilst still retaining power to detect an association, are required, but most studies to date have been performed on small series of patients.
Future directions
Knowledge of other aspects of PsA pathogenesis may help to identify candidate genetic susceptibility factors for future investigation. For example, PsA, like psoriasis, exhibits excessive paternal transmission, so evidence of genomic imprinting exhibited by a putative susceptibility gene would make it a stronger candidate [45].
Comparing RA to PsA, many of the differences observed appear to be quantitative rather than qualitative. PsA synovium does, however, have increased vascularity and fibrosis. E-selectin expression is also markedly reduced in PsA and, while interleukin-2 is absent from RA synovium, it is detectable in PsA synovium [46]. Genes encoding these factors are, therefore, potential candidate PsA susceptibility genes.
Linkage to the long arm of chromosome 17 (17q25) [13, 18] and the short arm of chromosome 6 (6p) [13–19] have been replicated in psoriasis-affected sibling pair families. Examination of genes mapping to these regions may reveal evidence for a unique genetic contribution to PsA. For example, the linkage to 17q25 has been found to be stronger in psoriasis families with joint complaints (again, no attempt was made to make a formal diagnosis of PsA in these families) [18]. Recently, this region has been linked to RA by two independent groups, which suggests that a general arthritis susceptibility locus may map to the region [47, 48]. Linkage to the pericentric region of chromosome 16 has been reported in Crohn's disease [49], psoriasis [20] and RA [48], again suggesting that a generalised arthritis susceptibility locus may map to this region.
Recent work showing response of PsA patients to treatment with soluble recombinant TNF receptor (TNFR)-2 fusion protein lends support for a role for this pathway in PsA pathogenesis [50]. Although association to TNF-α polymorphism has been demonstrated, polymorphism in other genes involved in this pathway (e.g. TNFR1, TNFR2, TNF cleavage enzyme gene [TACE]) may predispose some groups of patients to susceptibility and are worthy of investigation.
Conclusion
The investigation of PsA is challenging, not only because it represents a 'disease within a disease' (PsA within psoriasis), but also because neither a disease definition nor classification criteria have yet been universally agreed upon. Furthermore, there remains an ongoing debate regarding the features that distinguish PsA from other inflammatory arthropathies that may coincide with the presence of psoriasis. There is evidence for a strong genetic contribution to PsA, which may be greater than that for RA or psoriasis alone but few studies have attempted to identify genetic susceptibility factors; those which have consistently demonstrate an association between HLA and PsA, but it is unclear whether the primary association is between psoriasis and the major psoriasis susceptibility locus Cw6. Association with 2 genes, MICA and the TNF-α locus (which map within MHC but which are not classical HLA genes) has now been replicated, independently of HLA Class I associations. Studies of other candidate genes have either not shown association or demonstrated association, which could not then be replicated. The majority of studies have used a case control design, but sample sizes have generally been small and the studies were under-powered. Further research into this fascinating area is required but, in attempting to study the genetics of PsA, it is important to control carefully for psoriasis and its known genetic factors. It is also important to study genes that are potentially associated with other inflammatory arthropathies, to determine whether unique PsA susceptibility factors exist.
Abbreviations
- λs:
-
= sibling recurrence risk
- HLA:
-
= human leukocyte-associated antigen
- IA:
-
= inflammatory arthritis
- kb:
-
= kilobase
- LD:
-
= linkage disequilibrium
- MHC:
-
= major histocompatibility complex
- MICA :
-
= MHC chain-related gene A
- PsA:
-
= psoriatic arthritis
- RA:
-
= rheumatoid arthritis
- TNF:
-
= tumour necrosis factor
- TNFR:
-
= tumour necrosis factor receptor.
References
Moll JMH, Wright V: Psoriatic arthritis. Semin Arthritis Rheum. 1973, 3: 55-78. 10.1016/0049-0172(73)90035-8.
Cats A: Psoriasis and arthritis. Cutis. 1990, 46: 323-329.
Kay LJ, Parry-James JE, Walker DJ: The prevalence and impact of psoriasis and psoriatic arthritis in the primary care population in North East England [abstract]. Arthritis Rheum. 1999, 42: s299-10.1002/1529-0131(199902)42:2<299::AID-ANR12>3.0.CO;2-R.
Harrison BJ, Silman AJ, Barrett EM, Scott DGI, Symmons DPM: Presence of psoriasis does not influence the presentation or short-term outcome of patients with early inflammatory polyarthritis. J Rheumatol. 1997, 24: 1744-1747.
Hellgren L: Association between rheumatoid arthritis and pso-riasis in total populations. Acta Rheumatol Scand. 1969, 15: 316-326.
Baker H: Prevalence of psoriasis in polyarthritic patients and their relatives. Ann Rheum Dis. 1966, 25: 229-234.
McGonagle D, Conaghan PG, Emery P: Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum. 1999, 42: 1080-1086. 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7.
Gladman DD: Psoriatic arthritis. In Ballieres Clinical Rheumatology. Classification and assessment of rheumatic disease: Part 1. Edited by: Silman AJ, Symmons DPM. 1995, London: Balliere Tindall, 319-329.
Fournie B, Crognier L, Arnaud C, Zabraniecki L, Lascaux-Lefebvre V, Marc V, Ginesty E, Andrieu V, Dromer C, Fournie A: Proposed classification criteria of psoriatic arthritis: a preliminary study in 260 patients. Rev Rhum Engl Ed. 1999, 66: 446-456.
Barton AC, Bruce IN, Silman AJ: Genetic studies of psoriatic arthritis: dissecting joints and skin. J Rheumatol. 2001, 28: 3-5.
Bruce IN, Silman AJ: The aetiology of psoriatic arthritis. Rheumatology. 2001, 40: 363-366. 10.1093/rheumatology/40.4.363.
Vyse TJ, Todd JA: Genetic analysis of autoimmune disease. Cell. 1996, 85: 311-318.
Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, Westphal E, Guo SW, Christophers E, Voorhees JJ, Elder JT: Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel regions (16q and 20p) by genome-wide scan. Hum Mol Genet. 1997, 6: 1349-1356. 10.1093/hmg/6.8.1349.
Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, Barker JN: Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by two stage genome-wide search in psoriasis. Hum Mol Genet. 1997, 6: 813-820. 10.1093/hmg/6.5.813.
Burden AD, Javed S, Bailey M, Hodgins M, Connor M, Tillman D: Genetics of psoriasis: paternal inheritance and a locus on chromosome 6p. J Invest Dermatol. 1998, 110: 958-960. 10.1046/j.1523-1747.1998.00213.x.
Capon F, Novelli G, Semprini S, Clementi M, Nudo M, Vultaggio P, Mazzanti C, Gobello T, Botta A, Fabrizi G, Dallapiccola B: Searching for psoriasis susceptibility genes in Italy: genome-scan and evidence for a new locus on chromosome 1. J Invest Dermatol. 1999, 112: 32-35. 10.1046/j.1523-1747.1999.00471.x.
Balendran N, Clough RL, Arguello JR, Barber R, Veal C, Jones AB, Rosbotham JL, Little AM, Madrigal A, Barker JN, Powis SH, Trembath RC: Characterization of the major susceptibility region for psoriasis at chromosome 6p21.3. J Invest Dermatol. 1999, 113: 322-328. 10.1046/j.1523-1747.1999.00710.x.
Samuelsson L, Enlund F, Torinsson A, Yhr M, Inerot A, Enerback C, Wahlstrom J, Swanbeck G, Martinsson T: A genome-wide search for genes predisposing to familial psoriasis by using a stratification approach. Hum Genet. 1999, 105: 523-529. 10.1007/s004390051141.
Lee YA, Ruschendorf F, Windemuth C, Schmitt-Egenolf M, Stadelmann A, Nurnberg G, Stander M, Wienker TF, Reis A, Traupe H: Genomewide scan in German families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. Am J Hum Genet. 2000, 67: 1020-1024. 10.1086/303075.
Enerback C, Martinsson T, Inerot A, Wahlstrom J, Enlund F, Yhr M, Swanbeck G: Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997, 77: 273-276.
Nair RP, Stuart P, Henseler T, Jenisch S, Chia NVC, Westphal E, Schork NJ, Kim J, Lim HW, Christophers E, Voorhees JJ, Elder JT: Localization of psoriasis-susceptibility locus PSOR1 to a 60-kb interval telomeric to HLA-C. Am J Hum Genet. 2000, 66: 1833-1844. 10.1086/302932.
Gladman DD, Anhorn KA, Schachter RK, Mervat H: HLA antigens in psoriatic arthritis. J Rheumatol. 1986, 13: 586-592.
Beaulieu AD, Roy R, Mathon G, Morissette J, Latulippe L, Lang JY, Mathieu JP, Brunet D, Hebert J, Archambault H: Psoriatic arthritis: risk factors for patients with psoriasis – a study based on histocompatibility antigen frequencies. J Rheumatol. 1983, 10: 633-636.
Murray C, Mann DL, Gerber LN, Barth W, Perlmann S, Decker JL, Nigra TP: Histocompatibility alloantigens in psoriasis and psoriatic arthritis. Evidence for the influence of multiple genes in the major histocompatibility complex. J Clin Invest. 1980, 66: 670-675.
Sakkas LI, Loqueman N, Bird H, Vaughan RW, Welsh KI, Panayi GS: HLA class II and T cell receptor gene polymorphisms in psoriatic arthritis and psoriasis. J Rheumatol. 1990, 17: 1487-1490.
Espinoza LR, Vasey FB, Oh JH, Wilkinson R, Osterland CK: Association between HLA-BW38 and peripheral psoriatic arthritis. Arthritis Rheum. 1978, 21: 72-75.
Lopez-Larrea C, Torre Alonso JC, Rodriguez Perez A, Coto E: HLA antigens in psoriatic arthritis subtypes of a Spanish population. Ann Rheum Dis. 1990, 49: 318-319.
Gladman DD, Farewell VT: The role of HLA antigens as indicators of disease progression in psoriatic arthritis. Arthritis Rheum. 1995, 38: 845-850.
Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C: Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol. 1991, 30: 245-250.
Trabace S, Cappellacci S, Ciccarone P, Liaskos S, Polito R, Zorzin L: Psoriatic arthritis: a clinical, radiological and genetic study of 58 Italian patients. Acta Derm Venereol Suppl (Stockh). 1994, 186: 69-70.
Armstrong RD, Panayi GS, Welsh KI: Histocompatibility antigens in psoriasis, psoriatic arthropathy, and ankylosing spondylitis. Ann Rheum Dis. 1983, 42: 142-146.
Gladman DD, Cheung C, Ng CM, Wade JA: HLA-C locus alleles in patients with psoriatic arthritis (PsA). Hum Immunol. 1999, 60: 259-261. 10.1016/S0198-8859(98)00123-2.
Gladman DD, Farewell VT, Kopciuk KA, Cook RJ: HLA markers and progression in psoriatic arthritis. J Rheumatol. 1998, 25: 730-733.
O'Donnell BF, O'Loughlin S, Codd MB, Powell FC: HLA typing in Irish psoriatics. Irish Med J. 1993, 86: 65-67.
Gonzalez S, Martinez-Borra J, Torre-Alonso JC, Gonzalez-Roces S, Sanchez del Rio J, Rodriguez Perez A, Brautbar C, Lopez-Larrea C: The MICA-A9 triplet repeat polymorphism in the transmembrane region confers additional susceptibility to the development of psoriatic arthritis and is independent of the association of Cw*0602 in psoriasis. Arthritis Rheum. 1999, 42: 1010-1016. 10.1002/1529-0131(199905)42:5<1010::AID-ANR21>3.0.CO;2-H.
Gonzalez S, Brautbar C, Martinez-Borra J, Lopez-Vazquez A, Segal R, Blanco-Gelaz MA, Enk CD, Safriman C, Lopez-Larrea C: Polymorphism in MICA rather than HLA-B/C genes is associated with psoriatic arthritis in the Jewish population. Hum Immunol. 2001, 62: 632-638. 10.1016/S0198-8859(01)00242-7.
Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD: Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994, 96: 146-151.
Hoehler T, Grossann S, Stradmann-Bellingghausen B, Kaluza W, Reuss E, De Vlam K, Veys E, Maerker-Hermann E: Differential association of polymorphism in the TNF-alpha region with psoriatic arthritis but not psoriasis. Arthritis Rheum. 2000, 43 (suppl): s265-
Hoehler T, Kruger A, Schneider PM, Schopf RE, Knop J, Rittner C, Meyer zum Buschenfelde KH, Maerker-Hermann E: A TNF-alpha promoter polymorphism is associated with juvenile onset psoriasis and psoriatic arthritis. J Invest Dermatol. 1997, 109: 562-565.
Hamamoto Y, Tateno H, Ishida T, Muto M: Lack of association between promoter polymorphism of the tumor necrosis factor-alpha gene and psoriatic arthritis in Japanese patients. J Invest Dermatol. 2000, 115: 1162-1164. 10.1046/j.1523-1747.2000.0202a-5.x.
Hoehler T, Schneider PM, Rittner C, Hasenclever P, Meyer zum Buschenfelde KH, Maerker-Hermann E: LMP polymorphisms do not influence disease expression in psoriatic arthritis. Clin Exp Rheumatol. 1996, 14: 661-664.
Hoehler T, Weinmann A, Schneider PM, Rittner C, Schopf RE, Knop J, Hasenclever P, Meyer zum Buschenfelde KH, Maerker-Hermann E: TAP-polymorphisms in juvenile onset psoriasis and psoriatic arthritis. Hum Immunol. 1996, 51: 49-54. 10.1016/S0198-8859(96)00156-5.
Sakkas LI, Demaine AG, Panayi GS, Welsh KI: Arthritis in patients with psoriasis is associated with an immunoglobulin gene polymorphism. Arthritis Rheum. 1988, 31: 276-278.
Sakkas LI, Marchenosi A, Kerr LA, Ranza R, Colombo B, Welsh KI, Panayi GS: Immunoglobulin heavy chain gene polymorphisms in Italian patients with psoriasis and psoriatic arthritis. Brit J Rheumatol. 1991, 30: 449-450.
Rahman P, Schentag CT, Gladman DD: Excessive paternal transmission in psoriatic arthritis. Arthritis Rheum. 1999, 42: 1228-1231. 10.1002/1529-0131(199906)42:6<1228::AID-ANR20>3.0.CO;2-3.
Wong WM, Howell WM, Coy SD, Cawley MI, Smith JL: Inter-leukin-2 is found in the synovium of psoriatic arthritis and spondyloarthritis, not in rheumatoid arthritis. Scand J Rheumatol. 1996, 25: 239-245.
Barton A, Eyre S, Myerscough A, Brintnell B, Ward D, Ollier WER, Lorentzen JC, Klareskog L, Silman A, John S, The Arthritis and Rheumatism Campaign National Repository, Worthington J: High resolution linkage and association mapping identifies a novel rheumatoid arthritis susceptibility locus homologous to one linked to two rat models of inflammatory arthritis. Hum Mol Genet. 2001, 10: 1901-1906. 10.1093/hmg/10.18.1901.
Jawaheer D, Seldin MF, Amos CI, Chen WV, Shigeta R, Monteiro J, Kern M, Criswell LA, Albani S, Nelson JL, Clegg DO, Pope R, Schroeder HW, Bridges SL, Pisetsky DS, Ward R, Kastner DL, Wilder RL, Pincus T, Callahan LF, Flemming D, Wener MH, Gregersen PK: A genomewide screen in multiplex rheumatoid arthritis families suggests genetic overlap with other autoim-mune diseases. Am J Hum Genet. 2001, 68: 927-936. 10.1086/319518.
Cavanaugh J: International collaboration provides convincing linkage replication in complex disease through analysis of a large pooled data set: Crohn's disease and chromosome 16. Am J Hum Genet. 2001, 68: 1165-1171. 10.1086/320119.
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ: Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000, 356: 385-390. 10.1016/S0140-6736(00)02530-7.
Acknowledgements
Dr A Barton is in receipt of an MRC Clinical Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barton, A.C. Genetic epidemiology: Psoriatic arthritis. Arthritis Res Ther 4, 247 (2002). https://doi.org/10.1186/ar415
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar415