Abstract
Introduction
Disease remission has become a feasible goal for most rheumatoid arthritis (RA) patients; however, patient-reported symptoms, such as pain, may persist despite remission. We assessed the prevalence of pain in RA patients in remission according to the Disease Activity Score (DAS28-CRP4) and the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) remission criteria.
Methods
Data were analyzed from RA patients in the Brigham Rheumatoid Arthritis Sequential Study with data at baseline and 1 year. DAS28 remission was defined as DAS28-CRP4 <2.6. The ACR/EULAR remission criteria included (a) one or more swollen joints, (b) one or more tender joints, (c) C-reactive protein ≤1 mg/dl, and (d) patient global assessment score ≤1. Pain severity was measured by using the pain score from the Multi-Dimensional Health Assessment Questionnaire (MDHAQ). The associations between baseline clinical predictors and MDHAQ pain at baseline and 1 year were assessed by using multivariable linear regression.
Results
Among the 865 patients with data at baseline and 1 year, 157 (18.2%) met DAS28-CRP4 remission criteria at both time points. Thirty-seven (4.3%) met the ACR/EULAR remission criteria at baseline and 1 year. The prevalence of clinically significant pain (MDHAQ pain ≥4) at baseline ranged from 11.9% among patients meeting DAS28-CRP4 remission criteria to none among patients meeting ACR/EULAR remission criteria. Patient global assessment, MDHAQ function, MDHAQ fatigue, MDHAQ sleep, and arthritis self-efficacy were significantly associated with MDHAQ pain in cross-sectional (P ≤ 0.0005) and longitudinal analyses (P ≤ 0.03). Low swollen-joint counts were associated with high MDHAQ pain in longitudinal analyses (P = 0.02) but not cross-sectional analyses. Other measures of inflammatory disease activity and joint damage were not significantly associated with MDHAQ pain at baseline or at 1 year.
Conclusions
Clinically significant pain continues among a substantial proportion of patients in DAS28 remission but not among those in ACR/EULAR remission. Among patients in DAS28 remission, patient global assessment, disability, fatigue, sleep problems, and self-efficacy are strongly associated with pain severity at baseline and 1 year, whereas inflammatory disease activity and joint damage are not significantly associated with elevated pain severity at either baseline or 1 year.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes significant pain and disability. Sixty-eight percent of RA patients rate pain as their highest priority for improvement, and 90% of RA patients rate pain as one of their top three priorities [1]. With the earlier initiation of disease-modifying therapies and the development of targeted immunomodulating agents [2], an increasing number of RA patients are able to achieve minimal disease activity, and some are able to achieve complete disease remission [3–5]. However, on a population level, mean pain ratings have remained the same over the past 20-year period [6], and 82% of RA patients who consider their disease to be "somewhat to completely controlled" continue to report moderate to severe pain levels [7].
In a study of Disease Activity Score (DAS28) remission in the Arthritis and Rheumatology Clinic of Kansas and the Rheumatoid Arthritis Evaluation Study databases, mean and median pain scores were 2.43 and 1.50 on a 0-to-10 visual analogue pain scale [8]. Although these values were relatively low, they were higher than the cut-point of 1.25, which separated patients who were satisfied with their health from those who were not. This observation indicated that, even among patients in DAS28 remission, many still have enough pain to negatively affect health satisfaction. It was not clear whether this pain was due to residual inflammatory disease activity or whether this pain was not inflammatory.
Presented with a patient with tender joints and/or pain but no other evidence for inflammatory disease, physicians must decide whether to escalate or add other disease-modifying therapy. If they decide not to increase immunomodulation, they are left with the dilemma of how to address the pain. Currently, very little data exist to guide this treatment decision. The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) consensus conference for the definition of remission recognized the potential impact of non-inflammatory causes of pain and called for studies to investigate the role of non-RA pain among patients in remission [9].
In this article, we examine the prevalence of clinically significant pain among RA patients in remission according to the DAS28-CRP4 and the ACR/EULAR criteria. Among patients who met the DAS28-CRP4 criterion for remission, we also assess whether pain severity is associated with inflammation or other factors, such as fatigue and sleep problems, which may be indicative of a non-inflammatory chronic widespread pain syndrome.
Materials and methods
Study population
The primary cohort includes the 157 RA patients in the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study (BRASS) who met DAS28-CRP4 remission criteria [10, 11] at both study entry and 1-year follow-up. BRASS is an observational, hospital-based cohort of 1,118 individuals with established RA. Inclusion criteria include age 18 years or older and a diagnosis of RA made by a board-certified rheumatologist. Additional details can be found in a past publication [12]. The Partners Institutional Review Board approved the study, and written informed consent was obtained from all participants.
Remission definitions
The DAS28-CRP4 was calculated from the tender-joint count, swollen-joint count, C-reactive protein (CRP), and patient global assessment [13]. DAS28-CRP4 remission was defined as a score <2.6 [10]. To satisfy the ACR/EULAR remission criteria, participants had to have: 1) ≤1 swollen joint, 2) ≤1 tender joint, 3) CRP ≤1 mg/dl, and 4) patient global assessment score ≤1 (of 10) [14]. The patient global score was taken from the Multi-Dimensional Health Assessment Questionnaire (MDHAQ) item stating, "Considering all the ways that your illness affects you, rate how you are doing."
Study procedures
Participants provided demographic and clinical information, including detailed lists of comorbidities and medication histories. A modified Charlson comorbidity index was constructed by using available data [15]. Hemiplegia and peripheral vascular disease were omitted from the index because these data were not collected. We also did not have data to distinguish between mild liver disease and moderate/severe liver disease, uncomplicated diabetes versus diabetes with end-organ damage, and metastatic versus nonmetastatic tumors. In these cases, point values were assigned based on the less-severe condition (for example, all cases of liver disease were assigned 1 point; all cases of diabetes were assigned 1 point; all tumors were assigned 2 points).
Samples of blood were collected for a standard laboratory panel, including the CRP. Baseline hand radiographs were obtained, and Sharp scores were calculated based on joint-space narrowing and erosions. A physician performed a 28-joint count and assessment of disease activity. Participants also completed the MDHAQ, Mental Health Index-5 (MHI5) and the Arthritis Self Efficacy Scale (ASES). The MDHAQ is a validated instrument that includes assessments of pain, fatigue, and function, scored on 0-to-10 scales (high scores indicate more pain, more fatigue, and poor function) [16]. Specifically, the MDHAQ asks, "How much pain have you had because of your illness in the past week?" and "How much of a problem has fatigue or tiredness been for you in the past week?" The MDHAQ also includes a question regarding sleep problems, scored on a 0-to-3 scale (high scores indicate more sleep disturbances). The MHI5 is a validated five-item assessment of mental health, scored on a 1-to-100 scale (high scores indicate good mental health). The ASES is scored on a 10-to-100 scale (high scores indicate more self-efficacy) [17].
Statistical analyses
Means and standard deviations (SDs) were calculated for normally distributed variables. Median values and interquartile ranges (IQRs) were determined for variables that were not normally distributed. Continuous independent variables were categorized based on tertiles.
To examine the prevalence of pain, clinically significant pain was defined as an MDHAQ pain score ≥4. This threshold was determined based on literature reports requiring pain-severity levels ≥4 of 10 or 40 of 100 for inclusion in clinical trials of pain medications to treat painful conditions, including arthritis [18].
Multivariable associations between baseline clinical variables and baseline MDHAQ pain score were examined by using multivariable linear regression. All multivariable models were adjusted for age and gender. The same methods were used to examine the longitudinal relation between baseline clinical variables and 1-year pain severity, except, in the primary multivariable analyses, baseline MDHAQ pain score was also included as a covariate. Secondary analyses were also conducted, adjusting for age and gender but excluding baseline MDHAQ pain score as a covariate. All statistical analyses were performed by using the SAS 9.2 software package (SAS Institute, Cary, NC, USA).
Results
At baseline, the prevalence of DAS28-CRP4 remission was 24.9%, and the prevalence of ACR/EULAR remission was 8.8% among the 1,118 individuals in the BRASS cohort. The prevalence of remission did not differ significantly between those with 1-year follow-up data (25.3% met DAS28-CRP4 criteria; 8.6% met ACR/EULAR criteria) and those who were lost to follow-up (17.1% met DAS28-CRP4 criteria; 13.0% met ACR/EULAR criteria).
Among the 865 patients with both baseline and 1-year data, 157 (18.2%) patients remained in sustained DAS28-CRP4 remission at both baseline and 1 year (Figure 1). Thirty-seven (4.3%) also met ACR/EULAR criteria for remission at baseline and 1 year. All patients who met ACR/EULAR revised criteria for remission also met DAS28-CRP4 remission criteria.
Study disposition. Flow diagram of the number of participants included in this study. ACR, American College of Rheumatology; BRASS, Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study; DAS28, disease activity score in 28 joints; DAS28-CRP4, disease activity score in 28 joints calculated by using CRP and patient global assessment; EULAR, European League Against Rheumatism.
Baseline characteristics of patients in sustained remission
Among the 157 patients in sustained DAS28-CRP4 remission, mean age was 51.8 years; 135 (86.0%) were female patients (Table 1). Median disease duration was 6 years (IQR 2.0, 17.0), and 113 (72.4%) were seropositive. The median MHI5 score was 80.0 (IQR 70.0, 90.0) on a scale of 0 to 100, with 100 being perfect mental health. The median MDHAQ fatigue score was 2.5 (IQR 1.0, 5.0) on a scale of 0 to 10, with 10 being severe fatigue. Eighty-two participants (52.2%) were taking methotrexate; 71 (45.2%) were taking a biologic disease-modifying antirheumatic drug (DMARD), and 22 (14.0%) were taking a nonmethotrexate, nonbiologic DMARD. Thirty-six (22.9%) were taking a combination of methotrexate and a biologic DMARD. Four (2.6%) were taking a combination of methotrexate and a nonmethotrexate, nonbiologic DMARD, and seven (4.5%) were taking a combination of a biologic DMARD and a nonmethotrexate, nonbiologic DMARD. Eighty (51.0%) were taking nonsteroidal anti-inflammatory drugs, eight (5.1%) were taking opioid pain medications, and 16 (10.2%) were taking other pain medications (for example, tramadol and acetaminophen).
Pain severity at baseline and 1 year
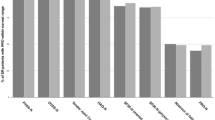
Among patients in sustained DAS28-CRP4 remission, the distribution of MDHAQ pain scores was skewed at baseline and 1 year, with the majority of patients having MDHAQ pain scores ≤2 (Figure 2). At baseline, 11.9% had MDHAQ pain scores ≥4, and 12.5% had MDHAQ pain scores ≥4 at 1 year. None of the 37 patients in sustained ACR remission had a MDHAQ pain rating ≥4 at baseline, and only two (5.7%) had MDHAQ pain ratings ≥4 at 1 year.
Distribution of MDHAQ pain scores at baseline and 1 year. The numbers depicted are among (a) RA patients in DAS28-CRP4 remission (n = 157), and (b) RA patients in ACR/EULAR remission (n = 37). ACR, American College of Rheumatology; DAS28-CRP4, disease activity score in 28 joints calculated by using CRP and patient global assessment; EULAR, European League Against Rheumatism; MDHAQ, Multi-Dimensional Health Assessment Questionnaire; RA, rheumatoid arthritis.
Cross-sectional associations between baseline clinical variables and baseline pain
In multivariable analyses adjusted for age and gender, baseline patient global assessment ratings (P < 0.0001) and MDHAQ function scores (P < 0.0001) were the only disease-related variables significantly associated with high baseline MDHAQ pain scores (Table 2). Swollen-joint count, tender-joint count, and CRP were not significantly associated with MDHAQ pain scores. Sleep and psychosocial variables, including the baseline MDHAQ fatigue score (P < 0.0001) and the MDHAQ sleep-problems score (P = 0.0005) were strongly associated with high baseline MDHAQ pain scores. Lower ASES scores (P = 0.0005) and MHI scores (P = 0.004) were also significantly associated with high baseline MDHAQ pain scores.
Longitudinal analyses examining associations between baseline clinical variables and pain at 1 year
In multivariable analyses adjusted for age, gender, and baseline MDHAQ pain score, baseline MDHAQ function score (P = 0.008), disease duration (P = 0.02), and patient global assessment (P = 0.03) were the only disease-related variables positively associated with MDHAQ pain scores at 1 year. Baseline swollen-joint count was negatively associated with MDHAQ pain scores at 1 year (P = 0.02). Baseline MDHAQ fatigue score (P = 0.0001), MDHAQ sleep-problems score (P = 0.001), and ASES score (P = 0.01) were all significantly associated with 1-year MDHAQ pain score (Table 3). In secondary analyses, excluding baseline MDHAQ pain score as a covariate, baseline patient global assessment, baseline MDHAQ function score, baseline MDHAQ fatigue score, baseline MDHAQ sleep-problems score and baseline ASES score all remained significantly associated with 1-year MDHAQ pain score (P < 0.0001).
Discussion
Among patients in sustained DAS28-CRP4 remission for longer than 1 year, the prevalence of clinically significant pain was 11.9% at baseline and 12.5% at 1 year. No markers of inflammatory activity were associated with increased pain severity at baseline or 1 year. Patient global assessment, disability, fatigue, sleep problems, and self-efficacy were strongly associated with pain at both baseline and 1 year.
Although rheumatologists and patients typically think of RA remission as a painless state [19–21], our study shows that remission does not always equal a pain-free state. These findings are significant because pain is considered the most important patient-reported outcome in rheumatology [22], yet it persists in >10% of patients who are classified in remission by DAS28-CRP4 criteria.
The cause of pain in these patients may arise from inflammation, structural joint damage, and/or non-disease-related factors. Our data suggest that inflammation and structural joint damage are not major causes of pain, because CRP, swollen-joint count, tender-joint count, and Sharp scores were not significantly associated with increased pain severity at baseline or 1 year. However, patient global assessment and MDHAQ function scores were strongly associated with increased pain severity at baseline and 1 year. Disease duration was also significantly associated with pain severity at 1 year, although not at baseline.
The patient global assessment and MDHAQ function scores are often interpreted as measures of RA-related joint inflammation and damage, but these measures also reflect a wide range of non-inflammatory factors. Other studies have reported that the explained variance in patient global scores by inflammation is low (6.7%) [23], and MDHAQ function scores are elevated in a wide range of other conditions, including fibromyalgia, a non-inflammatory condition that is not associated with joint damage [24]. RA patients with fibromyalgia have significantly worse physical function compared with RA patients without fibromyalgia, even though sedimentation rates are only marginally higher [25]. Given these observations and the lack of association between Sharp scores, CRP, swollen-joint count, tender-joint count, and high MDHAQ pain scores, we believe that the associations between patient global assessment/MDHAQ function and MDHAQ pain may be attributed to non-RA-related factors, and that the likelihood that inflammation and joint damage are major contributors to pain is low.
The association between disease duration and 1-year MDHAQ pain may reflect joint damage that accrues with increasing disease duration, or it may reflect time-dependent changes in pain processing that lead to a hyperalgesic state. However, disease duration was not significantly associated with baseline MDHAQ pain. This finding weakens the argument that disease duration acts as a proxy for joint damage and raises the possibility that the association between disease duration and 1-year MDHAQ pain may be attributable to chance. Alternatively, these observations are compatible with the hypothesis that increased pain is due to a hyperalgesic state, such as fibromyalgia, that is established and/or enhanced between the baseline and 1-year visits.
The strong association between baseline fatigue, sleep problems, poor self-efficacy, and baseline and 1-year pain severity also supports the existence of an enhanced, non-inflammatory pain state among these patients with pain despite DAS28-CRP4 remission. Fatigue, sleep problems, and poor self-efficacy are part of a noninflammatory symptom cluster associated with "symptom intensification" syndromes [26–28]. These syndromes encompass conditions such as fibromyalgia, which manifest with a broad range of symptoms, including widespread pain, fatigue, sleep problems, and cognitive difficulties. The etiology of these syndromes is unclear but may be associated with deficits in central pain-processing mechanisms, such as central sensitization, that result in hyperalgesia and allodynia [26]. Thus, therapeutic strategies targeted toward correcting central pain mechanisms and improving sleep and self-efficacy may be more effective at decreasing pain than strategies focused on intensifying disease-modifying therapies.
Limitations of this study include the use of the DAS28-CRP4 threshold of 2.6 as the primary criterion for remission. Although the DAS28-CRP4 remission criterion is the most commonly used definition for remission, it is not a perfect measure of inflammatory disease activity. Critics have noted that the DAS28-CRP4 remission threshold of 2.6 permits patients to have multiple tender and swollen joints [29]. In this study, the maximum number of tender joints was three, and the maximum number of swollen joints was 10, confirming these assertions. However, neither tender-joint count nor swollen-joint count was significantly associated with pain severity in cross-sectional analyses. In longitudinal analyses, swollen-joint count was associated with low pain levels, rather than high pain levels. Together with the finding that CRP levels were not significantly associated with pain scores, these observations argue against inflammation as a cause of pain among patients in DAS28-CRP4 remission.
In recognition that the DAS28-CRP4 remission criterion may be too permissive of residual inflammation, we also examined the prevalence of clinically significant pain among patients meeting 2010 ACR/EULAR remission criteria. The prevalence of clinically significant pain in this group was much lower, none at baseline and 5.7% at 1 year. This low prevalence is likely due to the ACR/EULAR criterion limiting the patient global assessment score to ≤1. Because the patient global assessment is heavily influenced by pain [30], this criterion essentially excludes patients with high pain scores. It is not clear whether omitting these patients is appropriate because the ACR/EULAR remission criteria exclude patients with pain due to residual, undetected inflammation or is inappropriate because the ACR/EULAR criteria include patients with non-inflammatory causes of pain. Our data do not allow us to distinguish between these two possibilities because we do not have measurements of joint tenderness and swelling in the feet and ankles. We also do not have radiographic measures to assess subclinical inflammation. Future studies including detailed assessments of inflammation in the lower extremities and radiographic assessments of synovitis are needed to elucidate the relation between inflammation and pain severity among patients in DAS28-CRP4 remission.
An additional limitation of this study is the homogeneity of the study population. The study was conducted at a single academic center, and 93.6% of this population was Caucasian. Other studies have reported higher rates of pain among racial minorities [31], and associations between pain, sleep problems, fatigue, and self-efficacy may differ depending on race. Future studies are needed to evaluate these relationships among different populations.
Conclusions
Pain continues to persist in a substantial subgroup of patients who meet the DAS28-CRP4 threshold for remission, whereas nearly all patients with clinically significant pain are excluded by the ACR/EULAR remission criteria. Among RA patients who meet the DAS28-CRP4 threshold for remission, pain was not associated with inflammatory markers and did not diminish despite continued control of inflammation over a 1-year period. Pain was associated with fatigue, sleep problems, and self-efficacy. These findings stress the importance of assessing alternative causes of pain and modifying therapy to address specific modulators of pain, rather than assuming that pain is an indicator of inflammation that should be treated by increasing immunosuppressive therapies.
Abbreviations
- ACR:
-
American College of Rheumatology
- ASES:
-
Arthritis Self Efficacy Scale
- BRASS:
-
Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study
- CRP:
-
C-reactive protein
- DAS28:
-
disease activity score in 28 joints
- DAS28-CRP4:
-
disease activity score in 28 joints calculated by using CRP and patient global assessment
- DMARD:
-
disease-modifying antirheumatic drug
- EULAR:
-
European League Against Rheumatism
- IQR:
-
interquartile range
- MDHAQ:
-
Multi-Dimensional Health Assessment Questionnaire
- MHI5:
-
Mental Health Index-5
- RA:
-
rheumatoid arthritis
- SD:
-
standard deviation.
References
Heiberg T, Finset A, Uhlig T, Kvien TK: Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann Rheum Dis. 2005, 64: 191-195. 10.1136/ard.2004.022699.
Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A: Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J Rheumatol. 2009, 36: 1611-1617. 10.3899/jrheum.080889.
Keystone E, Genovese MC, Klareskog L, Hsia EC, Hall S, Miranda PC, Pazdur J, Bae SC, Palmer W, Xu S, et al: Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010, 69: 1129-1135. 10.1136/ard.2009.116319.
Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, Dougados M, Baldassare A, Ferraccioli G, Chubick A, et al: Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007, 56: 3896-3908. 10.1002/art.23059.
Pincus T, Sokka T, Kavanaugh A: Relative versus absolute goals of therapies for RA: ACR 20 or ACR 50 responses versus target values for "near remission" of DAS or single measures. Clin Exp Rheumatol. 2004, 22: S50-S56.
Welsing PM, Fransen J, van Riel PL: Is the disease course of rheumatoid arthritis becoming milder? Time trends since 1985 in an inception cohort of early rheumatoid arthritis. Arthritis Rheum. 2005, 52: 2616-2624. 10.1002/art.21259.
Taylor P, Manger B, Alvaro-Gracia J, Johnstone R, Gomez-Reino J, Eberhardt E, Wolfe F, Schwartzman S, Furfaro N, Kavanaugh A: Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res. 2010, 38: 1213-1224.
Wolfe F, Michaud K: Proposed metrics for the determination of rheumatoid arthritis outcome and treatment success and failure. J Rheumatol. 2009, 36: 27-33.
van Tuyl LH, Vlad SC, Felson DT, Wells G, Boers M: Defining remission in rheumatoid arthritis: results of an initial American College of Rheumatology/European League Against Rheumatism consensus conference. Arthritis Rheum. 2009, 61: 704-710. 10.1002/art.24392.
Fransen J, Creemers MC, Van Riel PL: Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford). 2004, 43: 1252-1255. 10.1093/rheumatology/keh297.
Fransen J, van Riel PL: DAS remission cut points. Clin Exp Rheumatol. 2006, 24: S29-S32.
Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, Solomon DH, Weinblatt ME, Shadick NA: Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford). 2011, 50: 40-46. 10.1093/rheumatology/keq263.
Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel PL: Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009, 68: 954-960. 10.1136/ard.2007.084459.
Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH , Funovits J, Aletaha D, Allart CF, Bathon J, Bombardieri S, et al: American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011, 63: 573-586. 10.1002/art.30129.
Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987, 40: 373-383. 10.1016/0021-9681(87)90171-8.
Pincus T, Swearingen C, Wolfe F: Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999, 42: 2220-2230. 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5.
Lorig K, Chastain RL, Ung E, Shoor S, Holman HR: Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989, 32: 37-44. 10.1002/anr.1780320107.
Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, Bennett RM, Collins H: Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009, 146: 253-260. 10.1016/j.pain.2009.06.024.
Wolfe F, Boers M, Felson D, Michaud K, Wells GA: Remission in rheumatoid arthritis: physician and patient perspectives. J Rheumatol. 2009, 36: 930-933. 10.3899/jrheum.080947.
Soubrier M, Zerkak D, Gossec L, Ayral X, Roux C, Dougados M: Which variables best predict change in rheumatoid arthritis therapy in daily clinical practice?. J Rheumatol. 2006, 33: 1243-1246.
Aletaha D, Smolen JS: Remission of rheumatoid arthritis: should we care about definitions?. Clin Exp Rheumatol. 2006, 24: S45-S51.
Borenstein D, Altman R, Bello A, Chatham W, Clauw DJ, Crofford LJ, Croft J, Hassett A, Kozin F, Pisetsky D, et al: Report of the American College of Rheumatology Pain Management Task Force. Arthritis Care Res (Hoboken). 2010, 62: 590-599.
Kievit W, Welsing PM, Adang EM, Eijsbouts AM, Krabbe PF, van Riel PL: Comment on the use of self-reporting instruments to assess patients with rheumatoid arthritis: the longitudinal association between the DAS28 and the VAS general health. Arthritis Rheum. 2006, 55: 745-750. 10.1002/art.22225.
Pincus T, Sokka T: Can Multi-Dimensional Health Assessment Questionnaire (MDHAQ) and Routine Assessment of Patient Index Data (RAPID) scores be informative in patients with all rheumatic diseases?. Best Pract Res Clin Rheumatol. 2007, 21: 733-753. 10.1016/j.berh.2007.02.006.
Wolfe F, Michaud K: Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol. 2004, 31: 695-700.
Bradley LA, McKendree-Smith NL: Central nervous system mechanisms of pain in fibromyalgia and other musculoskeletal disorders: behavioral and psychologic treatment approaches. Curr Opin Rheumatol. 2002, 14: 45-51. 10.1097/00002281-200201000-00009.
Staud R: Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002, 4: 299-305. 10.1007/s11926-002-0038-5.
Wolfe F, Rasker JJ: The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol. 2006, 33: 2291-2299.
Makinen H, Kautiainen H, Hannonen P, Sokka T: Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis?. Ann Rheum Dis. 2005, 64: 1410-1413. 10.1136/ard.2005.037333.
Lati C, Guthrie LC, Ward MM: Comparison of the construct validity and sensitivity to change of the visual analog scale and a modified rating scale as measures of patient global assessment in rheumatoid arthritis. J Rheumatol. 2010, 37: 717-722. 10.3899/jrheum.090764.
Bruce B, Fries JF, Murtagh KN: Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. J Rheumatol. 2007, 34: 1475-1479.
Acknowledgements
YCL is supported by the NIH (AR 057578) and has received a grant from Forest. DHS receives salary support from the NIH (AR 047782, AR 055989) and the Agency for Healthcare Research and Quality. He has also received grants from Amgen and Abbott and is an unpaid member of committees overseeing pain-medication trials sponsored by Pfizer. The Brigham Rheumatoid Arthritis Sequential Study (BRASS) is supported by Biogen Idec and Crescendo Biosciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
YCL has stock in Merck, Novartis, and Elan Corporation and has a research grant from Forest. NAS receives research grant support from Biogen Idec, Crescendo Biosciences, and Amgen. MEW receives support from Biogen Idec and Crescendo Biosciences. DHS receives research support from Abbott and Amgen. No other authors have competing interests.
Authors' contributions
YCL conceived the hypothesis for the manuscript, participated in data collection, conducted the initial statistical analyses, wrote the first draft of the manuscript, and had primary responsibility for the manuscript process. JC, BL, and MLF contributed to data management and statistical analyses. CKI, NAS, and MEW participated in the design of the study and the interpretation of data. DHS participated in study design and analysis and interpretation of data. All authors critically reviewed, contributed to, and approved the final manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lee, Y.C., Cui, J., Lu, B. et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 13, R83 (2011). https://doi.org/10.1186/ar3353
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar3353