Abstract
All electron density functional theory (DFT) calculations have been carried out for calcium-doped porphyrin-incorporated(5, 5) carbon nanotube (Ca-PICNT) to investigate the formation energies, electronic properties of this system, and its application in hydrogen storage. It is found that the incorporation of porphyrin ring in carbon nanotube led to a decreased value of the highest occupied molecular orbital and lowest unoccupied molecular orbital gap and a strong binding of Ca over nanotube and consequently resulted to a drastic reduction of clustering of Ca atom over Ca-decorated carbon nanotube. The Ca-PICNT can bind a maximum of 4H2 with binding energy value of 0.105 eV per H2 molecule. Charge decomposition analysis indicated that the mode of hydrogen storage is via coulomb-electrostatic force, which is further supported by the natural bond orbital and partial density-of-states studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Carbon nanotubes (CNTs) have become very interesting systems due to their unique structure and electronic properties as well as their diverse applications. Their large surface area is very valuable for sensing gas molecules such as poisonous gas and H2 molecules by means of adsorption. Several groups have identified that metal decorated over CNT can absorb hydrogen non-dissociatively which is the requirement for operation of practical storage media at ambient thermodynamic conditions [1–5]. However, the hydrogen storage capability of metal-dispersed systems is limited due to cohesive interaction of metallic atoms. It has also been reported that the clustering of transition metal atoms can be overcome to a great extent by incorporating doping of boron or nitrogen in the systems such as fullerene, nanotubes, and graphene [6–8]. A range of biological organic molecules such as hemoglobin and chlorophyll in which metals linked with organic molecule are often used for catalysis or carrying gas molecules [9, 10]. It is the strong conjugation between metal ion and surrounding nitrogen atoms that prevent the clustering of metal atom. The dangling bonds of nitrogen acquire stabilization by receiving two s electrons from metal which also result in formation of metal ion. Experimentally, carbon vacancies can be created using high-resolution TEM [11] and proton irradiation technique [12, 13] among which formation of divacancy has been reported preferable over monovacancy [14]. The dangling-bonded carbon atoms near the divacancies are replaced by two N2 molecules from nitrogen gas in the presence of catalyst such as CoMo alloy or iron oxides. Using GGA exchange correlation functional natural Fe-porphyrin is found to adsorb hydrogen molecules with binding energy of 0.3 eV per H2[15]. Synthetic route of incorporating the natural stable porphyrin unit into vacancy-engineered defective graphenes is proposed by Choi et al. [6], and on rigorous evaluation, they found that the binding of H on porphyrin-induced graphene (Fe-PIG) is via Kubas interaction between H and Fe. Very recently, Lee and coworkers [16] synthesized Fe-porphyrin-like carbon nanotube from conventional plasma-enhanced chemical vapor deposition and anticipated it as prominent candidate for hydrogen storage. In continuation of earlier investigation [8], we have explored our study to formation of Ca-porphyrin-induced carbon nanotubes by using DFT. Adsorption of hydrogen molecules over these functionalized nanotubes is also reported.
Model system and computational method
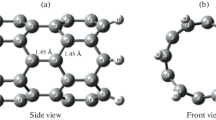
(5,5) CNT terminated with hydrogen atoms (C50H20), shown in Figure 1a, was taken as starting model system. A vacancy was created in CNT by removing two adjacent carbon atoms from its sidewall. It was followed by the substitution of four nearest neighboring carbon atoms by N atoms as shown in Figure 1b. Spin-restricted DFT calculations were carried out using generalized-gradient exchange-correlation PBEPBE as implemented in Gaussian 03 quantum chemical package [17]. Basissets selected for optimization were Gaussian-type orbitals 6–31 g(d,p) for C, N, and H, where as Ca was specified with 6-311++g(d,p) basis set. These specifications cut out the undesirable calculation and focus on the main fragments participating in hydrogen storage. All the structural parameters were fully optimized without any symmetry constraints. For Ca-incorporated porphyrin nanotube in order to confirm that the optimized geometries correspond to a real minimum at the potential energy surface vibrational frequency calculations were performed at the same level of theory. Subsequently, storage of hydrogen on the real minimum optimized structure was then analysed. For partial density-of-states (PDOS) analysis, single point energy calculations performed and implemented as input to AOMIX program [18].
Result and discussion
Incorporation of porphyrin ring in (5,5) CNT
Formation of highly N-doped carbon (CN4) nanotube has been carried out from its predecessor (5, 5). The optimized structure of the model is shown in Figure 1b in which C-N and some C-C bond lengths around the defective site of four nitrogen divacancy CNT (4ND-CNT) structure are also shown. The formation energy for 4ND-CNT structure is defined as Ef = Etot-(ENT + nNμN-6μC), where Etot is total energy of CN4 nanotube and μ is chemical potential, μC and μN being calculated from C in the corresponding pure CNTs and nitrogen in gas phase respectively, nN is number of N atoms, and 6 is the assignednumber of C atoms which are removed from pristine nanotube. The calculated formation energy of CN4 in the (5, 5) CNT is estimated to be 3.1 eV. Thus, nitrogen substitution reaction is thermodynamically favored with exothermic energy. It can be understood in terms of one extra valance electron of the substitutional N that stabilizes the unpaired electron of under-coordinated C atoms. The calculated highest occupied molecular orbital and lowest unoccupied molecular orbital (HOMO-LUMO) gap is reduced to 0.32 eV, which is markedly smaller as compared to pristine (5, 5) CNT having HOMO-LUMO gap of 1.52 eV. It can be understood in terms of the extra electrons provided by dangling nitrogen atom to the system which results in lower the HOMO-LUMO gap. It is pointed out here that the model system is very small. Because of the finite length of nanotube, the HOMO-LUMO gap is larger, and this gap reduces as the length of tube increases and the one with the smallest HOMO-LUMO gap may best represent the metallic character of this nanotube [19–21]. Mulliken charge analysis shows charge of order-0.346e on each of the four nitrogen atoms of 4NDV-CNT as a result this site of nanotube is very reactive and it can act as strong oxidizing agent for divalent metal atoms which is typically observed in organic molecules [9, 10].
Ca-decoratedporphyrin-induced CNT
On dispersing single Ca atom on divacancies nanotube, we found that Ca atom sits on the hollow site of the 4ND structure leading to formation of Ca-porphyrin-like structure. As the frequency calculation revealed zero value for imaginary frequency, the calculation assured this geometry holds local minima at potential energy surface. The adsorbed Ca atom on this structure is buckled out of plane due to its relative large size; the calculated adsorption energy of Ca over the CN4 nanotube is found to be-5.23 eV, which is much larger as compared to decoration of Ca atom over pristine CNT [22]. It thus prevents clustering of Ca atoms over Ca-decorated pristine carbon nanotube. Figure 2 shows the optimized structure of Ca-decorated porphyrin-induced CNT (Ca-PICNT) showing Ca-N bond lengths. After the Ca incorporation over central part of 4NDV, the HOMO-LUMO gap of system is now of the order of 1.41 eV. The strong binding of Ca atom with the nitrogen atoms of CN4 nanotube is further studied in terms of Mulliken and natural bond orbital (NBO) charge analysis. It has been mentioned above that in CN4 nanotube with 4ND structures; metal atoms are coordinated to four nitrogen atoms, which therefore reduce the Lewis acid character of metal atoms. The charges on N45, N46, N47, and N48 predicted by both techniques are negative while the metal charge on Ca atom is positive. We have tabulated the calculated charges obtained by both Mulliken and NBO method for Ca-PICNT in Table 1. The natural charges and Mulliken charges listed in Table 1 show that Ca-N bonds are highly polarized and are responsible for large shift of charge from metal to its neighbors. Without any ambiguity, the difference of charges on central metal atom and attached nitrogen atoms indicate that Ca-N bond are relatively ionic in nature. We further focus on the stabilization energy E(2) calculated from second order perturbation theory analysis of the Fock-matrix. Table 2 enumerates electron donor orbital i, electron acceptor orbital j, and stabilization energy E(2) between their interaction. It is well known that larger is the E(2), the stronger is the interaction between them and the greater the tendency that i provide electron to j, means more charge transferred [20]. It can be seen from Table 2 that the estimated value of E(2) between filled (donors) Lewis-type NBOs (LP(1)N(45), LP(1)N(46), LP(1)N(47), and LP(1)N(48)) and empty(acceptor) non-Lewis NBOs (LP(1)Ca(69)) are very large showing that electron density is significantly delocalized from nitrogen atoms to the metal atoms. In addition, the natural charges and Mulliken charges listed in Table 1 show that Ca-N bonds are highly polarized and are responsible for large shift of charge from metal to its neighbors. Also shown in the Table 2 is the donor-acceptor back-bonding interactions considering the nitrogen and its nearest neighboring carbon atoms from Ca-PICNT tagged with super-script ∑ as the first fragment (donor) and metal atom as the second fragment (acceptor) and viceversa. It is found that there is small back-bonding interaction for Ca which account for the fact that it has large charge distribution and smaller covalent contribution, this also supports the high adsorption energy of Ca.
Hydrogen adsorption on Ca-decoratedporphyrin-induced carbon nanotube
Adsorption of single hydrogen molecule
We further explore the hydrogen storage capacity of Ca-PICNT. The binding energy of single H2 molecule with Ca-PICNT is estimated to be 0.17 eV, indicating that the mode of interaction is strong physisorption. Figure 3 shows the bonddistance of adsorbed H2 with Ca atom over Ca-PICNT. In the case of 1H2-adsorbed Ca-PICNT, according to NBO analysis, the Ca center has 1.581e positive charge, slightly less than that of Ca in the bare Ca-PICNT (1.647e). The two H atoms of the adsorbed H2 carry natural atomic (NA) charges of 0.026e and-0.016e, respectively, indicating negligible charge transfer interaction between H2 molecule and Ca-PICNT. However, it can be considered as polarization of H2 molecule in the presence of electric field developed by cationic Ca atom of functionalized nanotube. The electronic configuration of the Ca center after hydrogen adsorption is [Argon]4 s0.163d0.124p0.16, thus almost resemble [Argon]4 s0.143d0.104p0.12 before the adsorption of H2. The NBO analysis also suggested that interaction of H2 over Ca-PICNT is almost free of charge transfer interaction. The calculated partial density-of-states considering Ca 3d,4 s, and H2σ as individual fragment are shown in Figure 4, from which it is evident that there is feeble change in electronic population of 4 s orbital, which is a result of very small electronic charge transferred by H2. There is almost negligible role of valence 3d orbital of Ca as the DOS remains unaffected in the presence of H2 molecules. The present study suggests that the interaction of Ca-PICNT with H2 is only via strong electrostatic charge interaction, and there is no Kubas-type interaction in contrast to the Kubas-type interaction observed in Ca-decorated CNT [19]. CDA calculation, which also give qualitative feature of energy decomposition analysis, carried over the H2 adsorbed Ca-PICNT by taking Ca-PICNT as the first fragment and H2 as the second, predicted that it is the manifestation of inter-frontier orbitals within the fragments in presence of each other which induce self-polarization. However, donation and back donation between occupied fragment orbitals of one and unoccupied fragment orbitals of other have no significant value, hence refer this system to adsorb hydrogen molecules by electrostatic interaction.
Adsorption of multiple hydrogen molecules
Subsequent addition of the second H2 molecule to one H2-adsorbed Ca-PICNT resulted in a binding energy per H2 molecule of-0.145 eV on optimization. NBO analysis performed over the system predicted small change of electronic configuration of central Ca atom. Natural charge on Ca atom is found to be 1.522e, where as the Hatoms of each H2 molecule exhibit NBO charges of the order-0.009e and 0.025e, respectively, with only 0.015e charge transferred to system. However, the decrease of positive electronic charge over Ca atom is result of electronic charge transferred from Natoms to central Ca atom which can be explained as manifestation of electronic configuration over the Ca-N parts of porphyrin ring in the presence of H2 molecule. In order to examine the maximum number of H2 molecule adsorbed over Ca-PICNT, we subsequently added more H2 on the system and found that this system can bind maximum of four H2 with binding energy value-0.105 eV/H2 molecule. On further evaluating the Gibbs free energy correction for the binding energy formulation of this maximum adsorbed hydrogen molecules system, the resulting formation energy comes out-0.097 eV/H2. The optimized structures obtained after the optimizations of the multiple H2 adsorbed are shown in Figure 5. The bond lengths of adsorbed H2 molecules as well as their distances from central metal atom (Ca) of porphyrin rings are presented in Table 3. It is observed that on increasing H2 molecules, the distances of H2 molecules with surface of nanotube tend to increase which corresponds to decrease in binding energy per H2 molecule. These calculations suggest that the hydrogen storage is possible over Ca-PICNT but at the cost of low temperature [23].
Conclusions
Present investigations show that dispersing Ca over porphyrin-induced carbon nanotube led to their strong binding over nanotube, which prevents clustering of Ca atoms over the surface of the nanotube. DFT calculations predicted the binding energy value for hydrogen storage in this system is suitable at low-temperature condition. The Ca-PICNT can absorb maximum of four H2 molecules. The NBO, CDA, and PDOS studies further confirmed the mode of storage in the system to be physisorption. These results recommend this model nanotube as promising candidate for storing hydrogen.
Acknowledgements
The author wants to thank the Department of Physics, Jamia Millia Islamia for its support.
References
Zhao Y, Kim YH, Dillon AC, Heben MJ, Zhang SB: Hydrogen storage in novel organometallic buckyballs. Phys. Rev. Lett. 2005, 94: 155504–155507.
Yildirim T, Ciraci S: Titanium-decorated carbon nanotubes as a potential high-capacity hydrogen storage medium. Phys. Rev. Lett. 2005, 94: 175501–175504.
Lee H, Choi WI, Ihm J: Combinatorial search for optimal hydrogen-storage nanomaterials based on polymers. Phys. Rev. Lett. 2006, 97: 056104–056107.
Sun Q, Wang Q, Jena P, Kawazoe Y: Clustering of Ti on a C 60 surface and its effect on hydrogen storage. J. Am. Chem. Soc. 2005, 127: 14582–14583. 10.1021/ja0550125
Li S, Jena P: Comment on “Combinatorial search for optimal hydrogen-storage nanomaterials based on polymers”. Phys. Rev. Lett. 2006, 97: 209601.
Choi WI, Jhi SH, Kim K, Kim YH: Divacancy-nitrogen-assisted transition metal dispersion and hydrogen adsorption in defective graphene: a first-principles study. Phys. Rev. B 2010, 81: 085441–085444.
Khan MS, Khan MS: Computational study of hydrogen adsorption on potassium-decorated boron nitride nanotubes. Nano Lett. 2012,2(5):1–5.
Khan MS, Khan MS: Comparative theoretical study of iron and magnesium incorporated porphyrin induced carbon nanotubes and their interaction with hydrogen molecule. Phys. E. 2012, 44: 1857–1861. 10.1016/j.physe.2012.05.010
Lavan DA, Cha JN: Approaches for biological and biomimetic energy conversion. Proc. Natl. Acad. Sci. U.S.A. 2006, 103: 5251–5255. 10.1073/pnas.0506694103
Sono M, Roach MP, Coulter ED, Dawson JH: Heme-containing oxygenases. Chem. Rev.. 1996,96(2888):2841.
Hashimoto A, Suenaga K, Gloter A, Urita K, Iijima S: Direct evidence for atomic defects in graphene layers. Nature (London) 2004, 430: 870–873. 10.1038/nature02817
Lehtinen PO, Foster AS, Yuchen MA, Krasheninnikov AV, Nieminen RM: Irradiation-induced magnetism in graphite: a density functional study. Phys. Rev. Lett 2004, 93: 187202–187205.
Berbe S, Oshiyama A: Atomic and electronic structure of divacancies in carbon nanotubes. Phys. Rev. B 2008, 77: 165405–165413.
Saito M, Yamashita K, Oda T: Magic numbers of graphene multivacancies. Jpn. J. Appl. Phys 2007, 46: L1185-L1187. 10.1143/JJAP.46.L1185
Kim G, Park N, Jhi SH: Effective metal dispersion in pyridinelike nitrogen doped graphenes for hydrogen storage Appl. Phys. Lett. 2008, 92: 013106–013108.
Lee DH, Lee WJ, Kim SO, Kim YH: Theory, synthesis, and oxygen reduction catalysis of Fe-porphyrin-like carbon nanotube. Phys. Rev. Lett. 2011, 106: 175502–175505.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, et al.: Gaussian 03, revision B.02. Pittsburgh, PA: Gaussian, Inc; 2003.
Gorelsky SI: Program for molecular orbital analysis. Ottawa: University of Ottawa; 2007.
Teo BK, Huang SP, Zhang RQ, Li WK: Theoretical calculations of structures and properties of one-dimensional silicon-based nanomaterials: particularities and peculiarities of silicon and silicon-containing nanowires and nanotubes. Coord. Chem. Rev. 2009, 253: 2935–2958. 10.1016/j.ccr.2009.08.001
Lee H, Ihm J, Cohen ML, Louie SG: Calcium-decorated carbon nanotubes for high-capacity hydrogen storage: first-principles calculations. Phys. Rev. B 2009, 80: 115412–115416.
Matsuo Y, Tahara K, Nakamura E: Theoretical studies on structures and aromaticity of finite-length armchair carbon nanotubes. Org. Lett. 2003, 5: 3181–3184. 10.1021/ol0349514
Lin JF, Wu CC, Lien MH: Ab initio study on the imine-enamine tautomerism of the alpha - substituted imines (XH2CCH:NH, X = H, BH2, CH3, NH2, OH, F, Cl, CN, NO). J. Phys. Chem. 1995, 99: 16903–16908. 10.1021/j100046a015
Schroeder DV: An introduction to thermal physics. MA: Addison-Wesley Reading; 2000.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
Both authors declare that they have no competing interests.
Authors’ contributions
All calculations and interpretation of the various results and their verifications were carried out by MSK and MSK. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khan, M.S., Khan, M.S. A DFT study of interaction of hydrogen molecules and (5,5) carbon nanotube with bioinspired functionalization. J Theor Appl Phys 7, 56 (2013). https://doi.org/10.1186/2251-7235-7-56
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7235-7-56