Abstract
This work investigated the optimization of biodiesel production from neem (Azadirachta indica) oil using a two-step transesterification process and determination of the qualities of the neem oil biodiesel. This was with a view to establish its production and viability potentials. Biodiesel production was carried using a two-step transesterification process. The first step was carried out using 0.60 w/w methanol-to-oil ratio in the presence of 1% w/w H2SO4 as an acid catalyst in 1 h at 50°C. The second step was base (NaOH) transesterification of the product from the first step using conditions specified in the optimization design. The central composite design optimization conditions for the second step were temperature (45°C to 65°C), catalyst amount (0.45% to 1.45% w/w), reaction time (45 to 65 min), and methanol/oil molar ratio (1.5 to 7.5). The physicochemical properties of the neem biodiesel were carried out using standard methods, while the fatty acid profile was determined using gas chromatography. Optimized biodiesel yield of 89.69% was produced at reaction time of 65 min, catalyst amount of 0.95 g, temperature of 55°C, and methanol/oil molar ratio of 4.5:1. The values for the physicochemical properties are 0.05% moisture content, 0.9 specific gravity at 25°C, 5.5 mm2/S kinematic viscosity, 207 mg KOH/g, 70.7 g I2/100 g iodine value, 55.31 cetane number, 39.85 MJ/Kg calorific value, 4 pour point, 8 cloud point, and 110 flash point. These values conform to international standards, in particular, American Society Testing Materials (ASTM).
It can be concluded that neem biodiesel showed a general compliance with known standards judging from its physicochemical properties and the engine test. These, coupled with its high yield, attested to the production viability and efficiency of neem biodiesel using two-step transesterification process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diesel fuels have an essential function in the industrial economy of developing and developed countries. The continued use of fossil fuel due to industrialization and domestic purposes especially in advanced nations resulted in environmental pollution which had contributed immensely to the present climate change, and its attendant effects had made it necessary to the development of alternative sources of energy [1]. This alternative fuel has been found to be technically feasible, economically competitive, environmentally acceptable, and readily available [1]. In addition, the fast depletion of fossil fuel and its high cost of procurement are factors that has made research into biodiesel, an alternative energy source that is clean, nontoxic, and a renewable necessity.
Biodiesel, either alone or in blends with fossil fuel, will help meet the world energy demands hitherto not yet sufficient. In addition, it is a renewable energy source which is unlike fossil fuels that is finite. Biodiesel obtained from energy crops also produces favorable effects on the environment, such as a decrease in acid rain and greenhouse effect caused by combustion [2]. Due to these factors and to its biodegradability, the production of biodiesel is considered an advantage than to that of fossil fuels [2]. Biodiesel is also nontoxic, eco-friendly, and useful in CO2 recycling over short periods [3, 4]. Biodiesel (fatty acid methyl esters (FAMEs)) derived from a variety of vegetable oils, animal fats, and waste oils may eventually be applicable as a petro-diesel and chemical substitute. Also, biodiesel evidences many salient properties, most notably cetane number, flash point, and volumetric heating value which have also been shown to be comparable to those of petro-diesel [5–8].
Many researchers have concluded that vegetable oils and their derivatives hold promise as alternative fuels for diesel engines with the only exception that the use of raw vegetable oils for diesel engines can cause numerous engine-related problems due to their high viscosity, low volatility, and high cetane number which can lead to severe engine deposits, injector coking, and piston ring sticking [9, 10]. However, these effects can be reduced or eliminated through transesterification of the vegetable oil to form an alkyl ester [9, 11].
The process of transesterification removes glycerin the triglycerides and replaces it with the alcohol used for the conversion process [12]. This process decreases the viscosity but maintains the cetane number and the heating value. It has also been shown that the use of biodiesel obtained from the mixture of oils for biodiesel production should be encouraged [13]. The result of the work showed that CO and HC emissions of the diesel engine when operated with the mixture as compared with high-speed diesel (HSD) were reduced by 10.97% to 21.16% and 38.76% to 47.6%, respectively. In addition, care has to be taken to ensure the storage stability of biodiesel when exposed to high temperature and air [14].
Biodiesel had been produced using homogeneous acid and base catalysts [15, 16]. A two-step process which involves acid-catalyzed transesterification process and followed by base-catalyzed transesterification process has been used in converting vegetable oil with high free fatty acid (FFA) value. The first step was acid esterification and pretreatment to remove FFA in the oil, which is mainly a pretreatment process and could reduce the FFA [17, 18].
The neem tree (Azadirachta indica) is a tropical evergreen with a wide adaptability, native to India and Burma, and been transplanted to Africa, the Middle East, South America, and Australia [19]. Neem (A. indica) tree is a native to tropical and semitropical regions with origin in Europe and later domesticated in Asia. It is extensively found in India and Indonesia [20]. It has been estimated that Indian neems bear about 3.5 million tons of kernels each year and that, in principle, about 700,000 tons can be recovered [4]. About 34 tons of neem seeds were exported from India in 1990 [21]. Neem oil has been extracted, and the yield is encouraging [20, 22].
Response surface methodology (RSM) is a useful statistical technique which has been applied in research into complex variable processes. It employs multiple regression and correlation analyses as tools to assess the effects of two or more independent factors on the dependent variables [23]. It has been used in methanolysis optimization of sesame (Sesamum indicum) oil to biodiesel [24].
This work seeks to optimize neem biodiesel production from neem oil using response surface methodology. The optimization of the neem biodiesel production via a two-step transesterification process will be designed using a central composite design model of the response surface methodology. The effects of variables such as the neem oil/methanol ratio, reaction time, reaction temperature, and catalyst amount on the biodiesel yield will as well be determined in order to find out the optimum conditions required for the neem biodiesel production. The physicochemical properties and fatty acid profiles of the neem biodiesel produced will be determined using appropriate established procedures. This will help in establishing the conformity of the neem biodiesel to standards, hence its suitability as alternative fuel source.
Methods
Raw materials and reagents
The major raw material used was neem oil which was purchased from Zaria, Nigeria. The reagents are all of analytical grade.
Biodiesel production from neem oil
Experimental design
In order to optimize the central composite experimental design (CCD), a five-level-four-factor central composite design was employed for this study, which generated thirty experimental runs. The factors investigated in this study were reaction time (minutes), catalyst amount (g), temperature (°C), and oil/methanol ratio. The coded and uncoded factors (X1, X2, X3, and X4), and levels used are shown in Table 1.
Preheating of neem oil
The neem oil was heated fairly strongly at 80°C for 30 min using Gallenkamp magnetic stirrer thermostat hotplate (Weiss Technik, England). The aim was to reduce the viscosity of the neem oil.
Transesterification reaction
A two-step transesterification reaction was used. This was due to the fact that neem oil had a high free fatty acid value; hence, a modified method of Hanny and Shizuko [17] was used. This method included acid transesterification followed by base transesterification.
Acid transesterification
The transesterification was carried out using 50 ml of methanol and 0.2 ml of concentrated H2SO4 mixed together inside a 250-ml conical flask. The conical flask was inserted into a water bath at 50°C. This mixture was later added to 200 ml of warmed (preheated) neem oil inside a 500-ml conical flask and placed on a magnetic stirrer with heater, continuously stirred, for 1 h for the acid transesterification to take place. The process was repeated for all the thirty samples.
Base transesterification
The product of the reaction in acid transesterification was used for the base transesterification. The calculated amount of NaOH (catalyst) and methanol was added for each reaction for the temperature and reaction time specified, as shown in Table 2.
Separation of biodiesel from glycerin
After the base transesterification process, the biodiesel was allowed to settle for at least 24 h inside a separating funnel to allow clear separation of the biodiesel from glycerin. The layer on the top is the biodiesel, while the bottom layer is the glycerin. The biodiesel separation was carried out by decantation as the glycerin was drained off while the biodiesel remained.
Biodiesel washing and drying
Warm distilled water at 50°C was added to the separated biodiesel, and the mixture was shaken vigorously. The water was allowed to drain through the bottom of the separating funnel. This was carried out five times until a clear biodiesel was obtained. Anhydrous CaCl2 was added to the biodiesel and heated gently at 50°C. The anhydrous CaCl2 was later separated from the biodiesel to obtain a clean dry neem biodiesel. The volume of the biodiesel obtained from each sample was determined, while the percentage of biodiesel was calculated.
Statistical analysis of neem biodiesel
The data obtained in the experiments (Table 2) were analyzed using response surface methodology so as to fit the quadratic polynomial equation generated by the Design-Expert software version 8.0.3.1 (Stat-Ease Inc., Minneapolis, MN, USA). The quality of the fit of the model was evaluated using analysis of variance (ANOVA). The fitted quadratic response model is as described in Equation 1.
where Y is response factor (% yield), and i and j denote linear and quadratic coefficients, respectively. bo is the intercept, b i is the first order model coefficient, k is the number of factors, and e is a random number.
Physicochemical analysis of neem oil biodiesel
The physicochemical analyses of the neem oil biodiesel were carried out using the Association of Official Analytical Chemists methods [25]. The analyses carried out include pour point determination (ASTM method), cloud point determination (ASTM), flash point determination (ASTM D93) using Pensky-Martens flash point tester, refractive index determination (using ATAGO Refractometer, RX-5000CX, Tokyo, Japan), specific gravity using specific gravity bottle according to AOAC method [25]), moisture content [25], viscosity (using Rion viscometer VTO4F, Netherlands), acid value [25], iodine value by Wijs method [25], peroxide value [25], saponification value [25], higher heating value (HHV) determination calculated model developed by Demirbas and Kara [26], cetane number (CN) determination using correlation reported by Patel [27] and Krisnangkura [28].
Characterization of neem oil and neem biodiesel
Fatty acid composition was determined by gas chromatography (HP 6890 powered with HP ChemStation Rev. A.09.01 [1206] software, Agilent Technologies, Inc., Santa Clara, USA). A 50 mg of the oil sample was hydrolysed for 5 min at 95°C with 3.4 ml of the 0.5 M KOH in dry methanol. The mixture was neutralized using titrimetric method by using 0.7 M HCI and then methylated using 3 ml of 14% boron trifluoride in methanol. The mixture was heated for 5 min at 90°C to achieve complete methylation process. The fatty acid methyl esters were extracted thrice from the mixture with redistilled n-hexane. The hexane extract was concentrated to 1 μl for gas chromatography analysis, and 1 μl was injected into the injection port of gas chromatography.
Results and discussion
Optimization of neem oil biodiesel yield via response surface methodology
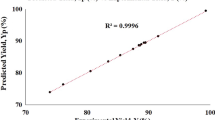
Table 2 showed the coded factors considered in this study, the experimental results, and the predicted values. The final equation in terms of coded factors for the central composite response surface quadratic model is shown in Equation 2.
The results of the ANOVA analysis for the response surface model (Equation 2) are shown in Table 3. The model's F-value of 292.39 implies that the model is significant. The p values <0.05 indicate that the model terms are significant. In this case, the linear coefficients X1, X2, X3, and X4; the cross-products, X1X2, X1X3, X1X4, X2X3, X2X4, and X3X4; and the quadratic coefficients, X12, X22, X32, and X42 are significant model terms (Table 3).
The effects of the factors such as reaction time, catalyst amount, temperature, and oil/methanol ratio on the percentage neem oil biodiesel yield were shown in Figures 1, 2, 3, 4, 5, and 6. Temperature had the greatest effect on the biodiesel yield, followed by the catalyst amount, reaction time, and oil/methanol ratio. The ANOVA analysis had also indicated the order of significance, giving temperature the factor that most significantly affected the oil yield, followed by catalyst amount, reaction time, and oil/methanol ratio.
This order of the effect of the factors on the percentage biodiesel yield agrees with that in the work of Jeong et al. [23], where their statistical response via a five-level-three-factor central composite ANOVA analysis showed that the temperature had the highest effect on the biodiesel yield with Prob > F value of 0.001, followed by catalyst amount with Prob > F value of 0.004, while neem oil/methanol oil ratio had Prob > F value of 0.072. In addition, the squares of the temperature, catalyst amount, and oil/methanol ratio were significant in the work by Jeong et al.[23], while only the squares of temperature and catalyst amount were significant in the present work. This could mean that those factors whose squares are significant have better effect on the biodiesel yield than those with only a linear factor that is significant. For example, reaction time, in this case, has a significant linear factor according to ANOVA, while its square factor is not significant. Also, all the cross-product terms were significant in this work and in the work of Jeong et al.[23]. In addition, a low lack of fit was noted according to the analysis of factors through ANOVA. This indicates that the model represents the actual relationship of all the parameters, which are well within the selected ranges (Table 2). In actual fact, the Prob > F value of 0.007 for the model is an indication of the significance of the model.
Figure 1 showed the effect of catalyst amount (X2) and reaction time (X3) on the biodiesel yield while keeping the other factors constant. The lowest yield was obtained around 56 min, irrespective of the catalyst amount. Highest yields were obtained at 50 and 60 min of base transesterification reaction. The catalyst amount of 0.70 and reaction time of 60 min gave the maximum yield of about 74%. At 60 min, the yield decreases with the increase of catalyst amount, while at 50 min, the yield increases slightly with the increase of catalyst amount. The effect of reaction time seems a not too important factor in biodiesel yield, as also observed by Zhang and Huang [29].
Figure 2 showed the effect of temperature (X1) and reaction time (X3) on the biodiesel yield. At 50 min, the yield was almost constant even with increasing temperature, but at 60 min, the yield decreases slightly with increase in temperature (from about 76% to 70%). At all temperature ranges, the effect of time on the yield is the same: high at 50 and 60 min, while low at 55 min. It can be deduced that temperature had a slight effect on biodiesel yield, with the lowest temperature having the highest yield. This is actually advantageous because biodiesel production especially from neem requires low temperature, hence low energy cost.
The effect of temperature (X1) and catalyst amount (X2) is shown in Figure 3. The effect of temperature is stronger than that of the catalyst amount, which is also in agreement with the result of ANOVA. As previously observed, the highest yields were obtained with lowest catalyst amount in all cases. The effect of catalyst amount is slight, except in Figure 4 where its effect is more pronounced in the presence of methanol/oil molar ratio.
The effect of catalyst amount on the biodiesel yield is slightly pronounced, as shown in Figure 4. The highest yield was obtained both at the lowest catalyst amount (0.70) and lowest methanol/oil molar ratio (3.0). Conversely, the lowest yield was obtained at highest methanol/oil molar ratio (6.0) and lowest catalyst amount (0.70). This implies that at lowest catalyst amount, increasing the methanol/oil molar ratio decreases the biodiesel yield.
Figure 5 showed the effect of oil/methanol ratio (X4) and reaction temperature (X1). The lowest conditions in both cases produced the highest yield. As the oil/methanol ratio increases, oil yield decreases. At the lowest methanol/oil ratio of 3.00, the yield is almost constantly high, irrespective of the temperature.
The highest reaction time of 60 min and the lowest methanol/oil molar ratio gave the highest yield of about 78%, as shown in Figure 6. At 60 min of base transesterification, the biodiesel yield decreases slightly with increasing methanol/oil ratio, while at 50 min, the yield is almost constant.
Taking together all these results, the optimized biodiesel yield of 89.69% was produced at reaction time of 65 min, catalyst amount of 0.95 g, temperature of 55°C, and oil/methanol ratio of 4.5. The result of this work showed that the maxima yield were obtained at lowest factor values as seen in Figures 1, 2, 3, 4, 5, and 6. This will definitely have economic advantage on neem oil biodiesel production as low energy cost, low catalyst amount, low methanol/oil ratio, and low temperature are able to produce high biodiesel yield. This was in agreement with the works of Meher et al. [1], Jeong et al. [23], and Marchetti and Errazu [30].
Physicochemical analysis of neem oil biodiesel
The properties of the biodiesel obtained from this work were shown in Table 4. It is compared with the American standard (ASTMD6751) and European specification (EN14214). The density, flash point, iodine number, cetane number, pour point, moisture content, and the calorific value conform to the approved international standards. The viscosity also conformed to the American standard. The amount and type of the fatty acid content in the biodiesel are the major factors that determine the viscosity of biodiesel. In addition, the neem oil and neem oil biodiesel compared with vegetable oil from other sources.
Gas chromatography analysis of neem oil biodiesel
The result of the chromatography analysis of neem oil biodiesel was shown in Table 5. The percentage of unsaturated fatty acid is 80.02%, while the percentage of saturated fatty acid is 19.97%. It has been shown that biodiesel fuel with more unsaturated fatty acid composition has more density but has less viscosity, lower cetane number, and heating value. It also has lower thermal efficiency compared to high saturated fatty acid composition. It emits lower HC and CO, and less smoke compared to highly saturated biodiesel fuel.
Conclusion
The maximum neem oil methyl ester (NOME) conversion yield was validated as 85.13% (w/w) under the optimal reaction condition of 3:1 methanol/oil molar ratio, 50°C reaction temperature, 0.70% catalyst concentration, and 60-min reaction time. The fuel properties of NOME satisfied both the ASTM D 6751 and DIN EN 1424 standards. Thus, the present study demonstrates the usefulness of RSM for optimum conversion of A. indica seed oil to NOME. It also suggests that the seed oil could be used effectively as feedstock for NOME production.
Authors’ information
OOA is a Lecturer II from the Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria. He holds a Ph.D. degree in Chemical Engineering from Obafemi Awolowo University, Ile Ife, Nigeria. His research interests are in the area of food rheology, renewable energy from food wastes and underutilized crops, and optimization of food processes and extrusion processes. SKL is a Professor of Chemical Engineering, Obafemi Awolowo University, Ile Ife, Nigeria, a Fellow of Nigerian Society of Chemical Engineers (FNSChE), a Fellow of Nigerian Society of Engineers (FNSE), and a member of Biochemical Engineering Subject Group (BESG) of Ichem E, UK. He studied Chemical Engineering (Diplom-Ingenieur) in the Polytechnic Institute of Bucharest Romania (1964 to 1970). In 1971, he proceeded to the University College London from where he bagged M. Sc. D.U.C in Biochemical Engineering in 1972. He obtained Ph.D. DIC in Biochemical Engineering (1975) from the Imperial College London. He is generally interested in reactors and bioreactors, in particular. This captures microbial reaction kinetics and its application in design, modeling, and optimization. It also includes microbial production of metabolites (bioethanol, biobutanol, etc.), biodiesel through alcoholysis and enzymatic route, biopolymer production, and bioremediation. He is currently interested in the development of processes for lignocellulosic bioethanol production which also has to do with hydrolytic enzyme production.
References
Meher LC, Vidya Sagar D, Naik SN: Technical aspects of biodiesel production by transesterification–a review. Renew. Sustain. Energy Rev. 2006, 10: 248–268. 10.1016/j.rser.2004.09.002
Antolin G, Tinaut FV, Briceno Y, Castano V, Perez C, Ramirez AI: Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83(2):111–114. 10.1016/S0960-8524(01)00200-0
Graboski MS, McCormick RL: Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energ. Combust. 1998, 24: 125–164. 10.1016/S0360-1285(97)00034-8
Jeong GT, Park DH, Kwang CH, Lee WT, Sunwoo CS, Yoon CH, Choi BC, Kim HS, Kim SU, Lee UT: Production of biodiesel fuel by transesterification of rapeseed oil. Biotechnol. Appl. Biochem. 2004, 114: 747–758. 10.1385/ABAB:114:1-3:747
Ma F, Hanna MA: Biodiesel production: a review. Bioresour. Technol. 1999, 70: 1–15. 10.1016/S0960-8524(99)00025-5
Lang X, Dalai AK, Bakhshi NN, Reaney MJ, Hertz PB: Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001, 80: 53–62. 10.1016/S0960-8524(01)00051-7
Usta N: Use of tobacco oil methyl ester in turbocharged indirect injection diesel engine. Biomass. Bioenerg. 2005, 28: 77–86. 10.1016/j.biombioe.2004.06.004
Jeong GT: Park, DH: Response surface methodological approach for optimization of enzymatic synthesis of sorbitan ethacrylate. Enzyme Microb. Technol. 2006, 39(3):381–386. 10.1016/j.enzmictec.2005.11.046
Perkins LA, Peterson CL: Durability Testing of Transesterified Winter Rape Oil (Brassica napus L.) as Fuel in Small Bore, Multi-Cylinder, DI, CI Engines. Warrendale: Society of Automotive Engineers; 1991.
Scholl KW, Sorenson SC: Combustion of Soybean Oil Methyl Ester in a Direct Injection Diesel Engine. Warrendale: Society of Automotive Engineers; 1993.
Zhang Q, Feldman M, Peterson C: Diesel Engine Durability when Fueled with Methyl Ester of Winter Rapeseed Oil. Washington, DC: American Society of Agricultural Engineers; 1988.
Van Gerpen JH, Hammond EG, Yu L, Monyem A: Determining the Influence of Contaminants on Biodiesel Properties. Warrendale: Society of Automotive Engineers; 1997.
Raheman H, Jena PC, Jadav SS: Performance of a diesel engine with blends of biodiesel (from a mixture of oils) and high-speed diesel. Int. J. Energy Env. Eng. 2013, 4: 6. doi: 10.1186/2251–6832–4-6 doi: 10.1186/2251-6832-4-6 10.1186/2251-6832-4-6
Mazumdar P, Borugadda VB, Goud VV, Sahoo L: Effect of storage parameters on stability of Jatropha -derived biodiesel. Int. J. Energy Env. Eng. 2013, 4: 13. doi: 10.1186/2251–6832–4-13 doi: 10.1186/2251-6832-4-13 10.1186/2251-6832-4-13
Noureddini H, Zhu D: Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74(11):1457–1463. 10.1007/s11746-997-0254-2
Narasimharao K, Lee A, Wilson K: Catalyst in production of biodiesel: a review. J. Biobased Mater Bio. 2007, 1: 19–20.
Hanny JB, Shizuko H: Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99: 1716–1721. 10.1016/j.biortech.2007.03.051
Awolu OO, Odedeji D: Production of fatty acid methyl ester (biodiesel) from waste cooking oil using transesterification methods. App. Trop. Agric. 2011, 16(1):23–29.
Ogbuewu IP M.Sc. thesis. In Physiological responses of rabbits fed graded levels of neem (Azadirachta indica) leaf meal. Owerri: Federal University of Technology; 2008.
Liauw MY, Nathan FA, Widiyanti DI, Indraswati N, Soetaredjo FE: Extraction of neem oil ( Azadirachta indica A. Juss ) using n-hexane and ethanol: studies of oil quality, kinetic and thermodynamic. J. Eng. Appl. Sci. 2008, 3(3):49–54.
National Research Council (NRC): Neem: A Tree for Solving Global Problems. Washington, DC: National Academic Press; 1992.
Awolu OO, Obafaye RO, Ayodele BS: Optimization of solvent extraction of oil from neem ( Azadirachta indica ) and its characterizations. J. Sci. Res. Rep. 2013, 2(1):304–314.
Jeong GT, Yang HS, Park DH: Optimization of transesterification of animal fat ester using response surface methodology. Bioresour. Technol. 2009, 100: 25–30. 10.1016/j.biortech.2008.05.011
Betiku E, Adepoju TF: Methanolysis optimization of sesame ( Sesamum indicum ) oil to biodiesel and fuel quality characterization. Int. J. Energy Env. Eng. 2013, 4: 9. doi: 10.1186/2251–6832–4-9 doi: 10.1186/2251-6832-4-9 10.1186/2251-6832-4-9
AOAC: Official Methods of Analysis. 17th edition. Maryland USA: Association of Official Analytical Chemists, Inc; 2000.
Demirbas A, Kara H: New options for conversion of vegetable oils to alternative fuels. Energ. Sour. Part A 2006, 28: 619–626. 10.1080/009083190951357
Patel V: Cetane Number of New Zealand Diesel Report. Wellington, New Zealand: Office of Chief Gas Engineer, Energy Inspection Group, Ministry of Commerce Press; 1999.
Krisnangkura K: A simple method for estimation of cetane index of vegetable oil methyl esters. J. Am. Oil Chem. Soc. 1986, 63(4):552–553. 10.1007/BF02645752
Zhang XW, Huang W: Optimization of the transesterification reaction from cottonseed oil using a statistical approach. Energ. Source Part A 2011, 33: 1107–1116. 10.1080/15567031003627971
Marchetti JM, Errazu AF: Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass. Bioenerg. 2008, 32: 892–895. 10.1016/j.biombioe.2008.01.001
Acknowledgements
Authors acknowledge Mr Igbe Festus of the Department of Biochemistry and Mr Oshosana of Department of Mechanical Engineering, Federal University of Technology, Akure, for assistance in carrying out some laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Both authors declare that they have no competing interests.
Authors’ contributions
OOA designed the work, carried out the laboratory work, analyzes and carried out the statistical analysis of the result, and wrote the manuscript. SKL supervised the work, and corrected the write ups. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Awolu, O.O., Layokun, S.K. Optimization of two-step transesterification production of biodiesel from neem (Azadirachta indica) oil. Int J Energy Environ Eng 4, 39 (2013). https://doi.org/10.1186/2251-6832-4-39
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-4-39