Abstract
This article studies the use of animal by-products and derived products not intended for humans as possible fuels in residential oil burning facilities. We first offer a chemical and physical description of the various types of animal by-products and derived products not intended for humans with a view to their possible use as fuels. Animal by-products and derived products not intended for humans have an extremely high viscosity for the pressure pulverisation burners used in residential oil burning equipment. We therefore mixed diesel with animal by-products and other derived products not intended for humans in different percentages so as to obtain suitable viscosity. To achieve this, we carried out a study of the miscibility of animal by-products in diesel. We subsequently performed a series of combustion experiments for mixtures of diesel and animal by-products, in varying (a) percentage of by-products in diesel, (b) injection pressure and (c) excess air in combustion. We analysed the experimental combustion results based on (a) energy efficiency of combustion, (b) CO and NO x emissions and (c) fossil-based greenhouse effect gases. Finally, we present the conclusions that combustion of mixed diesel with animal by-products and other derived products not intended for humans for use as boiler fuel low power does not require specific technology when using a conventional fuel oil burner for proper combustion. One only needs to adjust the burner factors: pressure and air flow. In the study of the combustion of mixtures, once the burner factor is adjusted, it appears that the combustion efficiency and greenhouse gas and emission gases are acceptable. The costs of removing fat through incineration or landfill range from 34 to 59 €/ton. The solution proposed in this work not only avoided the cost but also mentioned the value of residue use as fuel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In 2007, the Council of Europe set out a series of ambitious goals for 2020, given the name of ‘20-20-20’, which seek to cut greenhouse gas emissions by 20%, cut primary energy consumption by 20% through enhanced energy efficiency and promote up to 20% use of renewable final energy consumption [1].
Through the Directive 2009/28/CE, the European Parliament set a target for 2020 that 20% of final energy should be from renewable sources. The directive promotes energy generated from biofuels and bioliquids. For the latter to be considered sustainable, they must contribute to cutting greenhouse gas emissions by at least 35%. As of 1 January 2017, their contribution to emission reduction must reach 50% [2].
The directive stipulates that in order to be granted financial support, biofuels and bioliquids must be classified as ‘sustainable.’ This requires biofuels and bioliquids to be generated using raw materials from both outside as well as inside the various regions of UE. In addition, they must not originate from land that has high biodiversity value or large carbon reserves [3].
Bioliquids may be transformed for use in engines or used directly as fuels in burners. When used as fuels in burners, certain advantages are evident such as (a) requiring no specific transformation processes, thereby enabling them to be obtained relatively cheaply; (b) less rigorous specifications when used in burners than when used in engines; and (c) a wider range of technologies linked to burners than to heat engines. In addition, burners have a wider range of regulation vis-à-vis fuel than heat engines [4].
Some authors have explored the use of bioliquids. Batey [5] conducted studies into combustion of biodiesel and mixtures of oil and diesel in residential combustion facilities; Raheman et al. [6] evaluated a 10.3-kW single-cylinder water-cooled direct-injection diesel engine using blends of biodiesel (B10 and B20) obtained from a mixture of mahua and simarouba oils (50:50) with high-speed diesel (HSD) in terms of brake-specific fuel consumption; Vanlaningham et al. [7] analysed combustion of oil and soya seed for heating; San José et al. [8] studied combustion of mixtures of soya oil, rapeseed and sunflower oil with diesel in various proportions over a range of combustion parameters in a pressure pulverization burner and also studied combustion of biodiesel that does not meet biofuel specifications [9].
The fat used for heating purposes has been analysed at the University of Budapest by Lezsovits and Könczöl [10] who researched the use of animal fats in industrial steam generators that conventionally ran on diesel or natural gas. The use of this by-product was intended not only to remove it as a waste product but also to provide an alternative non-fossil fuel. In Germany, F. Pfab [11] treated animal fat from processing plants and subsequently used it in combustion to generate steam in a tubular boiler. Before feeding the fat into the burner, the burner must be cleaned with a decanter and separator. Our study breaks new ground in current technology by performing combustion of animal fat at an injection pressure below 10 bars, compared to conventional 30 bar pressure. In Spain, Dr. J. San José [12] has conducted a number of studies of the combustion of lard in a commercial burner and into the miscibility of lard in diesel. These studies posit an important field of study in the description of animal fats and their use as fuels in conventional diesel facilities.
Animal by-products and other derived products not intended for human consumption, which are recoverable in energy terms, are in abundant supply as waste and are found in the animal-based food production chain in (a) intensive livestock holdings, hen manure and other similar products, and in (b) slaughterhouses and retailers. The former are usually processed in the actual livestock holdings themselves, whereas the latter, which provide the case study for the present article, are dealt with by officially accredited firms.
The amount generated in slaughterhouses and by retailers in kilogram per animal slaughtered depends on the animal species. Studies carried out by the Ministry of the Environment, Rural and Marine Affairs in Spain put the estimates for mean production of waste per species at (a) bovines, 166.25 kg/animal; (b) dairy cows, 260.45 kg/animal; (c) swine, 10.79 kg/animal and (d) ovine, 8.05 kg/animal [13].
Handling these animal by-products as waste, together with the use of transformed animal fat as a biomass resource in combustion processes, is controlled under European Union Regulation 1774/2002. The said regulation lays down strict rules concerning animal welfare and public health to be applied when collecting, transporting, storing, handling, processing and using or eliminating this kind of by-product. These rules have been applicable throughout the whole of the EU since 1 May 2003 [14].
Processing any animal by-products and derived products not intended for human consumption generated in slaughterhouses and livestock resource processing companies firstly involves grinding the raw material followed by high-pressure heat treatment to remove excess moisture and kill microorganisms. One fraction of the subsequent by-product is in the form of flour and the other in the form of fat. In processing plants, the fraction of flour obtained accounts for between 25% and 40% wt. f.m. of the total processed by-product, whereas for the fat fraction this percentage ranges from 6% to 15% wt f.m. of the total by-product [15].
The amount of fat obtained in Europe is shown in Table 1[16]. This waste has a high-energy quality which ranges between 38.7 and 42.1 MJ/kg and which may be used as raw material to obtain biodiesel [17–19]. However, the economic crisis, the strict specifications imposed by the European Union on biodiesel and the investment required by such processing plants have made animal fat not intended for human consumption a waste product, the fat from which must be burnt in application of the current legislation.
The present article presents co-combustion of animal fats not intended for human consumption as an alternative fuel for conventional diesel facilities due to the following advantages:
-
It is a waste product which must be destroyed, as a result of which its calorific potential may be used and the waste thereby being removed.
-
It is biomass not intended for human consumption such that it does not cause price distortions in food products.
-
It allows for the use of conventional technology such that no major investments are required in equipment.
-
As it is biomass, it complies with all policies concerning reduction of greenhouse gas emissions and use of fossil fuel alternatives.
Methods
Animal fats are glycerine esters (triglycerides) and fatty acids. Fatty acids found in animal fats may be saturated CnH2nO2 and unsaturated [20]. One characteristic of saturated fatty acids is that at room temperature they are solid. Animal fats tend to be rich in this kind of acid. These include the following:
-
Formic or methanoic acid, with a single carbon and formula CH2O2, which in the developed form is H-COOH
-
Acetic acid or ethanoic acid with two carbons. It is an intermediate product of animal metabolism
-
Butyric acid or butanoic acid, C4H8O2 or in the developed form CH3-(CH2)2-COOH
-
Palmitic acid, C16H3O2, the developed formula which is CH3-(CH2)14-COOH
-
Stearic acid, C18H36O2, the developed formula which is CH3-(CH)16-COOH
Unsaturated fatty acids are generally liquid at room temperature. Due to their chemical structure, they can be mono- or poly-unsaturated. The following can be found in animal fat:
-
Oleic acid, C18H34O2, with the formula CH3-(CH2)7-CH=CH-(CH2)7-COOH
-
Linoleic acid with the formula CH3-(CH2)4-CH-CH2-CH=CH-(CH2)7-COOH
The chemical nature (composition) of fuels is insufficient when determining their behaviour or efficiency during combustion, as it is the physical-chemical characteristics which provide such knowledge. Table 2 shows the main physical-chemical characteristics of three fats: FF, food fat, NF1, non-food fat type 1 and NF2, non-food fat type 2.
The features of the three fats in Table 2 show that the three types of fat evidence very similar characteristics. The main differences with diesel are viscosity and percentage of oxygen, which means that a specific burner must be used for combustion of fats due to their high viscosity and that combustion must take place with lower excess air due to the oxygen content of the fat.
Since the present study seeks to achieve combustion of fats using conventional diesel combustion facilities which are normally mechanical pressure pulverization burners, we conducted a study of the mixture of fats with diesel in order to reduce the viscosity of the fats.
Analysis of the miscibility of the fats in diesel
Above their melting point, oils and fats are totally, and in all proportions, miscible with organic solvents [21, 22].
At room temperature, fats are doughy, preventing them from being handled and mixed for use as fuels. We carried out a melting analysis based on the principle of turbidity. Figure 1 shows a sample at room temperature and a melted sample.
After numerous tests, we concluded that not all fats undergo phase change at the same temperature, and we set 30°C as the minimum melting point temperature for all of them.
The miscibility and/or solubility of the diesel samples is tested by mass and because of the apolar nature of both fluids, dissolution is not a problem. Since there are no functional groups and the molecular structure is similar, there are no chemical reactions between the two products. In certain cases, some problems of stratification and turbidity of the fats could be seen at low temperatures. The conclusion is that for combustion assays using mixtures of animal fats and diesel, it is important to heat and stir the (animal fat-diesel) mixture before it is fed into the burner for subsequent use as fuel. During heating, the mixture must reach a temperature of above 30°C and the stirring must be constant to ensure that the mixture is homogeneously balanced.
A tank has been designed to ensure the balance of the mixture. This consists of a jacketed container which, together with the water pumped in by a thermostatic bath and coupled with an electrical stirrer, keeps the mixture warm, thereby ensuring that the animal fat-diesel mixture is kept uniform. Figure 2 shows a diagram of the tank in which the samples were kept.
Characteristics of the mixtures
The burner used has a limitation on fuel viscosity; this forced us to use mixtures of diesel and up to 40% lard. Our study of the mixture allows us to conclude that to have an even mixture of animal fat and diesel, the mixture cannot contain over 40% animal fat in mass. Each mixture is labelled with an identification code consisting of letters and two numbers: FC-XX, FN1-XX and FN2-XX.
The meanings of the letters are the following:
◦ FF refers to edible fat.
◦ NF1 refers to type 1 non-edible fat.
◦ NF2 refers to type 2 non-edible fat.
The numbers represent the percentage (%) of fat used in the mixture. Four mixtures in varying percentages (10, 20, 30 and 40) were prepared for each type of fat.
Describing each of the mixtures performed, based on the knowledge of the properties of the pure components that make up the (theoretical) mixtures, we use the additivity rule with regard to a physical property:
where Pmt(T) is the physical property of the theoretical mixture at temperature T, xa is the molar fraction of the fat in the mixture and Pa(T) and Pd(T) are the physical properties of the fat and diesel. A significant deviation can be seen in the viscosity calculated based on the values of the original compounds and their value percentage measured in the laboratory. As a result, the viscosity data are measured in the laboratory (empirical measurements) since the mixtures of fats and diesel cannot be considered as Newtonian liquid [23].
Table 3 shows the results of the mixtures of the various fats and diesel in different proportions.
The experimental facilities are divided into four parts: (a) the boiler-burner unit, (b) the fuel feed system, (c) the heat dissipation system and (d) the control and measuring system.
Boiler-burners. Combustion for heating is performed using burners prepared for the controlled and optimised combustion of a specific fuel. The basic conditions to be met by the burner are the following:
-
1.
It should adapt to the combustion chamber where it is to be used.
-
2.
It should be equipped with a suitable margin of regulation.
-
3.
It should be possible to control the shape and dimension of the flame.
-
4.
It should function in a stable manner.
-
5.
It should be equipped with safety and regulation systems to ensure automatic functioning
In the case of burners for liquids, the following features are also required:
-
1.
Capacity to achieve correct combustion intensity (amount of fuel per specific volume)
-
2.
Capacity to operate with a minimum amount of excess air (maximum flame temperature)
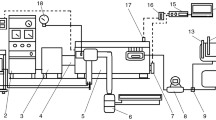
Burners for liquid fuels are based on two technologies: fuel vaporisation and fuel pulverisation in small drops. This study uses a pressure pulverisation burner, which can burn fuels with a viscosity between 1 and 5°E at 20°C, enabling regulation of the pressure between 10 and 20 atm and the air flow through which combustion is performed. If we add the mixture percentages to these two control parameters, we have the three parameters to be controlled and optimised in combustion for generating heat using mixtures of fat and diesel. The characteristics of the boiler and the burner are shown in Table 4. The burner and boiler are widely used commercial equipment in Spain. The system for feeding the fuel to the burner comprises two tanks, one containing the diesel used for the experiment to be considered stationary and the other containing the fuel mixtures. Figure 2 shows the system for feeding the fuel to burner.
Heat dissipation is through a two-tube radiator facility, with direct return. Figure 3 shows the layout of the facility.
The facility control parameters are shown in Table 5. These values should be kept within a specific interval for the test to be considered stationary.
The control and measuring system. The fume analysing equipment and the parameters obtained during each test are those used to describe the combustion and are shown in Table 6.
The results from the combustion of the mixtures were analysed based on three aspects:
-
Emissions of pollutant gases
-
Combustion efficiency
-
Greenhouse gases
Before analyzing the combustion of a mixture, it is found that the set boiler-burner works properly by an analysis of diesel fumes, which is considered patron fuel; Table 7 presents the reference values analysis of diesel fumes.
Experimental designs
Excess air, compared to the stoichiometric flow rate, is modified among values 1.2, 1.3 and 1.4 so as to establish the optimal combustion conditions for these mixtures in the burner used.
Having determined the variables, the test matrix to be conducted is posited to ensure that all the levels of all variables will be tested with the levels of the remaining variables in the same number of times. In other words, the design will maintain orthogonality. The order of the assays will therefore be carried out thus
-
1.
Three types of animal fat are studied (three levels).
-
2.
Four mixtures with different proportions of each animal fat with diesel are carried out (four levels).
-
3.
The injection pressure is varied using three values for each mixture (three levels).
-
4.
Excess air is varied using three values for a single sample of one type of fat at a specific percentage (three levels).
The number of tests to be conducted in order to maintain orthogonality coincides with the product of the number of levels of all the variables:
Finally, we present the procedure followed in the experimental tests and the requirements the facility must meet if results are to be accepted as valid (Figure 4).
Results and discussion
A total of 108 combustion tests were carried out in which the following conditions were met: (a) stable working conditions for the boiler, (b) air flow and fuel injection pressure adapted to the test, (c) uninterrupted running of the facility for each mixture and (d) the values for each test within the error limits for the measuring equipment.
Tables 8, 9 and 10 show the parts per million in CO fumes, the percentage of oxygen in fumes, fume temperature, air temperature or reference temperature and the concentration of nitrogen oxides in fumes obtained from the combustion.
With regard to the emissions of pollutant gases, we analysed emissions of carbon monoxide and nitrogen oxides. Combustions were considered acceptable when the concentration of fumes is less than 500 ppm of CO and/or is less than 300 ppm of NO x , the limits that are imposed under current legislation.
Combustion efficiency is defined by the following expression:
where Ps is sensible heat loss (MJ/kgcomb), Pi, is heat loss from incomplete combustion (MJ/kgcomb) and LHV is lower heating value (MJ/kgcomb). Sensible heat loss (Ps) corresponds to the energy the combustion gases have when extracted compared to the reference conditions of the combustion process. When these losses are higher, the internal energy of the flue gas (flue gas temperature) is greater. They also increase with flue gas flow, which increases with excess air, and this also increases the flow of flue gas, transporting a greater amount of heat.
Sensible heat loss is defined by the following percentage expression:
where M1Hs is mass of combustion of flue gas in (kg/kgcomb); C pA, heat capacity at constant pressure of flue gas heat (MJ/kg °Ccomb); LHV, lower heating value of the fuel in (MJ/kg); tH, temperature of flue gas at output in (°C); and tref, the ambient temperature in degrees Celsius (°C).
Heat loss from incomplete combustion (Pi) corresponds to the energy of the combustion products which have not been totally oxidised. This occurs in cases of incomplete combustion, the loss being greater with the higher amount of unburnt gas. When total carbon oxidation does not occur, the combustion product will be CO rather than CO2:
where V1Hs is the volume of dry of flue gas in (nm3/kgcom), LHVCO is the lower heating value of CO in (MJ/kgCO), ρco is the density of CO in (kg/m3) (CO), percent by volume in the flue gas (nm3CO/nm3) and LHV is the lower heating value of the fuel in megajoules per kilogram of combustion (MJ/kgcomb).
The combustion efficiency represents the amount of energy transferred to the heat system of the energy released by the fuel. The combustion performance values are shown in Figure 5 for FF, food fat, NF1, non-food fat type 1 and NF2, non-food fat type 2 mixtures in terms of the injection pressure, the mixture percentage and excess air during combustion.
As seen in Figure 5, the combustion efficiency is reduced when increasing the percentage of non-fat foot type 1, being very significant when it exceeds 30% in the mixture and also seen in Table 9, which increases the unburned (CO); this is due to be a fat containing many solid particles impinging on the phenomenon of the fuel spray.
The results are similar to those obtained in diesel combustion. It is worth noting that fats have a lower LHV than diesel and that the energy released per unit of mass in the combustion process is therefore lower. However, fats have a higher percentage of oxygen in their composition and thus require less airflow, which reduces loss due to fumes. These two phenomena together mean that the performance values of the mixtures and diesel are similar.
The average combustion performance values are shown for each fat in terms of injection pressure. For FN1, performance decreases as injection pressure increases. As a result, increasing injection pressure does not enhance combustion, as can be seen in Figure 6.
The average combustion performance values are shown for each fat in terms of the percentage of fat in the mixture (Figure 7). Combustion efficiency decreases as the percentage of fat in the mixture increases. This is because the fats have a lower calorific value and contain oxygen, which produces higher excess air during combustion.
The average combustion performance values are shown for each fat in terms of excess air (Figure 8). The performance follows a clearly linear trend, diminishing as the airflow involved in the combustion increases.
By increasing the percentage of fat in the fuel mix, an ascent is observed in the evolution of NO x . This is because one side has more oxygenated fuel component as we increase the percentage of fat, which increases the flame temperature and results to higher nitrogen content in the fat as we increase the percentage of NO x .
The greenhouse gases considered in this study were emissions of CO2 associated with diesel combustion, as it is a fossil fuel, since the CO2 generated by combustion of fats is renewable. The evaluation was carried out in terms of the basic composition of the diesel and fats and the mixture percentage. This value is shown in Table 11, which also shows the kilogram of CO2 emitted during full combustion per kilogram of fuel, distinguishing between the kilogram originating from fossil diesel and those from non-fossil fat.
Conclusions
Animal fat as an animal by-product not intended for human consumption is an abundant resource. Each year, Europe generates 1.35 million tons. Taking an average LHV of 42.1 MJ/kg, the energy potential per year is 56.8 million GJ/year which would mean a reduction in CO2 emissions of 4.43 million tons/year compared to diesel.
Providing a basic description of fats has enabled us to determine that their composition is similar to the composition of diesel, with a lower carbon and hydrogen content and higher oxygen content. This is due to the type of fatty acids contained in animal fats compared to the hydrocarbons that make up diesel. We also see that the LCV of fats is around 10% lower than that of diesel in all cases and that the density of fats is just under 10% higher than that of diesel. As a result, the energy flow by volume is similar in fats and diesel. The main difference between diesel and fats is in the viscosity. At 40°C, the viscosity of fats is 20 times higher than that of diesel. This property has a major impact on pulverization and proper combustion of liquid fuels and it should be remembered that at room temperature animal fats are mostly solid.
When fats are melted at a temperature above 38°C they are totally soluble in diesel, the mixture being fully homogeneous, and no dissolution limit having been found. Studies performed into the stability of the mixture and its homogeneity indicate that the mixture should be kept at 38°C and constantly stirred to ensure it does not lose its homogeneity.
We observed that combustion performance diminishes when the percentage of fat in the mixture is increased, which concurs with the lower LCV of fats compared to that of diesel.
We observed that combustion performance diminishes when pressure is increased, concurring with the theoretical studies carried out which indicate that above a certain injection pressure, no improvements in the pulverization process are seen, although it does contribute towards a detachment of the flame.
We also observed that performance diminishes when the combustion air flow is increased, which is accounted for by the amount of oxygen contained in the fats, meaning that they require less excess air for combustion.
References
Commission of the European Communities: GREEN PAPER, A European strategy for sustainable, competitive and secure energy; COM(2006) 105 final, Brussels, 8.3.2006.
Decision No 406/2009/EC, On the effort of Member States to reduce their greenhouse gas emissions to meet the Community’s greenhouse gas emission reduction commitments up to 2020 Off. J. Eur. Union 5.6.2009 5.6.2009
Directive 2009/28/EC, on the promotion of the use of energy from renewable sources and amending and subsequently repealing, the European Parliament and of the Council of 23.4.2009.
Ndayishimiye P, Masimalai S, Tazerout M: Preparation and properties analysis of methyl esters of palm oil and waste cooking oil mixture to use as fuel in diesel engines, 15th European biomass conference & exhibition. Hamburg Germany; 7–11.5. 2009 7–11.5. 2009
Batey J: Combustion testing of a bio-diesel fuel oil blend in residential oil burning equipment, Tech. rep., Energy Research Centre, prepared for: Massachusetts Oil hest Council & National Oil heat Research Alliance. 2003.
Raheman H, Jena PC, Jadav SS: Performance of a diesel engine with blends of biodiesel (from a mixture of oils) and high-speed diesel. Int. J. Energy Environ. Eng. 2013, 4–6.
Vanlaningham N, Gibson H, Kaufman B: Evaluation of soybean heating oil blends for use in residential applications, no. 046082. ASAE Paper, annual meeting held, 23–24. 8. 2004 ASAE Paper, annual meeting held, 23–24. 8. 2004
San José Alonso JF, López Sastre JA, Romero-Avila C: A note on the combustion of blends of diesel and soya, sunflower and rapeseed vegetable oils in a light boiler. Biomass Bioenergy 2008, 32: 880–886. 10.1016/j.biombioe.2008.01.007
San José J, Al-Kassir A, López Sastre JA, Gañán J: Analysis of biodiesel combustion in a boiler with a pressure operated mechanical pulverization burner. Fuel Process. Tech. 2011, 92: 271–277. 10.1016/j.fuproc.2010.05.020
Lezsovits F, Könczöl S: Animal-fat investigation and combustion test. J. Therm. Anal. Alorimetry 2012, 107: 271–278. 10.1007/s10973-011-1519-4
Pfab F: Processing and combustion of animal fat from animal waste rendering plants for steam generation in a smoke tube boiler. Rome: 2nd World Conference on Biomass for Energy, Industry and Climate protection; 2004.
San José J, Alonso S, Gobernado I: Study of thermal energy production by combustion of lard and diesel mixtures. J. Combustion 2012:
Ministerio de Medio Ambiente y Medio Rural y Marino: Anuario de estadística agroalimentaria y pesca 2007. Madrid; 2008.
Decision No 1774/2002/EC, laying down health rules concerning animal by-products not intended for human consumption Official Journal of the European Communities 3.10.2002 3.10.2002
Consejería de Sanidad: Agencia de Protección de la Salud y Seguridad Alimentaría, Datos estadísticos 2010. Junta de Castilla y León; 2010.
Issue number 6/2009, Main stages in the meat food chain in Europe. Euro stat Publish; 11.4 .2009 11.4 .2009
Ghassan M, Tashtoush , Mohamad I, Al-Widyan , Aiman M: Types of biofuels. J. Environ. Manage. 2007, 84: 401–411. 10.1016/j.jenvman.2006.06.017
Calais P, Clark T: Waste vegetable oil as a diesel replacement fuel. Perth, Australia: Environmental Science, Murdoch University; 2006.
Senthil Kumar M, Kerihuel A, Bellettre J, Tazerout M: Experimental investigations on the use of preheated animal fat as fuel in a compression ignition engine. Renew. Energy 2005, 30: 1443–1456. 10.1016/j.renene.2004.11.003
Madrid A: Aprovechamiento de los subproductos cárnicos. Capítulo 1. Los subproductos cárnicos: definición, composición y características. AMV Ediciones 1999, 17–22. Madrid Madrid
Bills CE, with the cooperation of McDonald F.G: Antiricketic substances: iii. The catalytic formation of an antiricketic cholesterol derivative. J. Biol. Chem. 1926, 67: 753–758.
Formo MW, Jungermmann E, Norris FA, Sonntag NO: Bailey’s industrial oil and fat products. Volume 1. 4th edition. Edited by: Swern D. New York, USA: John Wiley and Sons; 1997.
Flores Luque V, Gómez Herrera C, Tabernero de la Linde P: Estudio de volumen molar y refracción molar de miscelas de triglicéridos (triacetina, tributirina o tricaprilina) y alcoholes (etanol, 1-butanol o 1-hexanol). Grasas y aceites 1992, 4: 216–220.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
JFSNA coordinator working, IGA responsible for the installation and testing, SAM processing and analysis of experimental results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Alonso, J.F.S.J., Arribas, I.G. & Miñambre, S.A. Study of combustion in residential oil burning equipment of animal by-products and derived products not intended for human consumption. Int J Energy Environ Eng 4, 31 (2013). https://doi.org/10.1186/2251-6832-4-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-4-31