Abstract
In this study, a modified ecological function is presented for four-temperature-level absorption refrigerators, and an absorption refrigerator has been optimized using this function. An equivalent system was initially identified, followed by the application of the first and second laws of thermodynamics. Next, the optimization results were interpreted. The results of the optimization process, optimum point of xH, xR, aH, and aR, were defined as 0.87, 0.58, 2.41, 0.25, respectively. In addition to that, the effects of xH, xR, aH, aR, Ta, Tc, Te, and Tg were shown according to exergy destruction, ecological function, coefficient of performance, and cooling load. As a result, it was understood that especially the xH, parameter, and the generator temperatures are the variables of the system that require special attention because, at some points, they cause that generator and absorber transfer heat in the opposite direction they need to do.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Absorption refrigerators may provide a cooling effect using various energy resources in the system such as waste heat and renewable energy. However, the factor that makes them attractive is not only the diversity of energy resources used, but also their economic aspects and minimal pollution effect on the environment. In addition to these, it was demonstrated that absorption refrigerators are more economic when they are integrated with the combined heat and power stations. Finite-time thermodynamics have been used for the optimization of thermal systems since the 1970s. The ecological criterion [1] suggested by Angulo-Brown is another optimization method. This criterion is shown as for the finite-time Carnot machines. Here, TL is the cold reservoir temperature, and σ is the entropy production of the system. However, Yan demonstrated that showing the ecological criterion as would be more reasonable [2]. Here, T0 is the ambient temperature. There are many studies in the literature for the optimization of irreversible absorption refrigerators. Bhardwaj et al. optimized absorption refrigerators using finite-time thermodynamics [3, 4]. Huang et al. examined the surface area, coefficient of performance (COP), and ecological criteria for the irreversible four-temperature-level absorption refrigerator and used an ecological function based on energy analysis [5]. The ecological function they used is . Here, φC is the Carnot COP of the system, TL is the cold reservoir temperature, is the cooling load, and is the entropy production. Chen offered models for the optimization of absorption refrigerators, heat pumps, and cooling machines [6, 7]. Sun et al. examined the optimization of the relation between heating load and entropy production of the internal heating heat exchanger [8]. Chen et al. offered a model for the analysis of four-temperature-level absorption refrigerator cycles [9]. Chen and Yan made an optimization on the irreversible absorption heat exchangers for ecological criterion [10]. Yan and Chen [11] and Yan and Lin [12] optimized the three-temperature-level absorption cycles for the ecological criterion on the basis of energy and exergy output of the irreversible Carnot cycles. Fathi et al. offered a model for irreversible and solar absorption cooling [13]. In addition to these, except for the ones stated here, there are many performance optimization studies for absorption refrigerators [14–17]. In addition, new optimization methods called ‘advanced exergy-based analysis methods’ [18, 19] or ‘exergy-entropy relationship’ [20] of the system can be investigated for the future studies. Contrary to other studies in which cooling load is the only evaluation criteria, this study aimed to provide maximum cooling load, minimum operation input, and minimum entropy production in the system. Due to this reason, a modified ecological function was presented. Considering the energy requirements of the world, the purpose of this study is to contribute to the more effective operation of absorption refrigerators by presenting a modified ecological function.
Methods
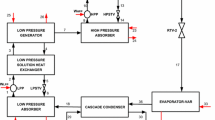
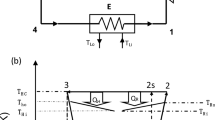
In this study, a four-temperature-level absorption refrigerator was optimized using a modified ecological function, and the results were presented. A vapor absorption refrigeration system consists of four components as generator, absorber, evaporator, and condenser. Working fluid in the system is made of a refrigerant and a solution that absorbs refrigerant. Refrigerant vapor comes from the evaporator and goes to the absorber, and it is absorbed in a liquid. The liquid is sent to the generator and is boiled out of the solution, taking the generator’s heat reservoir. It is sent to the condenser and becomes a rejected heat to the environment. Finally, working fluid comes back to the evaporator, and the cycle is completed. The system is the general absorption refrigerator (seen in Figure 1). Temperatures of heat reservoirs are TA, TC, TE, and TG in absorber, condenser, evaporator, and generator, respectively. Ta, Tc, Te, and Tg are working fluid temperatures in absorber, condenser, evaporator, and generator, respectively. In Figure 2, an equivalent system is defined. In the equivalent system, the absorber-generator assembly can be considered as heat engine part, and similarly, condenser-evaporator part can be considered as refrigerator part.
Generalized four-temperature-absorption heat pump system[3].
An equivalent system for generalized four-temperature-absorption heat pump system[3].
Thermodynamic analysis of system
Thermodynamic analysis and optimization were established for the equivalent system, and previous formulas offered by Chen et al. were used in the calculations [21]. The ambient temperature was assumed as 300 K.
Thermodynamic analysis of the absorber-generator assembly (heat engine part)
According to the first law of thermodynamics,
Here, (heat input from the generator heat source) and (heat output to the absorber heat sink) may be defined as
According to the second law of thermodynamics,
Here, IH is the internal irreversible parameter for absorber-generator assembly. IH > 0 for the irreversible cycles, and IH = 0 for the reversible cycles. Working fluid temperature ratio (xH), heat transfer surface area ratio (aH), and total heat transfer surface area (AT,H) for the absorber-generator assembly can be defined as following:
Tg, Ta, and can be defined using Equations (1) to (3):
Thermodynamic analysis of the condenser-evaporator assembly (refrigerator part)
According to the first law of thermodynamics,
Here, (heat rejection from the condenser) and (heat input to the evaporator) are
According to the second law of thermodynamics,
Here, I R is the internal irreversible parameter for condenser-evaporator assembly. IR > 0 for the irreversible cycles, and IR = 0 for the reversible cycles. Working fluid temperature ratio (xR), heat transfer surface area ratio (aR), and total heat transfer surface area (AT,R) for the condenser-evaporator assembly can be defined as following:
Tc, Tg, , and can be defined using Equations (8) to (11):
Optimum ecological function of the system
The modified ecological function, including the values of maximum cooling load in the absorption cooling system, maximum power output for the heat engine, and maximum energy destruction of the system is defined below:
Substituting (3) to (15) in (16) and setting Equation (16)'s derivation to zero with respect to , the value for which the optimum heat exchanger area was detected for the assembly of the absorber-generator and which corresponds to such area is as below:
Similarly, the optimum heat rate of the operation fluids is detected by decreasing the derivative of the ecological function to for the assembly of the absorber-generator, and the value corresponding to this rate is as below:
By applying the same calculations for the assembly of the condenser-evaporator ,
values are detected, and these values are as below,
Substituting (17) to (20) in (16), the optimized ecological function is calculated as
Optimum COP of the system
The COP of the absorption system can be defined as
The COP of the system is described by substituting Equations (7) and (15) into (22)
The optimized COP value is calculated as below, by putting the values in their places in Equation (23):
Optimum cooling load of the system
The optimum cooling load of the system is calculated by putting the optimized values in their places in Equation (15):
Results and discussion
This section presents an implementation for the absorption. The performance characteristics used for the numerical calculations are given in Table 1. The calculated optimum parameters for the system are listed in Table 2.
The ecological function and exergy destruction of the absorption cooling system and its change according to COP aH are given in Figure 3. Here, it can be seen that as the COP value aH increases, it decreases logarithmically, and it decreases to the value of 2.6 for the optimum aH value. However, this value is quite high for the currently used absorption cooling systems. In addition, it can be seen that the aH value is not very effective for the exergy destruction. While the aH value changes between 0.1 and 03, the increase seen for the exergy destruction is 3 kW.
The ecological function, exergy destruction, and cooling load of the absorption cooling system and its change according to COP aR are given in Figure 4. Here, it can be seen that the ecological function, exergy destruction, and cooling load increase logarithmically until optimum point with the increase of the aR parameter.
In Figure 5, the change of absorption cooling system according to xH is evaluated for the ecological function, exergy destruction, and COP. For the values of xH lower than 0.82, it can be seen that the generator radiates heat to the environment instead of removing heat from the environment, and the absorber removes heat from the environment instead of radiating heat to the environment. In other words, the heat machine part consumes power instead of producing power, and the system becomes unusable with these values. The COP of the system decreases with the increase of xH, and it requires an approximate value of 2.6 for the optimum xH. Exergy destruction also increases with the increase of xH, and an increase of 300 kW is seen with an exergy destruction in xH with a value of 0.2.
In Figure 6, the change of the absorption cooling system according to xR is evaluated for the ecological function, exergy destruction, cooling load, and COP. The COP of the system decreases with the increase of xR, and it approximately takes a value of 2.4 for the optimum xR. Exergy destruction and ecological function change drastically together with xR. The cooling load decreases with the increase of the value of xR. While the cooling capacity is 4917.49 kW when the xR value is 0.05, it decreases to 339.317 kW when the xR value is 0.65.
The effects of the absorber temperature on the system are given in Figure 7. As the absorber temperature increases, it can be seen that while the ecological function and exergy destruction values decrease, the COP value increases.
The effect of the generator temperature on the absorption cooling system is given in Figure 8. For temperatures lower than 335 K, the generator radiates heat to the environment instead of removing heat from the environment, so it cannot be used in lower temperatures. In addition to this, while the increase of the generator temperature increases ecological function and exergy destruction, it decreases the COP.
The effect of a condenser on the system is given in Figure 9. As seen here, as the condenser temperature increases, the ecological function, exergy destruction, cooling load, and COP values decrease.
In Figure 10, COP, ecological function, exergy destruction, and cooling load increase linearly with the increase of evaporator temperature.
Conclusions
The optimization of four-temperature-level irreversible absorption refrigerator was researched for modified ecological function in this study. By this method, it was attempted to establish a balance between the cooling load, the load supplied to the system, and entropy production. The effects of aH, aR, xH, and xR parameters and operation fluid temperatures on the system were studied, and the results were shown in Figures 3, 4, 5, 6, 7, 8, 9, and 10. Considering these results, the criteria that should be observed in designing an absorption cooling cycle were determined, and the parameters that should be observed were shown. This study will hopefully be a model for absorption refrigerator designs.
Abbreviations
Nomenclature
a, ratio of heat exchanger surface areas; A, heat exchanger surface area (m2); COP, coefficient of performance; , ecological function (kW); ExD, exergy destruction (kW); I, internal irreversibility parameter; m, ratio of environment temperature to absorber; n, ratio of environment temperature to generator; , heat (kW); T, temperature (K); , work (kW); x, ratio of temperatures; y, ratio of environment temperature to condenser; z, ratio of environment temperature to evaporator.
Subscripts
0, environment; 1, inlet, 2, outlet; a, absorber working fluid temperature; A, mean absorber heat source temperature; AB, evaporator; c, condenser working fluid temperature; C, mean condenser heat source temperature; CO, condenser; e, evaporator working fluid temperature; EC, ecological; E, mean evaporator heat source temperature; EV, evaporator; g, generator working fluid temperature; G, mean generator heat source temperature; GE, generator; H, heat engine part; R, refrigerator part; o, optimum; T, total.
Greek letters
α, total heat conductance of generator (kW m−2 K−1); β, total heat conductance of absorber (kW m−2 K−1); Φ, coefficient of performance of system (COP); μ, total heat conductance of condenser (kW m−2 K−1); θ, total heat conductance of evaporator (kW m−2 K−1); , entropy generation (kW K−1).
Authors’ information
EA is a research assistant in the Faculty of Engineering at Bilecik S.E. University. He received his BSc and MSc in the Engineering and Architecture Faculty in Eskisehir Osmangazi University in 2000 and 2004, respectively.
References
Angulo-Brown F: An ecological optimization criterion for finite-time heat engines. J. Appl. Phys. 1991, 69(11):7465–7469. 10.1063/1.347562
Yan Z: Comment on “Ecological optimization criterion for finite-time heat-engines”. J. Appl. Phys. 1993, 73(7):3583. 10.1063/1.354041
Bhardwaj PK, Kaushik SC, Jain S: Finite time optimization of an endoreversible and irreversible vapour absorption refrigeration system. Energ. Convers. Manage. 2003, 44(7):1131–1144. 10.1016/S0196-8904(02)00101-2
Bhardwaj PK, Kaushik SC, Jain S: General performance characteristics of an irreversible vapour absorption refrigeration system using finite time thermodynamic approach. Int. J. Ther. Sci. 2005, 44(2):189–196. 10.1016/j.ijthermalsci.2004.07.004
Huang Y, Sun D, Kang Y: Performance optimization for an irreversible four-temperature-level absorption heat pump. Int. J. Ther. Sci. 2008, 47(4):7479–7485.
Chen J: The optimum performance characteristics of a four-temperature-level irreversible absorption refrigerator at maximum specific cooling load. J. Phys. D: Appl. Phys. 1999, 32(23):3085–3910. 10.1088/0022-3727/32/23/315
Chen J: Optimal performance analysis of irreversible cycles used as heat pumps and refrigerators. J. Phys. D: Appl. Phys. 1997, 30(4):582–587. 10.1088/0022-3727/30/4/012
Sun F, Qin X, Chen L, Wu C: Optimization between heating load and entropy-production rate for endoreversible absorption heat-transformers. Appl. Energy. 2005, 81(4):434–448. 10.1016/j.apenergy.2004.09.003
Chen L, Zheng T, Sun F, Wu C: Irreversible four-temperature-level absorption refrigerator. Sol. Energy 2006, 80(3):347–360. 10.1016/j.solener.2005.01.013
Chen T, Yan Z: An ecological optimization criterion for a class of irreversible absorption heat transformers. J. Phys. D: Appl. Phys. 1998, 31(9):1078–1082. 10.1088/0022-3727/31/9/008
Yan Z, Chen T: Optimization of the rate of exergy output for an endoreversible Carnot refrigerator. J. Phys. D: Appl. Phys. 1996, 29(12):3017–3021. 10.1088/0022-3727/29/12/013
Yan Z, Lin G: Ecological optimization criterion for an irreversible three-heat-source refrigerator. Appl. Energy. 2000, 66(3):213–224. 10.1016/S0306-2619(99)00134-8
Fathi R, Guemimi C, Ouaskit S: An irreversible thermodynamic model for solar absorption refrigerator. Renew. Energ. 2004, 29(8):1349–1365. 10.1016/j.renene.2003.07.011
Gordon JM, Ng KC: Cool Thermodynamics. Cambridge: Cambridge International Science; 2000.
Ng KC, Chua HT, Han Q, Kashiwagi T, Akisawa A, Tsurusawa T: Thermodynamic modeling of absorption chiller and comparison with experiments. Heat Transfer Eng 1999, 20(2):42–51. 10.1080/014576399271574
Ng KC, Wang XL: Thermodynamic methods for performance analysis of chillers. P. I. Mech. Eng. E-J. Pro. 2005, 219(2):109–116.
Gordon JM, Ng KC: Predictive and diagnostic aspects of a universal thermodynamic models for chillers. Int. J. Heat Mass Tran. 1995, 38(5):807–818. 10.1016/0017-9310(94)00208-D
Morosuk T, Tsatsaronis G, Boyano A, Gantiva C: Advanced exergy-based analyses applied to a system including LNG regasification and electricity generation. Int. J. Energy Environ. Eng. 2012, 3(1):1–9.
Tsatsaronis G, Morosuk T: Advanced exergetic analysis of a refrigeration system for liquefaction of natural gas. Int. J. Energy Environ. Eng. 2010, 1(1):1–17.
Palazzo P: Thermal and mechanical aspect of entropy-exergy relationship. Int. J. Energy Environ. Eng. 2012, 3(4):1–10.
Chen L, Xiaoqin Z, Sun F, Wu C: Exergy-based ecological optimization for a generalized irreversible Carnot heat-pump. Appl. Energy. 2007, 84(1):78–88. 10.1016/j.apenergy.2006.04.003
Florides GA, Kalogirou SA, Tassou SA, Wrobel LC: Design and construction of a LiBr–water absorption machine. Energy Convers. Manage 2003, 44(15):2483–2508. 10.1016/S0196-8904(03)00006-2
Acknowledgments
The authors would like to express their sincere gratitude to the editor (Professor Farivar Fazelpour), anonymous reviewers for their invaluable comments and suggestions which substantially improved the overall quality of the manuscript, and to the language editor Sherlyn C Machica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Acikkalp, E. Modified thermo-ecological optimization for refrigeration systems and an application for irreversible four-temperature-level absorption refrigerator. Int J Energy Environ Eng 4, 20 (2013). https://doi.org/10.1186/2251-6832-4-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-4-20