Abstract
Background
Diabetes mellitus is a chronic metabolic disorder characterized by increased blood glucose level. It has become an epidemic disease in the 21st century where, India leads the world with largest number of diabetic subjects. Non-enzymatic glycosylation (glycation) is severe form of diabetes, occurs between reducing sugar and proteins which results in the formation of advanced glycation end products (AGEs) that leads to the other complicated secondary disorders. In this context, Mangifera indica (Mango), Syzygium cumini (Jambul), Vitis vinifera (Grapes), Citrus sinensis (Orange), Artocarpus heterophyllus (Jackfruit), Manilkara zapota (Sapodilla) seeds were evaluated for their antiglyation activity. Attempts were made to isolate the polyphenols in the seeds that have recorded the maximum activity.
Methods
Different extraction methods (shake flask, centrifugation and pressurized hot water) using various extractants (organic solvents, hot water and pressurized hot water) were adopted to investigate the in vitro antiglycation activity. Central composite (CCD) design based Response Surface Methodology (RSM) was espoused to optimize the extraction process of polyphenols from the fruit seeds that have recorded poor antiglycation activity. The PTLC analysis was performed to isolate the polyphenols (Flavonoids and phenolic acids) and LC-PDA-MS analysis was done for structure prediction.
Results
Pressurized hot water extraction of Artocarpus heterophyllus (87.52%) and Citrus sinensis seeds (74.79%) was found to possess high and low antiglycation activity, respectively. The RSM mediated optimization process adopted for the Citrus sinensis seeds have revealed that 1:15 solvent ratio (hexane to heptane), 6 minutes and 1:20 solid to liquid ratio as the optimal conditions for the extraction of polyphenols with a maximum antiglycation activity (89.79%). The LC-PDA-MS analysis of preparative thin layer chromatography (PTLC) eluates of Artocarpus heterophyllus seed has showed the presence of compounds like quercetin (301.2), 4-hydroxy phenyl acetic acid (149.0), rhamnosyl-di-hexosyl quercetin sulphate (857.6), quercetin-3-O-xyloside (428.2), rutin (613.4), diosmetin (298.1) and luteolin (283.0).
Conclusion
The Artocarpus heterophyllus was observed to possess a significant antiglycation activity and the activity of Citrus sinensis was improved after the optimization process, which proved that both the seeds may be used as a traditional medicine in the management of chronic diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus, a metabolic endocrine disorder, has become a common global health problem that affects >170 million people worldwide. It is one of the leading causes of death and disability. It is estimated that by 2030, the number will rise to 366 million. The majority of diabetes (~90%) is type 2 diabetes (T2D) caused by a combination of impaired insulin secretion from pancreatic beta cells and insulin resistance of the peripheral target tissues, especially muscle and liver [1]. Uncontrolled diabetes can complicate pregnancy and birth defects. Generally, the long-term complications of diabetes are categorized into macrovascular (coronary artery disease, peripheral arterial disease, and stroke) and microvascular (diabetic nephropathy, neuropathy, and retinopathy) [2].
High glucose level in the blood can be subjected for a spontaneous reaction that occurs between the aldehyde group and amino groups of the protein chain that leads to the formation of the Schiff base. This intermediate subsequently undergo a number of biochemical reactions to yield various fluorescent brown pigments known as advanced glycation end products [3]. The oxidation process is believed to play an important role in formation of AGEs. Further oxidation of AGEs leads to the formation of intermediate carbonyl compounds that can react with the nearby lysine or arginine residues to form protein crosslinks and therefore, agents with antioxidant property may retard the process of AGEs formation and their subsequent oxidation progression. Recently, role of fruits in the prevention of glycation activity has gained a substantial attention among scientific community and various families like Anarcardiaceae, Curcubitaceae, Moraceae, Annonaceae, Sapotaceae, Phyllanthus, Myrtaceae, Rutaceae etc. have been documented for their noteworthy role to own antioxidant and antiglycation activities. Several natural compounds like curcumin, rutin and garcinol have been reported to possess antioxidant property as well their role in the prevention of formation of AGEs in vitro and in vivo[4].
The investigation of antidiabetic agents of plant origin which are used in traditional medicine is of great importance. The seed kernel of Mangifera indica is one such herbal source which is mentioned in Ayurvedic literature for treating Diabetes mellitus. The kernel is astringent, anti-helmintic, stimulant, anti-inflammatory, antibacterial, antifungal, anti-pasmodic, anti-scorbutic and is administered in asthma, diabetes, nasal bleeding, diarrhea and ulcers [5]. The bark, leaves and seed extracts of Syzygium cumini have been reported to possess anti-inflammatory, anti-bacterial and anti-diarrheal effect [6]. Vitis vinifera seed have a variety of health benefits like antibacterial, antiviral, anticarcinogenic, anti-inflammatory, antioxidant and vasodilatory actions [7]. Similarly, Artocarpus heterophyllus possess numerous medicinal properties such as antibacterial, antioxidant, antidiabetic, anti-inflammatory, anti-diuretic, immunomodulatory and have been useful in the treatment of fever, skin diseases, convulsions, constipation, ophthalmic disorders and snake bite. The seeds were added to flour and baked or boiled/roasted and consumed as nutritious diet [8]. Citrus sinensis, belongs to Rutaceae family possess antioxidant, antibacterial, antifungal, anti-carcinogenic, anti-ulcer, anti-anxiety, antidiabetic and anti-inflammatory properties [9]. Manilkara zapota is a plant with traditional and medical importance. It contains several compounds like phenolics, alkaloids, epicatechin, leucocyanidin, leucodelphinidin, leucopelargonidin, chlorogenic acid, gallic acid, sugars, carotenoids and minerals like calcium, iron, zinc, copper and potassium. It has documented several properties like antibacterial, antifungal, antipyretic, antioxidant, antiulcer and used in the treatment of fever, wounds, diarrohea and pulmonary ailments [10].
Even though the antidiabetic activity has been reported in the plant species, no work on antiglycation has been explored in the above mentioned plants. In this milieu, our laboratory have focused on the evaluation of in vitro antiglycation activity in the seeds of Mangifera indica, Syzygium cumini, Vitis vinifera, Citrus sinensis, Artocarpus heterophyllus and Manilkara zapota.
Materials and methods
Chemicals
Bovine serum albumin (Fraction V), Glucose, Sodium azide and Nitro-blue tetrazolium was obtained from Himedia bioscience company Ltd., India.
Seed collection
Mangifera indica, Artocarpus heterophyllus, Manilkara zapota, Citrus sinensis, Syzygium cumini and Vitis vinifera fruits were obtained from the local market. The seeds were removed, dried at 60˚C for 5days, fine powdered and used for the experimental analysis.
Preparation of hot water extract (HWE)
In a clean dry 250 ml conical flask, 0.5 g of powdered material was weighed and extracted with 25 ml of distilled water by placing it in a boiling water bath at temperature 90˚C for 5 minutes. The content was filtered using Whatman No.2 filter paper, the filtrate obtained is precipitated by adding 10% of ammonium sulphate and centrifuged at 5000 rpm for 10 minutes. The supernatant were used to investigate the in vitro antiglycation activity.
Preparation of organic solvent extract
In a clean dry 250 ml of separating funnel, 5 g of powdered material was added to 20 ml of petroleum ether and shook thoroughly for 15 minutes. The resultant mixture was filtered and the filtrate was used for the antiglycation assay. The obtained residue was then re-extracted with chloroform, acetone, ethyl acetate, ethanol and distilled water, respectively and the process was repeated as mentioned above for each solvent extract. Each of the content was filtered using Whatman No.2 filter paper, the filtrate obtained is precipitated by adding 10% of ammonium sulphate and centrifuged at 5000 rpm for 10 minutes. The supernatant used for the experimental analysis [11].
Preparation of pressurized hot water extract (PHWE)
About 0.5 g of powdered material was weighed in a clean dry pressurized vessel and added 5 ml of distilled water. The vessel is placed in an oil bath at 180˚C, 1002 kPa for 10 minutes. The resultant mixture was filtered using Whatman No.2 filter paper, the filtrate obtained is precipitated by adding 10% of ammonium sulphate and centrifuged at 5000 rpm for 10 minutes and the supernatant was used for the assay.
In vitro antiglycation assay
A slightly modified method proposed by [12] was adopted for the evaluation of antiglycation activity. About 10 ml of BSA (20 mg/ml), 5 ml of glucose (500 mM) and 0.02% of sodium azide, dissolved in phosphate buffer (200 mM, pH 7.4) was added to 5 ml of the test sample and incubated at 37 C for 5 days. A mixture of BSA, glucose and sodium azide was used as a control along with the test sample. After 5days, 0.5 ml of the glycated material was mixed with 2 ml of nitro-blue tetrazolium (0.3 mM) dissolved in sodium carbonate buffer (100 mM, pH 10.35), incubated at room temperature for 15minutes and the absorbance was read spectrophotometrically (Beckman DU-530) at 530 nm.
Estimation of total phenolic content (TPC) by Folin-Ciocalteau method
A method proposed by [13] was adopted to determine the total phenolic content. To 0.1 ml of the extract, added 3.9 ml of distilled water and 0.5 ml of Folin’s reagent. The tube was incubated at room temperature for 3 minute. To this added 2 ml of 20% sodium carbonate and kept at boiling water bath for 1 minute. The blue color formed was read at 650 nm. Gallic acid was used as a standard for constructing a calibration curve.
Two-dimensional thin layer chromatography (TLC) and preparative thin layer chromatography (PTLC)
The glass plates (20 × 10 cm) were coated with the silica gel (0.1-0.2 mm thickness) with the help of an applicator and dried for 24 hrs to remove moisture or water and other adsorbed substances from the surface, in order to activate the plate. 40 μl of test sample was spotted from 2 cm above the base of the plate and a two dimensional thin layer chromatogram was developed using ethyl acetate: formic acid: acetic acid: distilled water (100: 11: 11: 26) and 15% acetic acid as mobile phase solvents. The plates were dried before spraying liquid ammonia (spraying agent) and visualized under long UV light at 360 nm to detect the presence of flavonoids [14]. The PTLC analysis was performed according to the procedure described by Meena and Patni, [15].
LC-PDA-MS (ESI+) analysis
The liquid chromatography electron spray mass spectrometry (LC-MS) analysis was performed on Varian Inc, (USA) 410 Prostar Binary LC with 500 MS IT PDA Detectors. The column was C18, 250 × 4.6 mm, i.d. 5 μm. The mobile phase A was made up of acetonitrile, while B was made of 0.1% formic acid (pH 4.0, adjusted with ammonium hydroxide). The gradient elution was performed at 1 ml/min with an initial condition of 12% of mobile phase A and 88% of mobile phase B for 10 min. The mobile phase A was linearly increased from 15% to 100% and analysis was performed from 20 minutes to 95 minutes. The eluates were monitored by PDA (Multi wavelength) detector at 260 nm. About 20 μl of the PTLC Artocarpus heterophyllus seed eluate was introduced into the ESI source and the mass spectra were scanned in the range 100–1000 amu and the maximum ion injection time was set at 200nS. Ion spray voltage was set at 5.3 KV and capillary voltage 34 V. The MS scan ran up to 26.67 minutes.
Optimization of polyphenols using response surface methodology (RSM)
The optimum extraction condition for the polyphenols (flavonoids and phenolic acids) was determined by central composite design (CCD) based response surface methodology that includes three variables and three factorial levels (For a total of 20 runs, 4 runs were dummy variables). The experimental design is a full factorial CCD with 8 factorial cube points, 6 axial points and 6 centre points in cube (Table 1). The independent variables used in this study were hexane: heptane ratio (v/v), extraction time (minutes) and solid: liquid ratio (g/ml). A single factor analysis of variance was adopted to investigate the effect of each factor on the extraction of polyphenols. Second-order polynomial equation was used to express the investigated responses (Y) as a function of the coded independent variables, where X1, X2…X3 are the independent variables affecting the responses Y, β0, βj, (i = 1, 2…k), βii (i = 1, 2…k), and βij (i = 1, 2…k; j = 1, 2…k) are regression coefficients for intercept, linear, quadratic, and interaction terms, respectively; k is the number of variables. Coded independent variables for our experiment are solvent ratio, time and solid: liquid ratio.
Statistical analysis
Statistical analysis was performed using Minitab 15 (trial version). The results were statistically tested by the analysis of variance (ANOVA) at 5% level of significance. Response surface and contour plots were generated and analyzed.
Results and discussion
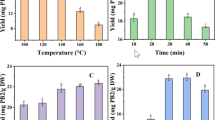
The experimental analysis on the evaluation of the antiglycation property has revealed that Artocarpus heterophyllus (PHWE: 87.52%) and Mangifera indica (PHWE: 84.76%) possess maximal activity, Vitis vinifera (solvent extraction: 83.31%) and Manilakara zapota (PHWE: 81.59%) own near maximal activity and Syzygium cumini (solvent extraction: 78.14%) and Citrus sinensis (solvent extraction: 75.65%) hold near moderate or considered as poor activity. On the comparison of different extraction methodologies like HWE, PHWE and solvent extraction, it was observed and recorded that HWE (41.65 ± 13.95%) was inferior, and both solvent based (77.7 ± 6.78%) and PHWE (76.70 ± 20.98%) was superior in the extraction of phytochemicals that were responsible for the antiglycation activity. A Comparison of in vitro antiglycation activity of six different seeds using different extraction methods has been depicted in Table 2. One way ANOVA analysis has revealed that there is a significant difference at 5% level (p < 0.05), between the seeds in the antiglycation activity (Table 3).
Medicinal plants, now-a-days, gain significant attention among research community due of its greater exploitation by the common public for the better prevention and cure of various ailments. Because of the adverse and or toxic effects of synthetic drugs sizeable awareness has been created on par with natural remedies which are safe, effective and non-toxic. The traditional techniques of solvent extraction of plant based materials are typically based on the extraction type, choice of right solvents, temperature, extraction type, agitation and material ratio for improved extraction efficiency as well as the mass transfer [16].
Artocarpus heterophyllus is generally recommended by traditional medical practitioners as a classical treatment for diabetes mellitus. Significant reduction of blood glucose (49%) through the administration of flavonoid fraction of the leaf in the alloxan induced diabetic rats has been reported. Moreover, no significant necrosis of hepatic, renal and cardiac tissues was observed and documented which, proved the non-toxicity of the leaves [17]. Similarly, investigation conducted by [18] has documented the efficacy of the hypoglycemic effect of Artocarpus heterophyllus leaves and the studies have proved a noteworthy reduction in the blood glucose and total cholesterol. Studies conducted by [19] have revealed the potent antidiabetic activity of Artocarpus heterophyllus fruits and its remarkable impact in the increase of glucose tolerance. Furthermore, investigation in the leaves of Artocarpus heterophyllus have classically indicated its efficiency in the attenuation of glycosylation of hemoglobin, enhancement in the transport of glucose across cells, stimulation of insulin release and inhibition of cholesterol biosynthetic enzymes [20, 21]. The present results on the significant and superior effect of the antiglycation property of PHWE of Artocarpus heterophyllus seeds was well corroborated with the above mentioned scientific documentation.
RSM is defined as a statistical technique adopted for any quantitative data from suitable experimental designs to determine and solve the multivariate equations [22]. Application of RSM in the optimization process may save cost, time and energy. Central composite design (CCD) is widely used for fitting a second order method in response surface method which consisted of four runs at the corners of the square, four runs at the center of this square and four axial runs [23]. It used to estimate parameters of a full second order polynomial model. CCD had been introduced by Box and William on 1957 which was divided into three point group of design; factorial, axial point and centre point.
In the present study, a suitable central composite based experimental design (CCD) has been formulated in order to develop an empirical model to investigate the interactions of the selected variables responsible for the extraction of polyphenols (flavonoids and phenolic acids) from the seeds of Citrus sinensis. The surface and contour diagrams depicted in Figure 1 have indicated that maximum antiglycation activity (89.79%) was obtained under the optimal conditions (extraction of polyphenols) at 1: 15 (hexane: heptane ratio, (v/v)), 6 minutes (Time), and 1: 20 (solid: liquid ratio (g/ml)) (Table 4). A significant increase (15%) in the antiglycation activity of the optimal seed extract of Citrus sinensis has been observed compared with hot water, pressurized hot water and solvent extracts (Figure 2). From multiple regression analysis, it was observed that the second-order polynomial equation generated from the experimental design can explain the antiglycation activity (%) regardless of the significance of coefficients:
where, Y represents the dependent variable (% antiglycation activity) and x1, x2 and x3 represents the independent ratio, time and solid–liquid ratio). The goodness of fit manifested by determination coefficient (R2 = 0.90) showed that the sample variation of 90% for antiglycation activity is characteristic to the independent variables and only 10% of the total variation cannot be explained by the developed model. The positive coefficient of hexane-heptane ratio (x1) and time (x2) revealed a linear effect in the increased antiglycation activity, whereas, the negative coefficient of solid: liquid ratio (x3) indicated an inverse effect (i.e., increase in the ratio leads to decreased antiglycation activity and vice versa). The cross-product results clearly showed that the most significant interaction has occurred between hexane-heptane ratio (x1) and time (x2), and also equally between time (x2) and solid: liquid ratio (x3). All the three variables mentioned above were found to significant at 5% level (p < 0.05) (Table 5).
Apparently, it has been proved that the experimental runs at centre points (run No.10 and 14–20) didn’t record the maximum antiglycation activity and this may be because of the inverse effect of solid: liquid ratio. The recovery yield of polyphenols through its solubility in the system can be highly influenced by the solvent system adopted. Solvent polarity plays a pivotal role in the increasing solubility of polyphenols. As per the previous reports by [24–27], mostly aqueous mixtures of ethanol, methanol, acetonitrile and acetone at various volume ratios were significant in the extraction of polyphenols. However, contradictory results in the selection of solvent, i.e., high non-polar solvents like hexane have been also explored and documented its efficiency in the extraction process [28]. A similar conflicting result was observed and recorded in our present investigation.
In order to understand the extraction kinetics of polyphenols effectively, it is an appropriate choice in the experimental design to incorporate the time variability parameter. This may avoid an increase loss of polyphenols, provides maximal antiglycation activity and also to find out the time range that leads to a minimal variability in the recovery of polyphenols [29]. Generally, longer the extraction time may leads to the loss of the polyphenols via oxidation. The oxidized products may polymerize to form insoluble compounds and inhibit the diffusion of the polyphenols. According to Fick’s law, this could be because of the existence of equilibrium between the solute and the solvent [30]. Hence, prolonged extraction time is futile, grab a lot of energy, cost and in our case, the extraction time was found to be very low (6 minutes). Previous reports [31, 32] on the recovery of flavonoids from the Citrus peels have revealed a maximum of 30 minutes and 3 hrs optimal extraction time, respectively and our present investigation in this dimension was well correlated with the above mentioned scientific findings.
The effect of solid/liquid ratio has a positive outcome in our present study, i.e., enhanced antiglycation activity due to increased extraction of polyphenols at higher ratio (1: 20). Mass transfer is the principle phenomenon, where a concentration gradient may be larger at high solid/liquid ratio, which greatly influences the dissolution of the solutes from the sample matrix to the solvent [33].
TLC is used for the analysis of several phytochemicals. The advantages of using TLC to construct the fingerprints of herbal medicines are its simplicity, versatility, high velocity, specific sensitivity and simple sample preparation. Thus, TLC is a convenient method of determining the quality and possible adulteration of herbal products [34]. Both the seed extracts viz., Artocarpus heterophyllus and Citrus sinensis, respectively has revealed the presence of polyphenols that includes flavonols, flavonoids glycosides and phenolic acids. Due to the maximal antiglycation activity, Artocarpus heterophyllus seeds were subjected for PTLC and further mass spectrometry analysis in the prediction of various polyphenolic compounds. The identification of flavonoids and phenolic acids in the 2D PTLC eluate of Artocarpus heterophyllus seeds was depicted in Figure 3 and the antiglycation activity was found to be 95.38%, which is about 8% increase in the activity compared to PHWE Artocarpus heterophyllus. This substantiates a strong role of polyphenols like flavonoids in the attenuation of glycation activity.
LC-PDA-MS (ESI+) Analysis of Artocarpus heterophyllus PTLC eluate have revealed the presence of compounds like rutin, quercetin, quercetin-3-O-xyloside, 4-hydroxy phenyl acetic acid, rhamnosyl-di-hexosyl quercein sulphate, luteolin and diosmetin (Table 6).
Artocarpus is an important genus in the Family of Moraceae and the phytochemical constituents distributed in this genus are mainly flavonoids, especially flavones, isoflavones and chalcones, xanthones and prenylated stilbenes. So far, seventy prenylated flavonoids have been isolated and possess a wide range of biological properties [35]. Previously, Omar et al., [36] has isolated isosquercitrin from the leaves of Artocarpus heterophyllus and explained its potent antioxidant, antidiabetic and antihyperlipidemic activities. Bourjot et al., [37] has isolated prenylated flavonoids like styracifolins, heterophyllin, artonins and artoheterophyllin with strong antiplasmodial and antitrypanosomal activities from the stem bark of Artocarpus styracifolius. Recently, Maneechai et al., [38] have reported the presence of certain prenylated derivatives of 5,7,2′,4′-tetrahydroxyflavones and 2,4,3′,5′-tetrahydroxystilbene (oxyresveratrol) with potent free radical scavenging activities from the Callus cultures of Artocarpus lakoocha. Compounds like artocarpusins A, B and C, chalcone and flavone, respectively, and known flavonoids with significant anticancer activities were isolated from the twigs of Artocarpus heterophyllus[39].
Previous scientific documentation by [40–44] on the identification and fragmentation pattern of flavonoids analyzed through LC-MS have clearly supported with our present recorded results (Figure 4).
Conclusion
From the present study, it was found that PHWE of Artocarpus heterophyllus possess a strong antiglycation activity. Flavonoid compounds like rutin, Quercetin, Quercetin-3-O-xyloside etc., may be responsible for the antiglycation activity. An improved antiglycation activity in Citrus sinensis seeds was observed and recorded after the CCD based RSM optimization process. Finally, it was concluded that both Artocarpus heterophyllus and Citrus sinensis can be used as a classical herbal medicine in the management of chronic diabetes mellitus.
References
Singh S: The genetics of Type 2 diabetes mellitus: a review. J Sci Res 2011, 55: 35–48.
Bastaki S: Diabetes mellitus and its treatment – a review. Int J Diabetes Metab 2005, 13: 111–134.
Esmaeili MA, Kanani MR, Sonboli A: Salvia reuterana extract prevents formation of advanced glycation end products: an in vitro s tudy. Iran J Pharm Sci 2010, 6(1):33–50.
Jedsadayanmata A: In vitro antiglycation activity of arbutin. Naresuan University Journal 2005, 13(2):35–40.
Jain N, Srivastava B, Sharma P, Khandelwal DK: Antihyperglycemic effect of Mangifera indica Linn (seed kernel). Asian J Biochem Pharm Res 2011, 3(1):195–200.
Kumar A, Illavarasan R, Jayachandran T, Deecaraman M, Aravindan P, Padmanabhan N, Krishnan MRV: Antidiabetic activity of Syzygium cumini and its isolated compounds against streptozotocin-induced diabetic rats. J Med Plants Res 2008, 2(9):246–249.
Hsi S-C, Kao Y-P, Wang P-Y, Chao H-M, Huang C-H, Liu H-S, Shih L-J, Tschen JS-M, Lin C-L, Kao Y-H: Grape seed procyanidins improve diabetic symptoms in mice with streptozotocin-induced diabetes. The Open Physiol Jl 2009, 2: 6–13. 10.2174/1874360900902010006
Prakash O, Kumar R, Mishra A, Gupta R: Artocarpus heterophyllus (Jackfruit): an overview. Phcog Rev 2009, 6: 353–358.
Milind P, Dev C: Orange: Range of Benefits. Int Res J Pharm 2012, 3(7):59–63.
Singh M, Shivhare Y: Manilkara Zapota : an overview. Herbal Tech Industry 2011, 8(6):13–16. June
Ghasemzadeh A, Jaafar HZE, Rahmat A: Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J Med Plants Res 2011, 5(7):1147–1154.
Majumdar M, Parihar PS: Antibacterial, antioxidant and antiglycation potential of Costus pictus from Southern region India. Asian J Plant Sci Res 2012, 2(2):95–101.
Singleton VL, Rossi JA: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 1965, 16: 144–153.
Ćetković GS, Đilas SM, Čanadanović-Brunet JM, Tumbas VT: Thin-layer chromatography analysis and scavenging activity of marigold (Calendula officinalis L.) extracts. APTEFF 2003, 34: 1–148.
Meena MC, Patni V: Isolation and Identification of Flavonoid “Quercetin” from Citrullus colocynthis (Linn.) Schrad. Asian J Exp Sci 2008, 22(1):137–142.
Thomas R, Tripathi R, Kamat SD, Kamat DV: Comparative study of phenolics and antioxidant activity of phytochemicals of T. chebula extracted using microwave and ultrasonication. Int J Pharm Sci Res 2012, 3(1):194–197.
Chandrika UG, Wedage WS, Wickramasinghe SMDN, Fernando WS: Hypoglycaemic action of the flavonoid fraction of Artocarpus heterophyllus leaf. Afr J Trad CAM 2006, 3(2):42–50.
Shahin N, Alam S, Ali M: Pharmacognostical Standardisation and Antidiabetic activity of Artocarpus Heterophyllus Leaves Lam. Int J Drug Dev Res 2012, 4(1):346–352.
Barik R, Jain S, Sharma R, Kumar P, Bhatt D: Effect of fruit extract of Artocarpus heterophyllus in alloxan induced diabetic rats. Int J Pharm Sci Res 2010, 1(11):59–64.
Chackrewarthy S, Thabrew MI, Weerasuriya MKB, Jayasekera S: Evaluation of the hypoglycemic and hypolipidemic effects of an ethylacetate fraction of Artocarpus heterophyllus (jak) leaves in streptozotocin-induced diabetic rats. Pharmacogn Mag 2010, 6(23):186–190. 10.4103/0973-1296.66933
Nair SS, Kavrekar V, Mishra A: Evaluation of in vitro antidiabetic activity of selected plant extracts. Int J Pharm Pharm Sci Invent 2013, 2(4):12–19.
Cochran WG, Cox GM: Experimental Designs. 2nd edition. New York: John Wiley and Sons, Inc; 1975.
Myers RH, Montgomery DC: Response Surface Methodology. 2nd edition. New York: John Wiley and Sons Inc; 2002.
Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, Perry NB: Extraction of phenolics and essential oil from dried sage ( Salvia officinalis ) using ethanol–water mixtures. Food Chem 2007, 101(4):1417–1424. 10.1016/j.foodchem.2006.03.050
Alothman M, Rajeev B, Karim AA: Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem 2009, 115(3):785–788. 10.1016/j.foodchem.2008.12.005
Tomsone L, Kruma Z, Galoburda R: Comparison of different solvents and extraction methods for isolation of Phenolic compounds from horseradish roots ( armoracia rusticana ). Eng Technol 2012, 64: 903–908.
Dent M, Dragovic-Uzelac V, Penic M, Brncic M, Bosiljkov T, Levaj B: The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage ( salvia officinalis L.) extracts. Food Technol Biotechnol 2013, 51(1):84–91.
Hsouna AB, Saoudi M, Trigui M, Jamoussi K, Boudawara T, Jaoua S, El Feki A: Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damage and renal failure in rats. Food Chem Toxicol 2011, 49(12):3183–3191. 10.1016/j.fct.2011.09.034
Rajaei A, Barzegar M, Hamidi Z, Sahari MA: Optimization of extraction conditions of phenolic compounds from pistachio (P istachia vera ) green hull through response surface method. J Agr Sci Tech 2010, 12: 605–615.
Chew KK, Khoo MZ, Ng SY, Thoo YY, Wan Aida WM, Ho CW: Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int Food Res J 2011, 18(4):1427–1435.
Londoño-Londoño J, Rodrigues de Lima V, Lara O, Gil A, Pasa TBC, Arango GJ, Pineda JRR: Clean recovery of antioxidant flavonoids from citrus peel: optimizing an aqueous ultrasound-assisted extraction method. Food Chem 2010, 119: 81–87. 10.1016/j.foodchem.2009.05.075
Zarina Z, Tan SY: Determination of flavonoids in Citrus grandis (Pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int Food Res J 2013, 20(1):313–317.
Radojkovic M, Zekovic Z, Jokic S, Vidovic S, Lepojevic Z, Milosevic S: Optimization of solid–liquid extraction of antioxidants from black mulberry leaves by response surface methodology. Food Technol Biotechnol 2012, 50(2):167–176.
Mohammad A, Bhawani SA, Sharma S: Analysis of herbal products by thin-layer chromatography: a review. Int J Pharma Biosci 2010, 1(2):1–50.
Syah YM, Achmad SA, Ghisalberti EL, Hakim EH, Mujahidin D: Two new cytotoxic isoprenylated flavones, artoindonesianins U and V, from the heartwood of Artocarpus champeden . Fitoterapia 2004, 75: 134–140. 10.1016/j.fitote.2003.11.005
Omar HS, El-Beshbishy HA, Moussa Z, Taha KF, Singab ANB: Antioxidant activity of Artocarpus heterophyllus lam. (jack fruit) leaf extracts: remarkable attenuations of hyperglycemia and hyperlipidemia in streptozotocin-diabetic rats. Sci World J 2011, 11: 788–800.
Bourjot M, Apel C, Martin M-T, Grellier P, Nguyen VH, Guéritte F, Litaudon M: Antiplasmodial, antitrypanosomal, and cytotoxic activities of prenylated flavonoids isolated from the stem bark of artocarpus styracifolius . Planta Med 2010, 76: 1600–1604. 10.1055/s-0030-1249777
Maneechai S, De-Eknamkul W, Umehara K, Noguchi H, Likhitwitayawuid K: Flavonoid and stilbenoid production in callus cultures of Artocarpus lakoocha . Phytochemistry 2012, 81: 42–49.
Di X, Wang S, Wang B, Liu Y, Yuan H, Lou H, Wang X: New phenolic compounds from the twigs of Artocarpus heterophyllus . Drug Discov Ther 2013, 7(1):24–28.
Schieber A, Berardini N, Carle R: Identification of flavonol and xanthone glycosides from mango ( Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 2003, 51: 5006–5011. 10.1021/jf030218f
Dubber M-J, Sewram V, Mshicileli N, Shephard GS, Kanfer I: The simultaneous determination of selected flavonol glycosides and aglycones in Ginkgo biloba oral dosage forms by high-performance liquid chromatography–electrospray ionisation–mass spectrometry. J Pharmaceut Biomed Anal 2005, 37: 723–731. 10.1016/j.jpba.2004.11.052
Bravo L, Goya L, Lecumberri E: LC/MS characterization of phenolic constituents of mate ( Ilex paraguariensis , St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res Int 2007, 40: 393–405. 10.1016/j.foodres.2006.10.016
Hong YJ, Tomas-Barberan FA, Kader AA, Mitchell AE: The Flavonoid Glycosides and Procyanidin Composition of Deglet Noor Dates ( Phoenix dactylifera ). J Agric Food Chem 2006, 54: 2405–2411. 10.1021/jf0581776
Sathishkumar T, Baskar R, Aravind M, Tilak S, Deepthi S, Bharathikumar VM: Simultaneous extraction optimization and analysis of flavonoids from the flowers of Tabernaemontana heyneana by high performance liquid chromatography coupled to diode array detector and electron spray ionization/mass spectrometry. ISRN Biotechnology 2013, 2013: 1–10.
Acknowledgement
The authors wish to express their sincere thanks to the SAIF, IIT-Mumbai for LC-MS analysis and Management of Kumaraguru College of Technology for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AS - Postgraduate student carried out the project work. TS - Project supervisor, framed the work design, contributed RSM design and LC-MS analysis, and prepared the manuscript. KK - First project review committee member and contributed extraction process. VS -Second project review committee member and contributed extraction process. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shakthi Deve, A., Sathish kumar, T., Kumaresan, K. et al. Extraction process optimization of polyphenols from Indian Citrus sinensis – as novel antiglycative agents in the management of diabetes mellitus. J Diabetes Metab Disord 13, 11 (2014). https://doi.org/10.1186/2251-6581-13-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-13-11